Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Heterotopias
Adenomatoid hyperplasia
Squamous metaplasia
Necrotizing sialometaplasia
Oncocytic changes (oncocytic metaplasia, oncocytosis)
Intercalated duct lesions (intercalated duct hyperplasia; [intercalated duct adenoma])
Lymphoepithelial cyst
Salivary duct cyst
Polycystic (dysgenetic) disease
Mucus extravasation phenomenon
Mucus retention cyst
Ranulas
Bacterial sialadenitis
Mumps
HIV salivary gland disease
Chronic sialadenitis
Nonobstructive
Infectious
Noninfectious
Obstructive
Sialolithiasis
Sialadenosis
IgG4-related sialadenitis
Lymphoepithelial sialadenitis
Sjögren syndrome
See Section 7, The Ear and Temporal Bone.
Definition: Salivary gland tissue located in sites other than those appropriate for the normal anatomic distribution of salivary glands.
Synonyms: Ectopic salivary glands; salivary gland choristoma
Majority of heterotopic salivary gland tissue occurs in head and neck sites.
Most common locations include the periparotid lymph nodes, the middle ear, and the lower neck; less frequent sites of occurrence include:
Upper neck
External ear (auditory canal), middle ear
Intraosseous sites (e.g., mandible)
Cerebellopontine angle
Pituitary gland
Salivary gland tissue has been reported in thyroglossal duct, capsules of the thyroid gland and parathyroid glands, mediastinum, tonsils, and gingiva, as well as more distant sites, including the prostate gland, vulva, and rectum.
Most of these examples occur in the lower anterolateral neck along the medial border of the sternocleidomastoid muscle.
Most common presenting symptom is a draining sinus on the anterior aspect of the neck along the medial border of the sternocleidomastoid muscle near the sternoclavicular joint.
Sinuses drain saliva-like material, usually a very limited quantity but the amount may increase during meals.
Localized nontender swelling associated with the sinus may be the only complaint.
Histologically, the tissue includes normal salivary gland tissue either purely serous or mixed serous and mucinous glands.
Not technically heterotopic but represents normal development
Generally accepted explanation for the occurrence of salivary gland tissue in periparotid lymph nodes is entrapment during embryonic development rather than true ectopia, although this issue is still the subject of debate.
Ontogenically, the parotid gland is the last of the salivary glands to be encapsulated, resulting in either incorporation/entrapment of lymphoid tissue within the parotid or incorporation/entrapment of parotid ducts and acini within the periparotid lymph nodes epithelium.
Histologically, the salivary gland tissue includes all components of the salivary gland unit including ducts and acini but more often consists of serous glands with scattered, well-formed ducts.
All pathologic lesions (non-neoplastic and neoplastic) may originate from the intranodal salivary gland tissues, including:
Cysts, metaplasia, hyperplasia, and benign epithelial ductal proliferations
Benign and malignant salivary gland neoplasms:
Represent primary intranodal neoplasms with the gland proper being devoid of tumor
A malignancy arising from intranodal salivary gland tissue:
May present as mass lesion within the node separate from adjacent major salivary glands
In the absence of an identifiable primary neoplasm from an adjacent salivary gland, these intranodal foci can be considered as the primary site for the development of the malignancy.
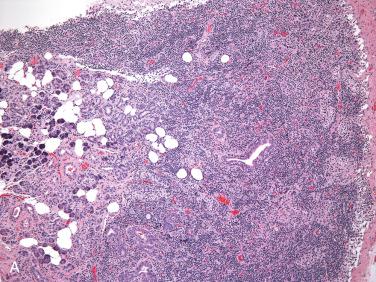
See Section 7, The Ear and Temporal Bone.
Heterotopic salivary gland tissue in the middle ear is discovered in the evaluation of patients for conductive hearing loss.
Most cases are associated with ossicular abnormalities of the incus and stapes.
Patients range in age from the first to sixth decades but most are under 21 years of age.
Because the ossicles originate from the first (malleus and incus) and second (stapes) branchial arch, the presence of salivary gland tissue is considered a developmental abnormality involving the branchial arches.
