Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter will:
List the nomenclature related to extracorporeal renal replacement therapies.
Provide a detailed description of the performance characteristics of membranes and filters.
Define and characterize transmembrane transport of solutes and fluid.
Name methods of measurement of prescribed and delivered treatment.
This chapter reports the conclusions of a consensus expert conference on the basic principles and nomenclature of renal replacement therapy (RRT) currently used to manage acute kidney injury (AKI). Common definitions, components, and modalities used to deliver extracorporeal therapies will be discussed, using a “machine-centric” rather than a “patient-centric” approach. A description of the performance characteristics of membranes, filters, transmembrane transport of solutes and fluid, flows, and methods of measurement of delivered treatment are provided, focusing on continuous renal replacement therapies (CRRTs), which are used in the management of critically ill patients with AKI. Devices and operations are classified and defined in detail to serve as guidelines for use of terminology in papers and research.
The main one-dimensional geometric characteristics of hollow fiber membranes are length (L), mean inner radius ( ![]() ), wall thickness (t), and number of pores (N p ). The membrane surface area depends on the number of fibers (N f ). Using these parameters, multidimensional characteristics can be expressed as listed in Table 176.1 .
), wall thickness (t), and number of pores (N p ). The membrane surface area depends on the number of fibers (N f ). Using these parameters, multidimensional characteristics can be expressed as listed in Table 176.1 .
| MULTIDIMENSIONAL CHARACTERISTIC | SYMBOL | FORMULA |
|---|---|---|
| Surface area | A | |
| Filter priming volume | |
|
| Total priming volume | |
|
| Membrane porosity | ρ | |
The performance characteristics define the potential applications of each membrane.
The membrane ultrafiltration coefficient (K UF ) represents the water permeability of the filter membrane per unit of pressure and surface. It depends on the dimensions of the membrane and the number of pores and is measured as
where Q UF is the ultrafiltration flow rate, TMP is the transmembrane pressure, and A is the membrane surface area. The unit of measurement is mL/hr/mm Hg/m 2 . Treatment parameters that enhance or reduce pore blockage induce changes in the K UF .
The filter ultrafiltration coefficient (DK UF ) is defined as the product of the K UF and membrane surface area (A):
The unit of measurement is mL/hr/mm Hg. Membrane manufacturers measure DK UF as the ratio of the Q UF per unit of applied TMP.
The K UF is used to define “high flux” or “low flux” membranes. Although there is no definitive consensus in the literature about the K UF cutoff value, it generally is assumed that a K UF less than 10 mL/hr/mm Hg/m 2 identifies a low-flux membrane, a K UF of 10 to 25 mL/hr/mm Hg/m 2 identifies middle-flux membranes, and a K UF greater than 25 mL/hr/mm Hg/m 2 identifies high-flux membranes.
The term high flux has been used generally to define a membrane with an ultrafiltration coefficient that exceeds 25 mL/hr/mm Hg/m 2 . This mainly describes the hydraulic permeability of the membrane (permeability to water). However, hydraulic permeability does not necessarily correspond to the permeability to solutes, which instead depends on the density of pores, the mean size of pores, and the distribution of pores. For this reason the terms high flux and highly permeable membrane are not interchangeable.
The mass transfer area coefficient (K 0 A) represents the overall capacity of the membrane to provide diffusive remove solute over the entire filter surface. It is defined as the product of the solute flux per unit of membrane area (K 0 ) and the membrane surface area. The unit of measurement is mL/min. The K 0 A value can change during dialysis as a result of changes in membrane permeability or a loss of membrane exchange surface area.
The sieving coefficient (SC) is the ratio of a specific solute concentration in the ultrafiltrate (removed only by a convective mechanism), divided by the mean plasma concentration in the filter:
where C UF is the solute concentration in the ultrafiltrate, and C pi and C po the plasma solute concentrations at the inlet and outlet of the filter, respectively. A true calculation would require to measure solute concentration in plasma water rather than plasma to avoid interference of proteins. Nevertheless, for practical purposes, plasma concentration normally is accepted.
SC is correctly measurable only in the absence of a gradient for diffusion (no concentration gradient through the membrane). Measurement of the SC varies during treatment because the characteristics of the membrane change. SC is specific for each solute and for every membrane ( Fig. 176.1 ). The formula is simplified commonly to the ratio between the concentration in the ultrafiltrate and the concentration in prefilter plasma. The rejection coefficient (RC) is defined as
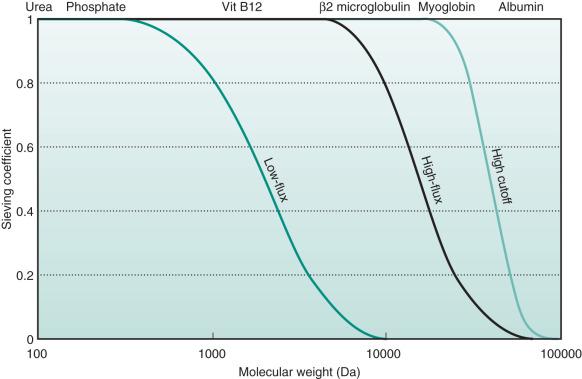
For a specific membrane, the cutoff represents the molecular weight of the smallest solutes retained by the membrane. Taking into account the normal distribution of membrane pore size, the statistical cutoff value is identified as the molecular weight of a solute with a SC of 0.1. For a specific membrane, the retention onset (cutoff 90% or 0.9) represents the molecular weight of a molecule with a SC of 0.9. For a complete understanding of the performance characteristics of a membrane, the cutoff value and the retention onset both must be taken into account, allowing evaluation of the profile of the SC curve for each membrane (see Fig. 176.1 ).
