Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Musculoskeletal pain of noninflammatory origin is common in childhood and is a frequent cause of referral to pediatric rheumatologists, orthopedic surgeons, sports medicine specialists, and primary care physicians. Noninflammatory causes of pain are more common than inflammatory ones, and early identification and differentiation from other causes of musculoskeletal pain, such as infection or malignancy, are essential to the institution of appropriate therapy and avoidance of inappropriate investigations. Children and adolescents with inflammatory arthritis may develop mechanical pain secondary to muscle tendon imbalances exaggerated by anatomical alignment, neuromuscular or proprioceptive deficits, rapid growth, or change in activity level.
The term joint hypermobility syndrome (JHS) was first described in 1967 as musculoskeletal symptoms associated with generalized hypermobility without any associated congenital syndrome or abnormality of connective tissue. Prevalence estimates vary from 7% to 36% depending on tests and criteria cutoff points. The criteria for hypermobility have evolved over the years with a systematic review recommending the 9-point Beighton score with a cutoff point of at least 6 for children older than 5 years of age ( Box 50.1 ). , Hypermobility is more common in girls and decreases with age. Asian and African races are more hypermobile than Caucasians. A family history of hypermobility is common.
≥6 points defines hypermobility:
Touch thumb to volar forearm (one point each for right and left)
Extend fifth metacarpophalangeal joint to 90 degrees (one point each for right and left)
>10-degree hyperextension of elbow (one point each for right and left)
>10-degree hyperextension of knee (one point each for right and left)
Touch palms to floor with knees straight (one point)
Some children with generalized joint hypermobility develop pain. The Brighton criteria are commonly used to diagnose benign JHS (BJHS) ( Box 50.2 ). However, the term benign may not be appropriate because many affected individuals have other symptoms. As such, JHS is preferred.
A Beighton score of 4/9 or greater (currently or historically)
Arthralgia for longer than 3 months in four or more joints
A Beighton score of 1, 2, or 3/9
Arthralgia in 1–3 joints or back pain or spondylolysis, spondylolisthesis
Dislocation in more than 1 joint, or on one joint on more than one occasion
Three or more soft tissue lesions (e.g., epicondylitis, tenosynovitis, bursitis)
Marfanoid habitus (tall, slim, span > height, upper segment/lower segment ratio less than 0.89, arachnodactyly)
Skin striae, hyperextensibility, thin skin, or abnormal scarring
Eye signs: drooping eyelids, myopia, or antimongoloid slant
Varicose veins or hernia or uterine/rectal prolapse
The 2017 International Classification of the Ehlers–Danlos Syndromes suggests JHS patients be included with the hypermobile Ehlers–Danlos syndromes (hEDS) group because they are essentially identical entities. hEDS has a clinical spectrum from asymptomatic joint hypermobility to nonsyndromic hypermobility symptoms. The molecular basis for hEDS is unknown, suggesting there is genetic heterogeneity. There are specific criteria to be met for a clinical diagnosis of hEDS, which include the simultaneous presence of criteria 1, 2, and 3. Criterion 1 requires generalized joint hypermobility with a Beighton score of at least 6 of 9 for prepubertal children and adolescents. Criterion 2 mandates at least two of the following features: (1) five of the following list: soft/velvety skin, mild skin hyperextensibility, unexplained striae, bilateral piezogenic papules of the heel, recurrent or multiple hernias, atrophic scarring, dental crowding, arachnodactyly, pelvic floor prolapse, arm span-to-height ratio 1.05 or more, aortic root dilation, and mitral valve prolapse; (2) positive family history with at least one first-degree relative meeting current criteria for hEDS; and (3) at least one of the following: musculoskeletal (MSK) pain in two or more limbs for 3 months or longer, chronic widespread pain for 3 months or longer, or recurrent joint dislocations without prior trauma. Criterion 3 recommends all of the following be met: absence of unusual skin fragility, exclusion of other acquired and heritable connective tissue disorders, and exclusion of alternate diagnoses such as other types of EDS, Marfan syndrome, Loeys–Dietz syndrome, and skeletal dysplasias ( Table 50.1 ). There are many other features of hEDS that are not in the criteria including fatigue, postural orthostatic tachycardia, dysautonomia, functional gastrointestinal disorders, anxiety, and depression. These features, in addition to pain, often reduce the patient’s quality of life. Premature osteoarthritis has been suggested to be a result of hypermobility, but longitudinal studies have not confirmed this association.