Heterotopic salivary gland tissue has been seen in association with anomalies that suggest the branchio-otorenal (BOR) syndrome; in addition, a syndrome including salivary gland choristoma in association with branchial arch abnormalities, most commonly the second, as well as abnormalities of the facial nerve, has been identified.
Salivary gland tissue in the middle ear:
May extend into the eustachian tube, involve the mastoid bone, and may be intimately associated with the facial nerve
Represents an admixture of seromucinous glands and adipose tissue; the latter contributes to the yellow appearance of the tissue
Recapitulates the acinar arrangement of normal salivary glands; ducts may be identified
Is situated within the submucosa
In general conservative surgical resection is curative; however, this tissue is slow growing and in conjunction with potential complications from surgery, particular to the facial nerve surgical intervention may not be advised.
A biopsy may be required to establish a diagnosis.
Intraosseous salivary gland tissue is uncommon and tends to occur in the posterior mandible near the angle beneath the mandibular canal and less frequently in the anterior mandible.
Intraosseous salivary gland tissue is asymptomatic and is an incidental radiographic finding.
No treatment is required.
Salivary gland neoplasms occurring in sites that normally do not contain salivary gland tissue are usually considered to originate from heterotopic salivary gland tissue.
An exception to the above statement includes those salivary gland tumors arising in periparotid lymph nodes without an identifiable parotid gland mass:
Clinical setting is one in which the intranodal salivary gland tumor, specifically a malignant tumor, is considered as being of “unknown primary origin.”
Clinical and radiologic search for the primary salivary gland tumor proves negative.
Entrapment of salivary gland parenchyma within lymph nodes during embryonic development accounts for the intranodal salivary gland tissue.
Salivary gland neoplasms, benign and malignant, may originate within periparotid nodal salivary gland parenchyma presenting as a mass separate from adjacent major salivary glands (i.e., parotid gland).
In the absence of an identifiable primary neoplasm from an adjacent salivary gland, these intranodal foci can be considered as the primary site.
A wide variety of tumor types occur in salivary gland heterotopia, including (but not limited to):
Pleomorphic adenoma, monomorphic adenomas (e.g., Warthin tumor), acinic cell adenocarcinoma, mucoepidermoid carcinoma, adenoid cystic carcinoma, adenocarcinoma not otherwise specified, carcinoma ex pleomorphic adenoma, sialoblastoma, others
Represent isolated lobules of parotid gland parenchyma separated from the main body of the gland situated along a major salivary duct
Incidence reported up to 56% in an autopsy series
Accessory parotid tissue is spheric or oblong, measuring from 0.5 to 3 cm in greatest dimension.
Accessory tissue can be seen anterior to the border of the masseter situated on the buccal fat pad.
Accessory tissue is normally connected to Stensen duct by a single accessory duct, although more than a single duct may be found.
Clinically, lesions of accessory parotid tissue present with mass in the cheek.
Histologically, accessory tissue is identical to the normally situated parotid tissue and reflects similar pathologic processes that the main gland may exhibit (e.g., inflammation, fatty infiltrate, other).
Benign and malignant salivary gland tumors may arise in accessory parotid glands:
Benign tumors may include pleomorphic and monomorphic adenomas, Warthin tumor, others.
Malignant tumors reported include a wide variety of types, including (but not limited to) mucoepidermoid carcinoma, acinic cell adenocarcinoma, adenoid cystic carcinoma, carcinoma ex pleomorphic adenoma, others.
Magnetic resonance imaging is helpful in the diagnosis and treatment of accessory parotid gland tumors.
Best surgical approach to tumors in the accessory parotid region is via a standard parotid incision and concomitant superficial parotidectomy; this approach to accessory parotid gland tumors is superior in that it provides a better margin of resection and minimizes functional and cosmetic deformities.
Definition: Hyperplastic or hamartomatous proliferation of the mucous acini of salivary gland tissue.
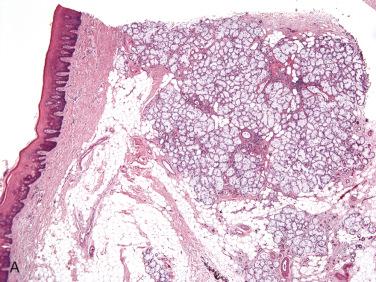
Relatively uncommon lesion that tends to occur slightly more often in men than in women
Occurs in all age groups, although it is more common in adults than in children
Majority of cases involve the hard or soft palates; less common sites of occurrence include the retromolar mucosa; involvement of major salivary glands has not been reported.