Clinically, the expression “high cutoff membranes” describes membranes with a cutoff value that approximates the molecular weight of albumin (before exposure to blood or plasma).
Solute transport occurs mainly by two phenomena: convection and diffusion. Fluid transport across semipermeable membranes is driven by ultrafiltration. Adsorption influences removal of hydrophobic (lipid-soluble) compounds by attachment of solute to the membrane. When solute removal rate (mass/time) is normalized by the concentration of blood/plasma entering the filter (mass/volume), the correct term is solute clearance, which is expressed in mL/min and describes the volume of blood completely purified by the solute in the unit of time.
Ultrafiltration describes the transport of plasma water (solvent, free of cells and colloids) through a semipermeable membrane, driven by a pressure gradient between blood and dialysate/ultrafiltrate compartments. It is influenced by the intrinsic properties of the filter, such as the DK UF , and the operating parameters (e.g., TMP). Quantitatively, ultrafiltration is defined by the ultrafiltration rate (Q UF ):
The term ultrafiltration requires some specifications, depending on the context in which it is used. When ultrafiltration is applied to a circuit or a CRRT treatment, specifications should be made using terms such as total ultrafiltration (UF, overall ultrafiltration volume produced during treatment) and net ultrafiltration (UF NET , net ultrafiltrate volume removed from the patient by the machine). In the first case, the overall volume can be replaced completely, partially replaced, or not replaced at all. UF NET is the difference between UF and the volume replaced in the circuit ( Table 176.2 ).
| FLOW RATE | SYMBOL | UNIT OF MEASURE | DEFINITIONS AND COMMENTS |
|---|---|---|---|
| Blood flow rate | Q B | mL/min | Depends on:
|
| Plasma flow rate | Q P | mL/min | Approximated as: Q P = (1−HCT) ∙ Q B where HCT: hematocrit |
| Ultrafiltration flow rate | Q UF | mL/hr | Total volume of fluid removed in the filter by positive TMP per unit of time: Q UF = Q UF NET + Q R. Depends on:
|
| Net ultrafiltration flow rate (Δ weight flow rate) (weight loss flow rate) |
Q UF NET | mL/hr | Net volume of fluid removed from the patient by the machine per unit of time |
| Plasma ultrafiltration flow rate | Q P-UF | mL/hr | Total volume of plasma removed in the plasma filter by positive transmembrane pressure (TMP) per unit of time |
| Replacement flow rate (or substitution flow rate or infusion flow rate) | Q R PRE Q R POST Q R PRE/POST |
mL/hr | Sterile fluid replacement can be
|
| Replacement plasma flow rate | Q P-R | mL/hr | Replacement of plasma downstream of the plasma filter |
| Dialysate flow rate | Q D | mL/hr | Volume of dialysis fluid running into the circuit per unit of time |
| Effluent flow rate | Q EFF | mL/hr | Waste fluid per unit of time coming from the outflow port of the dialysate/ultrafiltrate compartment of the filter: Q EFF = Q UF + Q D = Q UF NET + Q R + Q D |
When techniques are discussed, ultrafiltration may be isolated (no other mechanism is used in the treatment and only volume control is achieved), be used as part of hemofiltration (the ultrafiltrate is replaced partially or completely achieving volume and solute control), or combined with diffusion in treatments such as hemodialysis (HD) or hemodiafiltration (HDF). Different membranes are used for different techniques.
Convection is the process in which solutes pass through membrane pores, dragged by fluid movement (ultrafiltration) caused by a hydrostatic and/or osmotic transmembrane pressure gradient.
The convective flux (J c ) of a solute depends on the Q UF , the membrane surface area (A), the solute concentration in plasma (C Pi ), and the solute SC:
Compared with diffusive transport, convective transport permits the removal of higher molecular weight solutes at a higher rate.
In hollow fiber filters, the TMP is the pressure gradient across the membrane. The terms that define this gradient are the hydrostatic pressure in the blood compartment (P B ), the hydrostatic pressure in the dialysate/ultrafiltrate compartment (P D ), and the blood oncotic pressure (π B ). The TMP value varies with length (l) along the whole filter length:
Generally, TMP is expressed using a simplified formula:
where P Bi is the blood inlet pressure, P Bo the blood outlet pressure, P Di the dialysate/ultrafiltrate inlet pressure, P Do the dialysate/ultrafiltrate outlet pressure, π Bi the oncotic pressure of the inlet blood, and π Bo the oncotic pressure of the outlet blood. It must be stressed that the TMP* is a positive, averaged value along the length of filter, and does not reflect the true local pressure profile in the filter. In other words, a positive TMP* does not imply a positive TMP (l) at each point in the filter.
Furthermore, CRRT machines do not usually measure directly the P Di or the oncotic pressure, so the TMP is estimated using an even simpler formula:
where P PRE is the prefilter pressure, P OUT the postfilter pressure, and P EFF the pressure measured in the effluent line (all three measured by the machine). In the most common configuration, as blood flows down the filter, plasma water is removed and eliminated with the spent dialysate (if present), which flows in a countercurrent direction (see later). This ultrafiltration, called direct (or internal) filtration, identifies the one-directional movement of plasma water from the blood-side to the dialysate/ultrafiltrate compartment of the filter resulting from a local positive TMP (l):
At a critical point on the filter, where P B (l) = P D (l) + π B (l), equilibrium is achieved. After this point, the TMP (l) may become negative (even if TMP* is positive), allowing dialysate fluid to flow back into the blood compartment, resulting in so-called backfiltration. Backfiltration describes the movement of fluid from the dialysate compartment to the blood compartment.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here