| Clinical EDS Subtype | Abbreviation | IP | Genetic Basis | Protein | |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
||||
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|||||
|
|||||
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
||||
|
|
||||
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Heritable disorders of connective tissue (HDCT) must be considered in patients presenting with hypermobility. Several such syndromes are listed in Box 50.3 ; characteristic phenotypes and genetic testing help confirm the diagnosis. Reduced muscle strength, balance, and head and trunk stability are more prevalent in some hypermobile children, suggesting delayed locomotor development. In addition, children with developmental coordination disorder (with motor coordination below expected for chronological age and intelligence) are frequently hypermobile.
Tall and thin
Arm span greater than height
Lower ratio of upper-body segment to lower-body segment (long legs); normal ratio is 0.85 in whites and 0.92 in blacks
Arachnodactyly
Pectus excavatum or carinatum
Kyphoscoliosis
Dislocation of the lens of the eye
Aortic root dilatation
Heart murmurs, midsystolic click
Hernias
Autosomal dominant disorder resulting from mutations of fibrillin gene on chromosome 15
Marfanoid habitus
Major risk of thrombotic events
Autosomal recessive disorder usually associated with cystathionine β-synthase deficiency resulting from mutations of gene on long arm of chromosome 21
Marfanoid habitus
Typical facial appearance: malar hypoplasia, depressed nasal bridge, epicanthal folds, micrognathia
Cleft palate (Pierre Robin sequence)
Severe myopia (may lead to retinal detachment)
Sensorineural hearing loss
Mitral valve prolapse
Autosomal dominant disorder resulting from mutations of type II collagen gene on chromosome 12
(Mutation either TGFBR1 or TGFBR2 ; SMAD3 or TGFB2 genes)
Enlargement of the aorta which may develop aneurysms or dissection
Arterial tortuosity
Easy bruising
Translucent skin often with striae and visible underlying veins
Hypertelorism
Strabismus
Spontaneous pneumothorax
Hernias
Bifid uvula
Cleft palate
Craniosynostosis
Scoliosis
Pectus excavatum
Pectus carinatum
Club foot
Pes planus
Elongated limbs with contractures
Dural ectasias
Instability of C-spine
Osteoarthritis
Prone to food allergies, asthma, eczema and inflammatory bowel disease