Clinical presentation includes a localized painless mass often discovered during routine dental examination; ulceration of the overlying mucosa is not present:
Often mistaken for a salivary gland neoplasm
Cause is unknown and there is no association with sialadenosis or trauma.
Lesions are firm and sessile, varying in size from 0.5 to 3 cm.
Submucosal proliferations of hypertrophied and/or hyperplastic lobules of otherwise normal-appearing mucinous acini
No significant inflammation or fibrosis are seen.
Overlying epithelium is unremarkable, although pseudoepitheliomatous hyperplasia may be present.
Salivary gland neoplasms:
Submucosal lobular configuration composed of a single cell type (i.e., mucinous acini) should allow for differentiation from benign and malignant salivary gland neoplasms.
Surgical excision is curative.
Relatively common metaplastic changes that can be seen in a wide variety of lesions, including non-neoplastic lesions and neoplasms (benign and malignant)
May occur spontaneously or occur as secondary to a traumatic event such as fine-needle aspiration biopsy or biopsy
For illustrations see Chapter 20 under Pleomorphic Adenoma and Warthin Tumor.
Definition: Benign, self-healing (reactive) inflammatory process of salivary gland tissue, which clinically and histologically may be mistaken for a malignant neoplasm.
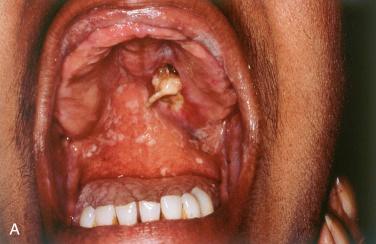
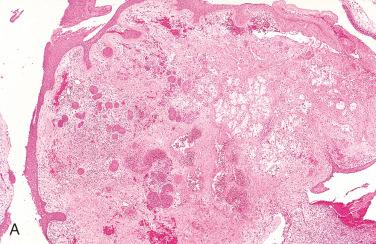
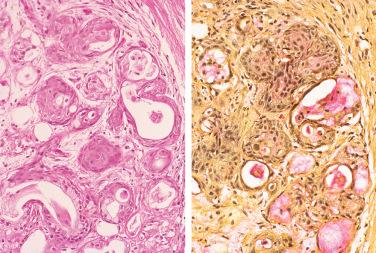
Synonym: Adenometaplasia
Tends to affect men more than women; occurs over a wide age range with the average age of occurrence in the fifth and sixth decades of life
Most commonly involves the intraoral minor salivary glands, particularly involving the palate:
Major salivary glands, as well as minor salivary glands of virtually every site in the upper aerodigestive tract, can be affected.
Larynx may be affected but is a rare site of occurrence.
Most common presenting problem is that of a painless ulcerated lesion or a nodular swelling, which is usually unilateral but may be bilateral:
May be associated with pain, numbness, or a burning sensation and dysphagia
Uncommonly, may present with anesthesia of the greater palatine nerves
Pathogenesis:
Believed to be secondary to trauma and/or an ischemic event with compromise of the vascular supply to salivary glands leading to ischemic necrosis:
Ischemia may be iatrogenically induced after an operative procedures (surgery, postintubation, postbronchoscopy), anesthesia, or radiotherapy.
Mean duration of 18 days from the time of the insult to the development of the lesion
In experimental studies on rat submandibular and sublingual glands, the induction of sialometaplasia occurred 6 to 8 days after arterial ligation.
Inciting event is primarily but not exclusively thought to be due to ischemia.
May occur de novo unassociated with a traumatic event or it may occur in association with other non-neoplastic lesions or in association with a neoplasm (benign or malignant)
Typically appears as a deep, crater-like ulcerative lesion, measuring from 1 to 3 cm:
May appear as a submucosal nodular swelling that may slough, leaving a crater-like ulcer
At low magnification there is preservation of the lobular architecture of the minor salivary glands.
