Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The World Health Organization (WHO) recognizes nodular lymphocyte–predominant Hodgkin's lymphoma (NLPHL) as a separate entity distinct from classical Hodgkin's lymphoma (CHL) in the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. This reflects the fact that there are clear and consistent histologic, epidemiologic, immunologic, and genetic differences between NLPHL and CHL. NLPHL is an indolent germinal-center (GC) B-cell malignancy, representing a nodular proliferation comprising a minority of large neoplastic centroblasts with multilobated nuclei, the so-called popcorn or lymphocyte-predominant (LP) cells (formerly called L&H cells [lymphocytic and/or histiocytic Reed-Sternberg cell variants] ), and a majority of reactive lymphocytes and histiocytes.
Several schemes have been used to classify Hodgkin's lymphoma (HL) since 1947. Jackson and Parker identified three subtypes termed Hodgkin's paragranuloma, Hodgkin's granuloma, and Hodgkin's sarcoma. Hodgkin's paragranuloma was characterized by obliteration of the normal lymph node architecture by abundant small lymphocytes, among which Hodgkin and Reed-Sternberg (HRS) cells were present as single cells or in small groups. In a study of follicular lymphomas, Hicks and associates described a nodular variant of paragranuloma.
In the classification of HL proposed by Lukes and Butler in 1966, six subgroups were identified. At the Rye conference on the staging of Hodgkin's disease, the six subclasses of Lukes and Butler were reduced to four, combining their lymphohistiocytic nodular and lymphohistiocytic diffuse types into one class designated lymphocyte predominant. This so-called Rye classification was in widespread use until the 1990s.
In 1979. Poppema and coworkers published a series of three papers on the histology, immunophenotype, and epidemiology of the nodular and diffuse lymphocyte-predominant subtype of Hodgkin's disease, indicating that NLPHL was a separate entity. In these papers, the association between progressively transformed germinal centers (PTGCs) and NLPHL was established, as well as the first documented cases of transition to diffuse large B-cell lymphoma (DLBCL). Further, it was established that NLPHL and its diffuse variant (nodular paragranuloma and diffuse paragranuloma) did not transform to other subtypes. In the 1980s, clinical studies delineated important differences in immunophenotype and clinical course between NLPHL and CHL.
A more formal distinction between NLPHL and CHL was proposed by the International Lymphoma Study Group in the Revised European American Lymphoma (REAL) classification. This proposal was adopted by the WHO classification, which emphasizes that NLPHL is biologically distinct from CHL. A category of lymphocyte-rich CHL (LRCHL) was proposed in the REAL classification as resembling the lymphocyte-predominant HL of the Rye classification, based on the abundance of normal lymphocytes, but being biologically and clinically more closely related to CHL. A nodular form of LRCHL was described by Ashton-Key and colleagues, which they termed follicular Hodgkin's lymphoma. Both nodular and diffuse forms of LRCHL are included in the WHO classification and are discussed more fully in Chapter 28 .
NLPHL accounts for 3% to 8% of HLs in Western countries. In older series, up to half the cases may in fact have been LRCHL. NLPHL occurs in all age groups, with a peak incidence in the fourth decade, in contrast to a peak incidence in the third decade for the nodular sclerosis subtype of CHL ( Fig. 27-1 ). NLPHL shows a male predominance of 2.4 : 1, different from the slight female predominance in nodular sclerosis CHL. There are no significant differences between cases that are exclusively nodular and those with prominent diffuse areas.

Familial cases of NLPHL have been reported. A recent Finnish population-based study indicated a high familial risk. First-degree relatives of NLPHL patients had a standardized incidence ratio for NLPHL of 19, compared with 5.3 for CHL and 1.9 for non-Hodgkin's lymphoma. Familial NLPHL affected males and females equally, in contrast to the male predominance of NLPHL overall. The reasons underlying the familial risk for NLPHL are unknown but may include both genetic and environmental factors, including infectious etiologies.
Immune responses to HL may be influenced by interindividual genetic variations. Variants in the KLHDC8B gene have been found in familial CHL but were not definitely associated with familial NLPHL. There have been numerous suggestions that HL is influenced by the human leukocyte antigen (HLA) class II region, and specifically by alleles at the HLA-DPB1 locus (DPB1*0301 associated with susceptibility, and DPB1*0201 with resistance), although the relative risks associated with these alleles were small. Taylor and colleagues also found that susceptibility to NLPHL is associated with the DPB1*2001 allele. NLPHL has been reported in two children with Hermansky-Pudlak type 2 syndrome, a primary immune deficiency associated with AP3B1 mutations. These patients were found to have reduced NK and NK T-cell subsets, suggesting a possible role for effector cell defects in NLPHL. The risk for HL in young adults decreases with an increasing number of C alleles at position –174 in the interleukin-6 promoter. A significant excess of G alleles at this position was observed in young adults with NLPHL. A truncating germline mutation in NPAT , a gene adjacent to ATM that encodes a nuclear protein associated with cell-cycle regulation, has been shown to segregate with NLPHL in an affected Finnish family; another germline variant in NPAT was found in several other HL patients.