(Mutations in MTHFR , MTR , MTRR , and MMADHC genes)
Marfanoid habitus
Major risk of thrombotic events
Autosomal recessive disorder usually associated with cystathionine β-synthase deficiency resulting from mutations of gene on long arm of chromosome 21
(Mutations in COL2A1 , COL11A1 genes)
Marfanoid habitus
Typical facial appearance: malar hypoplasia, depressed nasal bridge, epicanthal folds, micrognathia
Cleft palate (Pierre Robin sequence)
Severe myopia (may lead to retinal detachment)
Sensorineural hearing loss
Mitral valve prolapse
Autosomal dominant disorder resulting from mutations of type II collagen gene on chromosome 12
(Mutations in COL1A1 , COL1A2 , CRTAP , P3H1 genes)
Blue sclerae
Fragile bones with multiple fractures and deformities
Short stature
Spinal deformity
Different types; usually autosomal dominant inheritance
Involves abnormalities of type I collagen
Short stature
Characteristic elfin facial appearance
Hoarse voice
Friendly and loquacious
Developmental delay
Supravalvular stenosis
Occasionally hypercalcemia
Initially hypermobile but later become hypomobile without pain
Sporadic and inherited cases resulting from deletion of elastin allele on chromosome 7
Hypotonia
Developmental delay
Characteristic facial appearance; epicanthal folds
Short stature
Endocardial cushion defects
Broad hands with simian creases
Brushfield (depigmented) spots of the iris
Usually occurs in a sporadic fashion
Many other youth with hypermobility have higher motor proficiency and greater exercise capacity. They may sustain injuries like patellofemoral pain syndrome (PFP), frequent ankle sprains, and temporomandibular joint dysfunction with disc displacement. ,
Explanation of the problem with reassurance that there is no underlying disease is all that is needed in most cases. Although hypermobility may enable a child to be a good gymnast or ballet dancer, injuries may be more frequent. Supportive footwear is helpful for many. Some children benefit from a postactivity or evening dose of acetaminophen or a nonsteroidal antiinflammatory drug (NSAID). Older, more severely affected children may improve with formal physical therapy that focuses on overall reconditioning and the reestablishment of normal muscle power. Taping or bracing of troublesome joints may be advantageous. Orthotics have a