Histologic hallmark is squamous metaplasia of residual acinar and ductal elements:
Squamous cells are typically bland in appearance with uniform nuclei and abundant eosinophilic cytoplasm.
Preservation of ductal lumina and/or scattered mucocytes may or may not be identified.
Lobular architecture is maintained and metaplastic lobules vary slightly to moderately in size and shape, have smooth edges.
Metaplastic foci may be surrounded by granulation tissue and mixed acute and chronic inflammatory reaction.
In some examples, the squamous metaplastic foci may be atypical, including irregular contours of metaplastic lobules with nuclear hyperchromasia, increased nuclear-to-cytoplasmic ratio, dyskeratosis, and mitoses, suggesting a possible diagnosis of squamous cell carcinoma:
Preservation of lobular architecture of the involved minor salivary glands should allow for differentiation from carcinoma but in any given example it may be challenging differentiating NS from squamous cell carcinoma.
Low proliferation rate (<10%) by Ki67 staining and absence of p53 reactivity may support a diagnosis of NS but do not unequivocally differentiate NS from squamous cell carcinoma.
Presence of myoepithelial cells along the periphery of the metaplastic lobules as determined by reactivity for myoepithelial cell-related markers (e.g., p63, calponin, smooth muscle actin) and keratin subtypes in NS suggested as possible differentiating findings from squamous cell carcinoma but do not unequivocally differentiate NS from squamous cell carcinoma
Necrotic lobules consist of acinus-sized pools of mucin, which may extend into adjacent tissue, eliciting a granulation tissue reaction with associated acute and chronic inflammation:
Necrotic lobules may not be present in all cases so that the designation of sialometaplasia without “necrotizing” would be more appropriate in such a situation.
With regeneration, mitoses, individual cell necrosis, enlarged nuclei, and prominent nucleoli can be seen.
Associated findings include ulcerated mucosa and pseudoepitheliomatous hyperplasia (PEH):
PEH results when the metaplastic lobules present in excretory ducts and merge with surface epithelium.
This reaction may be so striking, presenting a diagnostic nightmare in separation from an infiltrating squamous cell carcinoma.
Histochemistry:
Intraluminal and/or intracytoplasmic mucicarmine and diastase-resistant, PAS-positive material may be identified.
Mucoepidermoid carcinoma
Adenosquamous carcinoma
Squamous cell carcinoma
NOTE: The retention of the overall lobular architecture, bland appearance of the squamous nests with rounded or smooth edges, and retention of residual ductal lumina and mucocytes help in differentiating NS from the malignant neoplasms listed above.
| NS | MEC, Low-Grade | SCC | |
|---|---|---|---|
| Architecture/growth | Retention of lobular architecture | Cystic and solid; may be circumscribed to encapsulated without invasion or show infiltrative growth | Haphazard, infiltrative growth |
| Cellular components | Smooth round to oval nests of metaplastic squamous epithelium with bland cytology; may show residual ductal lumina with mucous cells | Admixture of mucous, intermediate (“basaloid”) and epidermoid (squamous) cells; bland cytology; irregular cell nests | Nests and cords of squamous cells with irregular outlines and variable amount of cytologic atypia; may entrap residual glands but the tumor itself contains no mucin |
| Cyst formation | Absent | Present (prominent component) | Absent |
| Surface epithelium | May show PEH; usually not connected with NS | Uninvolved; not connected with tumor | Often dysplastic and/or in direct continuity with the carcinoma; may be ulcerated |
| Extravasated mucin | May be present | May be present | Absent |
| Necrosis of salivary gland lobules | May or may not be present | Absent | Absent |
| Inflammation | May be prominent | May be prominent with mucin extravasation | May be present; associated desmoplasia |
| Cytogenetics | None known | CRTC1-MAML2 translocation | None known |
NS are self-limiting lesions that heal by secondary intention:
Depending on the size of the lesion, the healing process in most cases occurs from 3 to 12 weeks.
Debridement and saline rinses may aid in the healing process.
Recurrences do not usually occur.
Nonspecific inflammatory condition of unknown cause affecting oral minor salivary glands:
Some authorities believe that SANS should not be included within the spectrum of necrotizing sialometaplasia and most likely represents an infectious process or perhaps an immune response to an unknown allergen.