There are several indications that HL may have an infectious cause, and there is extensive evidence that Epstein-Barr virus (EBV) plays a role in a major subset of CHL. Some studies have found evidence of EBV-positive NLPHLs, especially in developing countries, whereas other studies found only negative cases. While inclusion of LRCHL cases in older reports and early EBV infection as seen in developing countries might explain rare EBV-positive NLPHL in some series, two recent North American studies have confirmed the existence of true EBV-positive LPHLs. A possible role for other viruses, including human herpesvirus 6, has been studied but has not been demonstrated to date. Although CHL is seen with increased frequency in patients infected with human immunodeficiency virus (HIV), a risk for NLPHL has not been observed. Recently presented data showing that B-cell receptors from IgD-positive NLPHLs react to Moraxella catarrhalis indicate a possible bacterial pathogenesis of some NLPHLs.
Patients usually present with isolated lymphadenopathy of long duration. There is frequent involvement of cervical and axillary nodes, with less frequent inguinal or femoral nodal involvement. Mediastinal NLPHL is an unusual finding (7%). The most frequently involved primary extranodal sites include the tonsil, parotid gland, and soft tissue. The liver and spleen are common extranodal sites of high-stage node-based disease. B symptoms are uncommon and are found in only 10% of patients. Bone marrow involvement by NLPHL is extremely rare (2.5%) and is associated with aggressive clinical behavior and poor prognosis.
NLPHL typically presents as early-stage disease, with slow progression and an excellent outcome with standard therapy. Approximately 20% of patients have advanced disease at the time of presentation. Recurrences develop in a relatively high percentage (≈21%), regardless of original clinical stage, and multiple recurrences (27%) are not uncommon. In 65% of cases, the recurrence is local or regional, in 23% the recurrence is in a different region, and in 12% the disease is generalized.
NLPHL does not transform to other subtypes of HL, though clonally related NLPHL and CHL has been reported. Transformation to DLBCL has been reported to occur in 3% to 14% of cases. Less commonly, NLPHL and DLBCL are seen in the same site as composite lymphoma. The issues of transformation to DLBCL and the relation to T-cell/histiocyte–rich large B-cell lymphoma (THRLBCL) are discussed later.
At low magnification, complete obliteration of the lymph node architecture is usually evident. In some cases, a compressed rim of normal lymphoid tissue with reactive follicles is present in the periphery of the node, usually sharply demarcated from the tumor tissue. Fan and associates described six immunoarchitectural patterns of NLPHL: (1) classical B-cell-rich nodular; (2) serpiginous nodular; (3) nodular with prominent extranodular LP cells; (4) T-cell–rich nodular; (5) diffuse THRLBCL-like; and (6) diffuse with a B-cell–rich pattern. The histologic pattern, particularly in cases with variant pattern, should be reported. A mixture of patterns in a single biopsy is more commonly observed than a single, pure pattern. Neoplastic cells are found both within and outside the macronodules ( Fig. 27-2 ). The nodularity created by loose aggregates of follicular dendritic cells (FDCs) is generally easily appreciated in routine hematoxylin-eosin slides, but it may be visualized by immunohistochemistry. The nodules vary in size, but they are mostly large. A diffuse growth pattern can be seen focally; rarely, it may predominate.

The predominant cell population in the nodules is small lymphocytes. The presence of histiocytes and LP cells leads to a “moth-eaten” appearance ( Fig. 27-3 ). The number of epithelioid histiocytes varies, and in some cases they are the most conspicuous cells. This feature led to the original term lymphohistiocytic type of Hodgkin's disease . In some cases, groups of epithelioid cells may form a ring in a circular pattern around the nodules ( Fig. 27-4 ).


Scattered FDC nuclei can be identified; in some cases multinucleated, Warthin-Finkeldey–type giant cells are seen. These are most likely FDC multinucleated variants ( Fig. 27-5 ). The cellular composition of the nodules may vary within the same lymph node: nodules with a predominance of lymphocytes can be seen together with nodules showing a large proportion of epithelioid histiocytes.