positive influence on the immediate gait patterns in hypermobile patients. Those with more widespread pain may benefit from the addition of a psychologist-led cognitive behavioral therapy program. , Children who “crack their knuckles” are frequently hypermobile. Parents are often concerned that this activity might lead to joint damage, but it is probably not a cause of later osteoarthritis.
The flexible flat foot is common in infants. By the age of 2 years, toddlers develop a medial arch while seated that flattens on weight bearing. Many measures have been used to diagnose the pediatric flexible flat foot. The Chippaux-Smirak and Staheli arch indices (footprint measures) and the Foot Posture Index-6 (FPI-6) demonstrate validity and reliability. The reported prevalence of flexible flat feet is higher in young children (up to 60% of children 2 to 6 years old) but reduces to less than 19% by 8 to 13 years of age. A flexible flat foot usually resolves by 10 years of age, but it can persist into adolescence and adulthood. Factors associated with persistent flat feet include male sex, family history, generalized ligamentous laxity, and obesity. , A patient with flat feet and hind-foot valgus is shown in Fig. 50.1 .
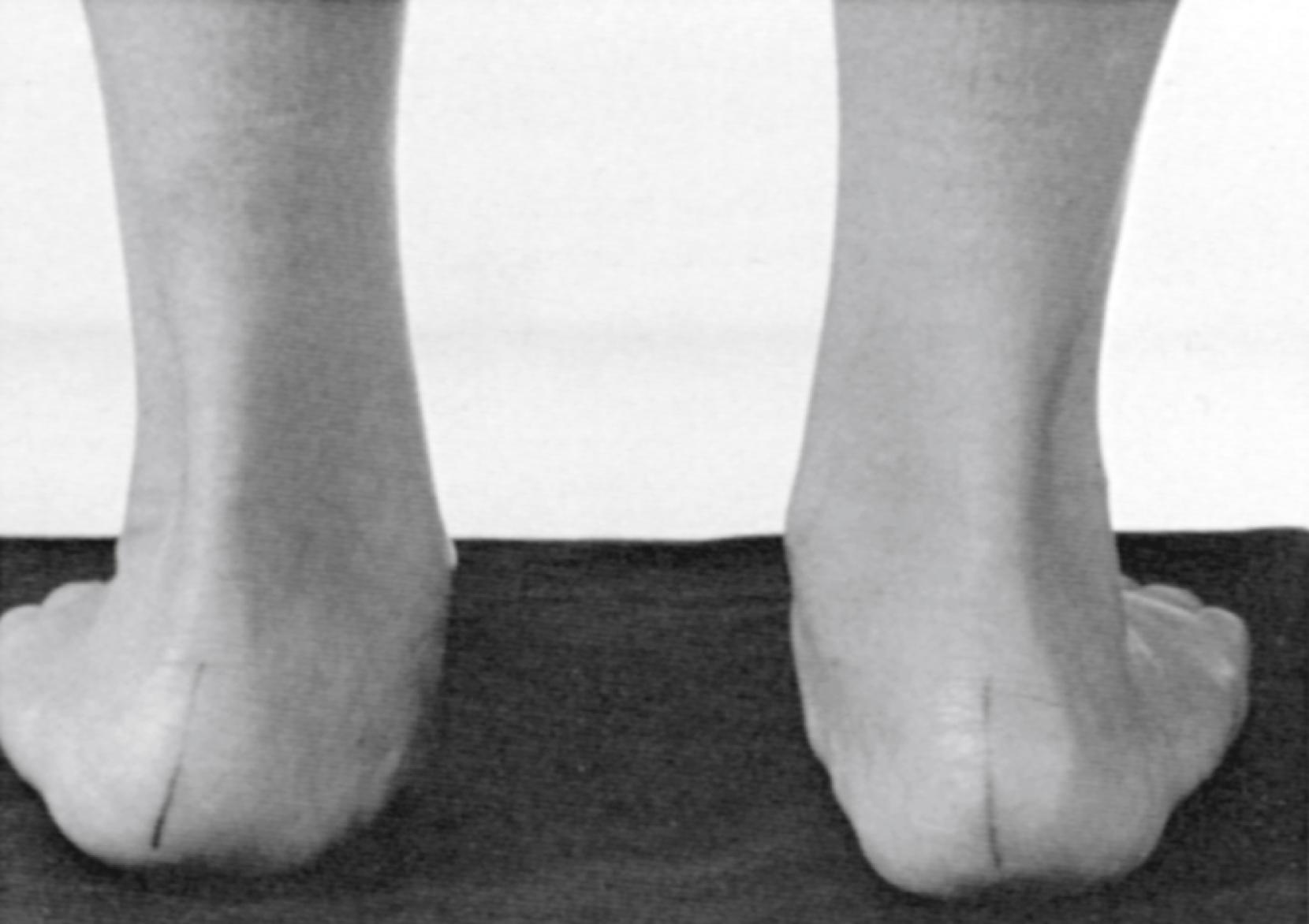
Children with flexible flat feet are often asymptomatic, but affected preschoolers seen in follow-up at 16 years of age had occasional pains in the neck, back, knee, leg, or foot. Anterior knee pain was more common in 17-year-old military recruits with flexible flat feet. The Framingham foot study found that men with pes planus had more arch pain and those with pronated feet had more generalized foot and heel pain. Interestingly, pes planus does not appear to be associated with significant lower extremity injuries in young adult athletes or impair athletic performance in children and youth. ,
Without pain, observation is an acceptable management of pediatric flat foot. Those who are symptomatic may benefit from activity modification, ice, massage, and supportive footwear, as well as stretching and strengthening exercises. Corrective orthotics may improve pain, foot posture, and gait, as well as static, dynamic, and functional balancing. Surgery is indicated only in those with persistent pain despite nonsurgical treatment but should be avoided in the pediatric age group.