Other authorities believe SANS may represent the early or minimal form of NS.
In either case, SANS most often is characterized by a localized palatal swelling, accompanied by an abrupt onset of pain.
Patients range in age from 15 to 45 years, with a mean age of 21.9 years.
SANS most often affects intraoral sites, including the hard palate, soft palate, buccal mucosa, and tonsils.
Lesions typically are nonulcerated swellings, develop over a short period of time (7 to 10 days), and range in size from 0.3 to 2.5 cm in diameter.
Histopathologic features include:
Diffuse involvement of minor salivary glands by lymphocytes, histiocytes, neutrophils, and variably by eosinophils
Loss of acinar cells, early acinar cell necrosis surrounded by a dense polymorphous inflammatory infiltrate, and atrophy of ductal cells
Squamous metaplasia is not usually seen.
Appears to be a self-limiting process with most cases resolving 2 to 3 weeks after biopsy without recurrences
Main differences between SANS and NS include:
SANS is usually a smaller-sized lesion than lesions of NS.
Scarcity of ulceration in SANS but typically present in NS
Absence of squamous metaplasia in SANS but present in NS
Oncocytic cells in the salivary glands occur in the following settings:
Oncocytic metaplasia
Oncocytosis (nodular or diffuse)
Oncocytoma; oncocytic carcinoma
Warthin tumor
Variety of other lesions/tumors that may have oncocytic cells including but not limited to:
Pleomorphic adenoma
Basal cell adenoma
Myoepithelioma
Canalicular adenoma
Mucoepidermoid carcinoma
Acinic cell carcinoma
Others
Non-neoplastic transformation of ductal and acinar epithelium to oncocytes:
Oncocytes are histologically characterized by cytoplasmic alteration (metaplasia) of epithelial and/or myoepithelial cells with swelling of the cytoplasm by mitochondrial hyperplasia giving the cell a characteristic granular eosinophilic appearance by light microscopy.
Represents an aging phenomenon:
Oncocytic metaplasia is generally not seen in patients less than 50 years of age from which time the percentage of the population with oncocytic metaplasia increases.
In contrast to oncocytoma (and oncocytosis), oncocytic metaplasia is non–mass-forming focal or limited changes in one or more areas within the salivary gland.
Appear as isolated clusters of oncocytic cells within otherwise normal-appearing parenchyma
Clear cell changes may be present.
Usually represents incidental histologic findings in salivary glands excised for other reasons
Oncocytic cells may be seen in other non-neoplastic processes (see oncocytosis immediately following), as well as in numerous salivary gland tumors, including pleomorphic adenomas, Warthin tumor, oncocytoma, mucoepidermoid carcinoma, acinic cell carcinoma, oncocytic carcinoma, others.
Oncocytosis (also referred to as oncocytic [adenomatous] hyperplasia) represents a non-neoplastic mass–forming proliferation of oncocytic cells within the salivary gland.
Typically appear as nodular foci, referred to as nodular oncocytic hyperplasia or nodular oncocytosis
Less commonly, may represent a diffuse alteration in the affected salivary gland referred to as diffuse oncocytosis
In either nodular or diffuse form may present as a clinically detectable mass lesion presenting difficulties in differentiation from oncocytoma
Histologically, oncocytotic foci:
Include multiple (often two or more) separate nodules
Unencapsulated
Gradual merge with and/or contain residual (nononcocytic) salivary gland parenchyma, including ductular epithelium and serous acinar cells
Such histologic findings assist in differentiating oncocytosis from oncocytoma, the latter entirely composed of oncocytes without identifiable residual normal parenchyma.
May show clear cell change
Differentiation of oncocytosis from oncocytoma may not be possible due to overlapping histologic features, and this differentiation may be more of an academic than practical issue because treatment and prognosis are essentially similar (i.e., cured by excision).