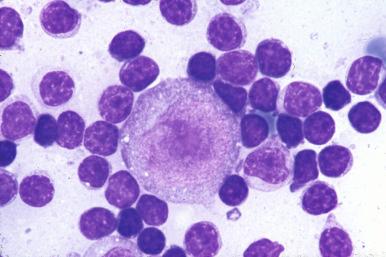
Occasionally, only a small number of LP cells are present; more often, they can be found with little difficulty. In rare cases, they form large clusters and are the most conspicuous cell type within some nodules. The clinical significance of this variation is not known. Classical HRS cells are not required to make a diagnosis of NLPHL, but neoplastic cells resembling classical HRS cells are not as infrequent as previously reported in the literature. The identification of classical HRS cells should always prompt careful immunohistochemical evaluation to exclude the possibility of LRCHL with a nodular pattern. However, in some cases of NLPHL the LP cells may mimic classical HRS cells while retaining the immunophenotype of LP cells.
The compressed internodular areas contain small lymphocytes and high endothelial venules. Plasma cells and eosinophils are characteristically scarce or absent. In some cases of NLPHL, there is a nodular sclerotic stromal reaction, particularly in large nodal masses. Because a documented history of long-term nodal enlargement is available in some of these cases, it is possible that this represents a chronic-phase tissue reaction to the NLPHL.
LP cells are large cells, with nuclei larger than those of normal centroblasts (see Fig. 27-5 ). Owing to their complex lobation, the term popcorn cells has been widely used. The nucleoli are medium-sized, generally basophilic, and smaller than those of classical HRS cells. The cytoplasm of LP cells is relatively sparse. In Giemsa-stained tissue sections and in Wright-stained imprints or smears, the cytoplasm may be moderately basophilic.
The absence of criteria by which a diffuse variant of NLPHL (pattern E in Fan et al) can be distinguished from THRLBCL with available methodologies has led to some controversy over whether the former actually exists. An argument in favor of the existence of a diffuse variant is that many cases of NLPHL have diffuse areas and other cases transform to a diffuse morphology. Therefore it appears likely that there are also primary diffuse variants. The presence of numerous LP cells outside the nodules may predict for progression to a diffuse THRLBCL-like pattern. Metachronous occurrence of the diffuse variant after NLPHL has been described and should be considered THRLBCL-like transformation of NLPHL.
A precise definition of the diffuse variant of NLPHL does not exist in the literature, but it is defined arbitrarily as a lymphoma with cytologic characteristics of NLPHL but lacking evidence of a nodular growth pattern either morphologically or with adjunctive immunophenotypic studies. In a review of a large case series by the European Task Force on Lymphoma, only 2% lacked nodular areas. In the largest series of NLPHL cases reviewed, only 7 cases out of 219 (3%) closely resembled THRLBCL by the presence of loosely distributed neoplastic cells in a background infiltrate of lymphocytes without evidence of nodularity. Many diffuse cases in studies from before the immunohistochemistry era likely would be classified today as the nodular type of NLPHL based on demonstration of FDC meshworks or as LRCHL.
The major diagnostic features of NLPHL are summarized in Table 27-1 .
| Feature | LP Cells | Background Cells |
|---|---|---|
| Morphology | Nuclei larger than centroblasts, hyperlobated nuclei, medium-sized nucleoli, sparse basophilic cytoplasm | Follicles with predominantly small lymphocytes, together with histiocytes and LP cells; “moth-eaten” appearance |
| Immunophenotypic features | CD45 + , CD20 + , CD15 – , CD30 – , BCL6 + , AID + , BSAP + , Oct-2 + , BOB.1 + , PU.1 + / – , MUM-1 + / – , T-bet + / – , HGAL + , BCL2 – , p53 – , CD10 – , CD138 – , EBV – | Predominantly CD4 + T cells; CD4 + , c-Maf + , CD57 + , PD-1 + T-cell rosettes around LP cells are present; low ratio of TIA-1 + to CD57 + T cells |
| Genetic and molecular findings | Clonal immunoglobulin gene rearrangements; ongoing mutations; BCL6 rearrangements in half of cases; BCL2 translocation usually not detected | Polyclonal B cells and T cells |
LP cells stain with antibodies to CD45, CD45RA, CD45RB, and CD45RC, but not CD45RO, in contrast to most classical HRS cells ( Table 27-2 ). There is consistent staining for pan–B-cell markers such as CD20 ( Fig. 27-6, A ), CD22, and CDw75. This profile differs from that of HRS cells in CHL, which typically show CD20 expression in only a subset of neoplastic cells and cases. CD79a is usually positive but varies in intensity. LP cells commonly lack CD19. LP cells also stain for CD40, CD70, CD80, CD86, HLA class II, and CD74 (the invariant chain of HLA class II). All of these are also expressed on normal GC blasts, with the exception of CD70, which is the receptor for CD27. In normal GCs, CD70 expression appears to be confined to GC blasts expressing only IgD, which can be seen sporadically in clusters in GCs of tonsil.