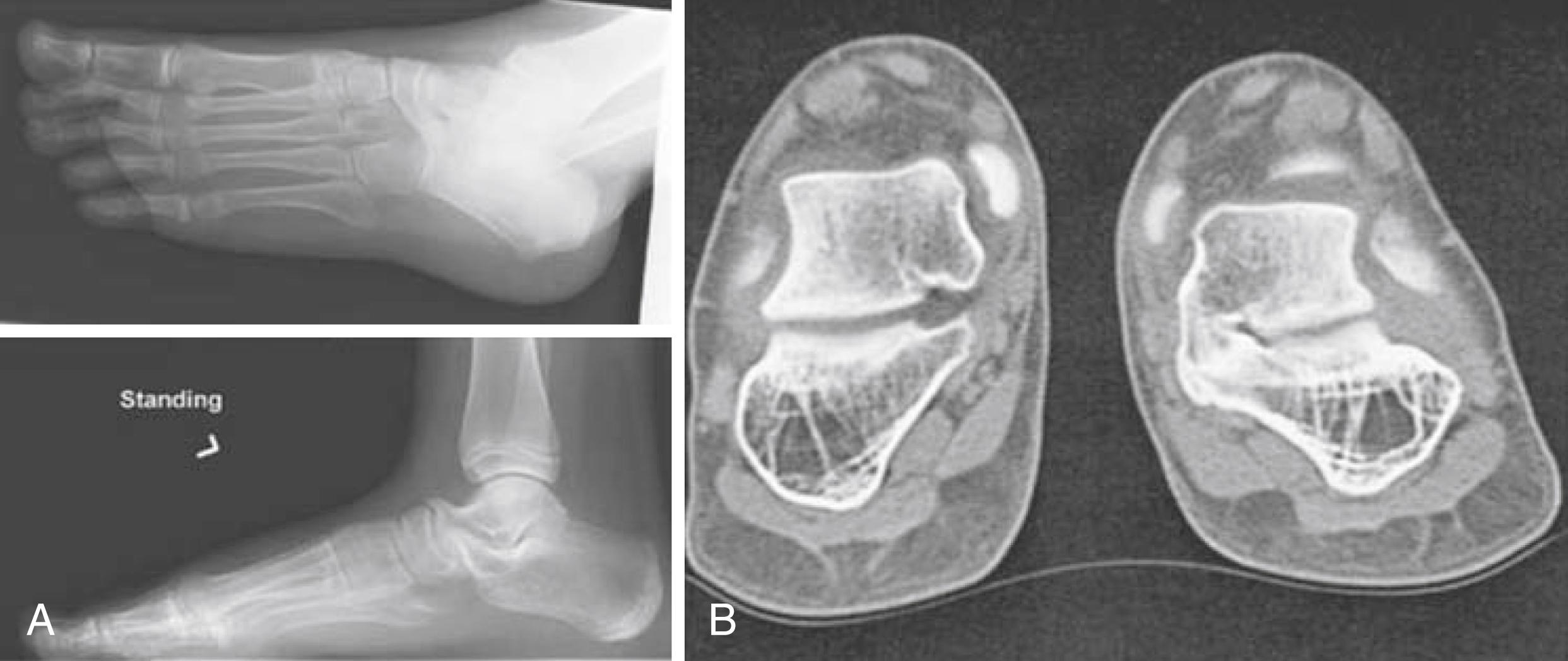
In contrast to the mobile flat foot, a rigid flat foot is always pathological. This occurs in 1% of the pediatric population and is defined by reduced range of motion of the tarsal and subtalar joints and a longitudinal arch that does not increase when standing on the toes. A rigid flat foot may result from tarsal coalition in which a fibrous or bony connection between two or more tarsal bones is present at birth ( Fig. 50.2 ). The prevalence of tarsal coalition was initially estimated at 1% to 2% of the population; however, affected individuals are not usually diagnosed until they become symptomatic, which does not occur until the second decade of life. Magnetic resonance imaging (MRI) and cadaveric studies have demonstrated a higher prevalence at 12% to 13%. This condition is bilateral in 50% of cases. Although tarsal coalition is usually an isolated abnormality, there is often a family history of first-degree relatives who are asymptomatic. About 90% of cases are calcaneonavicular and talocalcaneal coalitions. Children may report ankle pain that is aggravated by activity or frequent “ankle sprains.” On examination, restricted movement of the subtalar joint and/or midfoot with a valgus rear foot is typically noted. The peroneal muscles may spasm from adaptive shortening in response to heel valgus. Radiographs (oblique and axial views) may show calcaneonavicular coalitions but computed tomography (CT) or MRI is needed to assess fibrous or cartilaginous union and other tarsal coalitions ( Fig. 50.2 ). Children with symptomatic coalitions should receive nonoperative treatments for at least 6 months. This may include orthotics, walking boot, and immobilization of the foot, as well as NSAIDs and intraarticular steroid injections. Those who fail nonoperative therapy and have a single coalition without degenerative changes may undergo open resection.