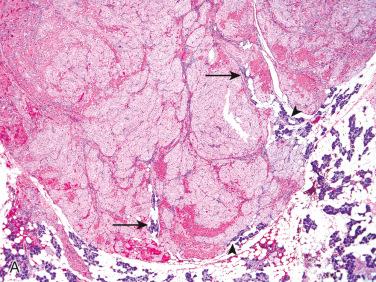
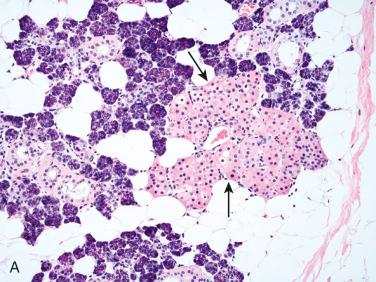
Definition: Rare lesions that include hyperplasia, adenoma, or both (hybrid) that may be a precursor lesion of salivary gland neoplasms:
Proposal that IDL may represent precursor lesion to salivary gland neoplasms based on presence of small foci of IDL in cases of basal cell adenoma, epithelial-myoepithelial carcinoma, pleomorphic adenoma, mucoepidermoid carcinoma, basal cell adenocarcinoma, Warthin tumor, acinic cell carcinoma, others
Role as a precursor lesion not definitively proven
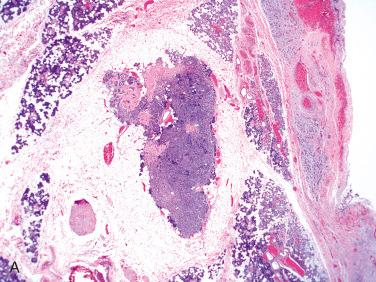
Rare lesion
More common in females than in males; occur over a wide age range from second to ninth decades with a mean in the sixth decade
Majority occur in the parotid gland > oral cavity > submandibular gland.
Typically represent incidental finding found in association with another lesion
Usually a small lesion measuring less than 5 mm in greatest dimension
Most common type of IDL
Characterized by unifocal or multifocal (and diffuse) unencapsulated proliferation of small ducts with minimal intervening stroma merging/blending imperceptively with acinar and mucous cells of surrounding salivary gland parenchyma
At low magnification appear as irregular pale foci contrasted with the surrounding darker-appearing salivary gland parenchyma:
In multifocal lesions, foci vary in size and shape.
Intercalated ducts are lined by single layer of cuboidal to columnar cells with small round nuclei and eosinophilic to amphophilic cytoplasm:
There is an absence of nuclear pleomorphism or increased mitotic activity.
Acinic cells may be incorporated within the proliferation and/or also identified at the periphery of most lesions.
Myoepithelial cells are consistently present around the ducts but are not discernible by light microscopy requiring immunohistochemical staining with p63 or other markers (e.g., calponin, CK14, others) for identification.
Stromal hyalinization that may be periductal can be identified.
Other uncommon features may include:
Perilesional follicular lymphoid hyperplasia
Clear myoepithelial cells
Entrapment of nerve within the lesion
Presence of focal luminal eosinophilic secretions
Cystic change
Basal lamina-like material with a cribriform pattern
Absence of fat cells or intralesional inflammatory infiltrates
Presence of discrete, rounded, partially to completely encapsulated nodules with well-defined contours
Fibrous capsule may vary in thickness and may contain entrapped, irregular-appearing ducts.
Composed of intercalated ducts lined by a single layer of cuboidal to columnar cells with small round nuclei and eosinophilic to amphophilic cytoplasm:
There is an absence of nuclear pleomorphism or increased mitotic activity.
Minimal intervening stroma present
Occasionally acinar cells may be interspersed among the ductular structures.
Least common type of IDL
Partially round, encapsulated adenoma-like appearance admixed with irregular hyperplasia-like areas
Give the impression of a transition from hyperplastic intercalated ducts to adenomatous areas
Alternatively may include a completely encapsulated adenoma with separate discrete, hyperplastic foci immediately adjacent to the capsule.
Entrapped irregular ductal structures can be identified within capsule of the adenoma.
Clear intercalated duct cells may be present.
Immunohistochemistry
CK7, S100 protein, estrogen receptor, lysozyme positive; progesterone receptor negative
Myoepithelial cells positive for calponin and CK14
No specific treatment required
Given their usual small size typically cured in the excision without known untoward biology
More critical issues relative to treatment and prognosis relate to associated neoplasms some of which include malignant salivary gland neoplasms (see under definition earlier).
Most cystic lesions of major salivary glands represent cystic neoplasms.
True cysts differ from pseudocysts by the presence of an epithelial lining.
Most true cysts of major salivary gland arise in the parotid gland and are divided into three general categories:
Lymphoepithelial cyst
Salivary duct cyst
Polycystic (dysgenetic) disease
Definition: Benign true cystic lesion of the parotid gland of uncertain origin with characteristic histology and unrelated to human immunodeficiency virus (HIV) infection.
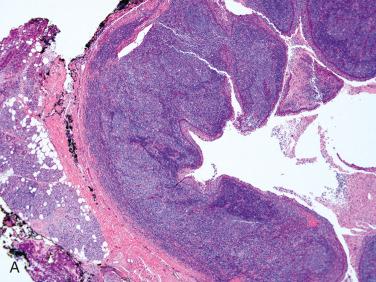
Uncommon acquired parotid gland cyst
Slightly more common in men than in women; occurs over a wide age range including at birth to the eighth decade of life; the average age is in the fifth decade of life
May be present at birth but the clinical manifestations may not become apparent until adult life
Presents as painless swelling of the parotid gland
Majority are unilateral although bilateral lesions may occur.
May occasionally be tender or painful, which may be due to secondary infection
Rarely, may be associated with facial paralysis
Histogenesis remains controversial:
Suggested to originate from the branchial apparatus and/or develop from salivary gland inclusions in lymph nodes
Designation of lymphoepithelial cyst is histologically accurate and is more appropriate than branchial cleft cyst.
Some authors believe that LECs, as well as salivary duct cysts and dysgenetic or congenital cysts, arise from salivary ducts and have no relation with the branchial apparatus.
Cyst is usually sharply circumscribed, fluctulent and unilocular, rubbery to firm ranging in size from 0.5 to 6 cm in greatest dimension
Multilocular cysts are uncommon.
On cut section, small intracystic protrusions may be present, imparting a granular appearance to the lesion; the latter correlate to the presence of the lymphoid component in the cyst wall (see later).
Usually contain caseous material with a yellow-white appearance
Sharply circumscribed from the surrounding parotid parenchyma and is separated from the parotid tissue by fibrous tissue
Lined by a variety of epithelial cell types, including squamous, cuboidal, columnar, and pseudostratified types; mucous (goblet) cells, sebaceous cells, and oncocytic metaplasia may be present.
Cyst wall composed of an abundant mature lymphoid cell proliferation with readily identifiable germinal centers
Immunohistochemistry:
Lymphoid component shows expression for B-cell (CD20, others) and T-cell (CD3, others) markers.
Absence of Epstein-Barr virus (e.g., in situ hybridization for Epstein-Barr encoded RNA)
Absence of p24 staining (marker for HIV infection)
Other salivary gland true cysts, as well as cystic neoplasms:
LECs may be mistaken for cystic lymphoepithelial sialadenitis (LESA):
In contrast to LECs, the cystic LESA has:
Characteristic lymphoepithelial cell lesions
Tendency of the lymphocytes to migrate through the epithelial component
Cystic LESA lack features that can be seen in LECs, including mucous (goblet) cells.
HIV-salivary gland disease (see later in this chapter):
Histology of LECs is similar to cystic lymphoepithelial lesions in HIV-associated salivary gland disease.
Absence of features that might suggest associated with HIV infection including absence of:
Florid follicular hyperplasia
Multinuleated giant cells
Lymphoepithelial cell islands
Presence of p24 immunoreactivity in HIV-associated lesions
Marked decrease in interfollicular CD4:CD8 ratio observed in HIV+ compared with the HIV negative cases
Among the cystic neoplasms of the parotid that LECs may be confused with is Warthin tumor and cystic mucoepidermoid carcinoma (cystic MEC):
In contrast to Warthin tumor, LECs are unilocular, lack papillary architecture, and lack an epithelial cell component with oncocytic cytoplasmic changes.
Cystic MECs are usually low-grade malignancies characterized by the presence of an admixture of mucous cells, epidermoid cells, and intermediate cells.
These three cell types are absent in LEC.