Genu recurvatum, like pes planus, can be part of a generalized hypermobility syndrome or may occur as an isolated phenomenon. Some reported causes include fibrocartilaginous or spondyloepiphyseal dysplasias and trauma or surgery to the growing proximal tibia, as well as neuromuscular and genetic disorders. Symptoms range from pain to weakness and instability, which worsen with standing or walking and are relieved by rest. Athletes may have particular difficulty. Adolescent girls are more commonly affected with an associated increased incidence of anterior cruciate ligament injury. Obese children are also more likely to be affected and suffer lower extremity pain. Treatment includes orthotic correction of biomechanical faults, improving knee proprioception, and physiotherapy exercises to develop better muscle control, gait, and maintenance of good knee alignment during functional activities.
Symptomatic generalized hypomobility (JHypoS) is an entity in which decreased ranges of joint motion and pain in periarticular tissue is probably caused by an increased stiffness in joint ligaments. Physical activity induced lower extremity pain and habitual toe walking is typically associated with JHypoS, and boys appear to be more frequently affected. Reduced exercise tolerance is associated, which is likely from a pain-related deconditioned state. Although familial hypomobility has been described, prevalence data are not yet available. This condition may be caused by changes in collagen metabolism. There are other uncommon disorders, including hyalinosis and familial fibrosing serositis , where pain may relate to very stiff joints ( Box 50.4 ). Some children are born with multiple joint contractures (arthrogryposis) secondary to decreased movement in utero. Most causes of arthrogryposis are hereditary neuromuscular disorders. These children, along with those having other neurological or genetic disorders such as Williams and Angelman syndrome, do not have seem to have arthralgia, so careful evaluation for other explanations of pain needs to be undertaken. Treatment of these conditions requires an effective stretching exercise program to allow plastic deformation of collagen tissue.
Diabetes mellitus (diabetic cheiroarthropathy)
Tightening of skin and soft tissues of fingers
Short stature
Scleroderma and scleroderma-like conditions
Mucopolysaccharidoses and mucolipidoses with dysostosis multiplex
Autosomal recessive inheritance (except in Hunter syndrome, which is X-linked)
Hyalinosis
Familial fibrosing serositis
Progressive contractures of fingers and toes
Fibrosing pleuritis and constrictive pericarditis
Probably autosomal recessive inheritance
Camptodactyly syndromes (several familial conditions including Blau syndrome)
Flexion contractures of fingers
Beals contractural arachnodactyly syndrome
Marfanoid features
Crumpled ears
Cardiac abnormalities unusual
Linked to fibrillin-L-like gene on chromosome 5 (autosomal dominant inheritance)
Winchester syndrome
Multicentric osteolysis particularly of fingers, starting in infancy
Autosomal recessive inheritance
Arthrogryposis
Multiple joint contractures from poor fetal movement in utero
Numerous underlying genetic neuromuscular disorders
Angelman
Stiff jerky gait, joint contractures, scoliosis, mental retardation, absence of speech, seizures, bouts of laughter
Mutations in UBE3A gene
PFP is a common cause of chronic knee pain. It is more common in girls and is most common during the adolescent growth spurt with an annual prevalence of 29% in adolescents ( Table 50.2 ). Symptoms usually affect both knees with one side more affected. PFP presents as peripatellar or retropatellar pain during patellofemoral joint loading activities (e.g., running, climbing stairs, squatting). Pain may be present with prolonged sitting with the knee in flexion (“theatre sign”). The etiology of PFP is unknown and believed to result from biomechanical factors, malalignment of the patella relative to the femoral trochlea, and excessive mechanical loading. , True instability does not occur, but patients often report “giving way” because of pain-related reflex inhibition of the quadriceps muscle or deconditioning. On examination, children may have lower extremity malalignment (genu valgum, varum, or recurvatum; leg length discrepancy; femoral anteversion; external tibial torsion; laterally displaced tibial tubercle; pronated subtalar joint), vastus medialis wasting, increased Q-angle (angle formed between the line joining the anterior superior iliac spine and the center of the patella and the latter and tibial tuberosity), patellar facet tenderness, tightness (hamstrings, quadriceps, iliotibial band, gastrocnemius) and weakness (quadriceps, hip external rotators, abductors, trunk muscles) of the lower extremity muscles. A painful quadriceps setting/grind test (suprapatellar resistance while the patient performs isometric quadriceps contraction with knee in full extension) and patellar compression test (direct compression of the patella into the trochlea) aid diagnosis.