Further, MECs tend to be infiltrative neoplasms, a feature that is not present in LECs.
Confusion with metastatic cystic squamous cell carcinoma may occur, but LECs lack the cytologic atypia and increased mitotic activity usually seen in metastatic cystic squamous cell carcinoma.
Surgical excision is curative.
Definition: Acquired cyst believed to develop due to ductal obstruction with marked cystic dilation of a salivary gland duct.
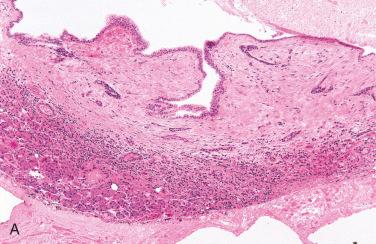
Synonyms: Acquired, simple, and retention cyst
Most common salivary gland cysts
Represent approximately 2% to 3% of all parotid gland lesions
No gender predilection; occur over a wide age range from early childhood to older adults but most patients are over 30 years of age
Clinical presentation includes unilateral painless swelling.
Approximately 85% occur in the parotid gland with 10% in submandibular gland; remainder in various other salivary gland sites.
Obstructive changes that may result in the development of the salivary duct cyst include neoplasms, postinflammatory strictures, calculi, and mucus plugs.
Well-circumscribed and are usually uniloculated containing thin, watery, to viscous brown fluid
Majority are 1 to 3 cm, although may reach as large as 10 cm.
Sharply demarcated from the adjacent salivary gland parenchyma
Epithelium lining of the cysts may be single or multilayered cuboidal, columnar, or squamous epithelium; mucus-containing goblet cells and oncocytic metaplasia may be seen.
Cyst wall is composed of collagenized connective tissue of varying thickness.
Sparse to minimal chronic inflammatory cell infiltrate can be present.
Granulomatous inflammation may be present in the cyst wall and in the adjacent gland.
Adjacent salivary gland parenchyma may show duct ectasia with inspissated secretions and chronic inflammation.
Lymphoepithelial cyst:
Marked inflammatory cell component typically seen in lymphoepithelial cysts is not seen in salivary duct cysts.
Cystic neoplasms in particular mucoepidermoid carcinoma:
In contrast to cystic mucoepidermoid carcinoma (MEC), salivary duct cysts lack the proliferative (hyperplastic) cellular features as well as combination of cell types (i.e., epidermoid cells, mucocytes and intermediate cells) seen in MEC:
Infiltrative growth would further support a diagnosis of a malignant neoplasm but a number of malignant salivary gland neoplasms including MEC may not be infiltrative and still represent a carcinoma (see Chapter 20 for more complete discussion).
Surgical excision is curative.
Rarely, salivary gland neoplasms have been associated with salivary duct cysts.
Definition: Rare developmental abnormality of the salivary gland duct system histologically similar to polycystic diseases of other organs (e.g., kidney, liver, pancreas, and lungs).
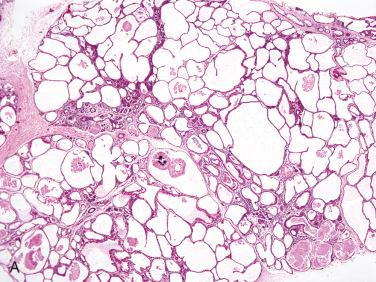
Polycystic disease represents approximately 0.2% of benign salivary gland cysts with less than 20 cases reported in the world literature.
The overwhelming majority of the reported cases involve the parotid gland with rare involvement of the submandibular gland.
Women are almost always affected and the majority of cases occur in childhood although patients range in age from the first to the seventh decade of life.
Most patients have bilateral gland involvement, but occasionally only a single gland is involved.
The clinical presentation includes recurrent, painless swelling with abnormalities in the flow of saliva.
Based on a single case of familial occurrence, an autosomal dominant mode of transmission has been suggested.
Aspirate smears characterized by relatively clean background, in which are distributed histiocytes, red blood cells, and small clusters of ductal epithelial cells
Characteristic eosinophilic laminated spheroliths lie in many of the cystic spaces.
On cut section the enlarged gland is spongy in consistency with apparent variable-sized cysts.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here