| Age at onset | Adolescence to young adulthood |
| Sex ratio | Girls > boys |
| Symptoms | Insidious onset of activity-related knee pain, difficulty descending stairs and squatting, need to sit with legs straight (“theatre sign”) |
| Signs | Vastus medialis atrophy, patellar facet tenderness, positive quadriceps setting/grind and patellar compression tests, lateral tracking of patella. Weakness of the quadriceps, hip external rotators and abductors, and trunk muscles |
The aim of treatment is to correct unbalanced tracking of the patella. Longer pain duration before initiation of treatment is associated with poorer long-term prognosis. Treatment includes activity modification; cryotherapy; short-term NSAID therapy, and physiotherapy. Physiotherapy focuses on patellar tracking exercises and flexibility and strengthening around the hip and knee. Foot orthoses, patellar taping, or manual therapy may reduce symptoms but have no benefit unless combined with exercise therapy. Box 50.5 shows guidelines for safe return to sport.
Pain free
No swelling
Full range of motion (compare the injured part with the uninjured opposite side)
Full or close to full (90%) strength (compare with the uninjured side)
For lower body injuries—able to perform full weight-bearing without limping
For upper body injuries—able to perform athletic movements (throwing, swimming, etc.) with proper form and no pain
Always start with aerobic exercise, followed by functional and sport-specific skills (jumping, pivoting, etc.), and practice before competitive play
Patellofemoral instability is more common in children with hypermobility and anatomical variants (ligamentous laxity, patella alta, trochlear dysplasia, external tibial torsion, or genu valgum). Children complain of anterior knee pain, episodic giving way and locking, and recurrent swelling. On examination, findings are similar to PFP and the patellar apprehension test (contraction of the quadriceps muscle when the examiner attempts to displace the patella laterally), or frank lateral dislocation may be elicited. Radiographs are recommended to rule out osteochondral fracture associated with patellar dislocation. Treatment is the same as for PFP. Patellofemoral orthoses (stabilization braces) may prevent recurrent episodes of instability. Orthopedic referral is recommended for acute patellar dislocation and recurrent instability. High-level evidence supports nonoperative treatment for first-time acute patellar dislocations, but there is emerging evidence that surgery may result in better short-term outcomes. A 2015 Cochrane review reports low quality evidence to support surgical over nonsurgical management of primary patellar dislocation. A systematic review found better outcomes for surgical stabilization in patients with recurrent instability.
Plicae are inward folds of the synovial lining and are present in most knees. They are thought to be residual embryonic remnants persisting from when the knee cavity was a septated structure. Occasionally, plica becomes symptomatic because of inflammation from acute trauma, repetitive microtrauma, or transient synovitis. The mediopatellar plica syndrome is most common; presenting with medial knee pain, patellar snapping, and catching during flexion. The plica may be palpable as a tender thickened band when pressed against the edge of the condyle, and there may be localized tenderness at the medial and inferior patellar border. A knee extension test (plica stutter test) may be positive. This test is conducted as follows: The patient is seated at the edge of the examination table, and the examiner stands or sits to the side being tested while gently palpating the patella. The patient is instructed to slowly flex and extend the knee. If the patella stutters or jumps during the course of movement—usually 45 to 70 degrees toward extension—it is indicative of a plica. MRI may show thickening of the plica and any associated synovitis or reactive changes in subchondral bone. Management includes patellar mobilization and massage, physiotherapy, NSAIDs, and intraarticular steroids. Surgical removal of the plica is reserved for recalcitrant symptoms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here