| Antigen | Significance | Findings |
|---|---|---|
| Lymphocyte-Signaling Molecules | ||
| CD45 (LCA) | All leukocytes Tyrosine phosphatase activity |
Positive |
| CD45RA (KIB3) | B cells, T-cell subsets, monocytes | Positive |
| CD45RB | Thymocytes, T cells | Positive |
| CD45RC | B cells, CD8-positive T cells | Positive |
| CD45RO (UCHL1) | Thymocytes, monocytes, macrophages, granulocytes | Negative |
| CD20 (L26) | B cells (not plasma cells) | ≈100% positive |
| CDw75 (LN1) | GC cells | Positive |
| CD79A (MB1) | Pan–B cells | Positive, but lower than CD20 |
| CD19 | B cells (not plasma cells) | Negative |
| CD40 | B cells, dendritic cells, macrophages | Positive |
| CD70 | Activated B cells and T cells, receptor for CD27 | Positive |
| CD80 | GC blasts and APC, receptor for CD28 and CTLA-4 | Positive |
| CD86 | GC blasts and APC, receptor for CD28 and CTLA-4 | Positive |
| MHC II (TAL1B5) | Control of immune responses through presentation of peptide antigens to T cells | Positive |
| CD74 (LN2) | B cells, invariant chain of MHC II | Positive |
| CD30 (Ki1/Ber H2) | Activated T cells and B cells | Generally negative |
| CD15 (Leu M1) | Myeloid cells | Negative |
| J chain | B cells | ≈60% positive |
| IgG, IgM, IgA, IgD | B cells | Variably positive |
| Igκ, Igλ | B cells | Variably positive |
| FREB | Leukocyte Fc receptor family, GC B cells | Positive |
| AID | Essential for SHM and CSR in GC B cells | Positive |
| GCET1 | GC B cells | Positive |
| HGAL (GCET2) | GC B cells | Positive |
| SWAP70 | B cells, specificity for the switch regions upstream of the constant region Ig genes | Positive |
| CD10 | GC B cells | Negative |
| Signaling Intermediates | ||
| NTAL | Adapter protein, linker for activation of B cells | Positive |
| CD138 (SDC1) | Post-GC terminal B cells, epithelial cells | Negative |
| LYN kinase | B-cell intracellular signaling molecule | Usually negative |
| JAK2 | B-cell intracellular non–receptor tyrosine kinase | Positive |
| Transcription Factors and Regulators | ||
| Oct-1 | Ig gene TF | Positive |
| Oct-2 | Ig gene TF | Positive |
| BOB.1 | Essential for response of B cells to antigens and formation of GC | Positive |
| BSAP/PAX5 | B-cell development and differentiation | Positive |
| ID2 | Negative regulation of E2A and PAX5 | Positive |
| PU.1 | Ig gene TF | Variably positive |
| MUM-1 | Subset of GC B cells, plasma cells | Inconsistently positive |
| BCL6 | TF expressed in GC cells | Positive |
| BLIMP1 | GC B cells showing plasma cell differentiation, plasma cells | Negative |
| FOXP1 | Mantle zone, some GC B cells | Negative |
| T-bet | Th1 cell development, role in Ig class switching | Half of cases positive |
| GATA3 | Th2 cell development | Negative |
| GATA2 | Development of hematopoiesis | Negative |
| c-Maf | Th2 cells, responsible for tissue-specific expression of IL-4 | Negative |
| NFATc1 | Normal homeostasis and differentiation | Usually cytoplasmic positive |
| REL (c-Rel) | NF-κB family member, antiapoptotic activity, function in lymphopoiesis | Negative ≫ positive |
| RELA | NF-κB family member, antiapoptotic activity, function in lymphopoiesis | Positive |
| BAFF-R (TNFRSF13C) | Mantle zone B cells, subset of GC B cells | Weakly positive or negative |
| JUNB | Component of AP1 transcription complex involved in cell proliferation and apoptosis | Negative |
| Cell-Cycle Proteins | ||
| Ki-67 (MKI67) | Marker of proliferation | Positive |
| PCNA | Proliferating cells | Positive |
| TOP2A | Cell proliferation marker | Positive |
| Tumor Suppressors and Apoptosis-Related Proteins | ||
| CASP3 | CD95-mediated apoptosis | Negative |
| c-FLIP | Competitive negative regulator of Fas-induced death | Negative ≫ positive |
| p53 | Apoptosis-related protein | Negative |
| TP73L (p63) | Subset of GC B cells | Positive |
| BCL2 | Represses cell death by apoptosis | Negative |
| BAX | Promotes cell death by apoptosis | Positive |
| A20 | Inhibits cell death by apoptosis induced by TNF | Variably positive |
| TRAF1 | Downstream component in CD30 signaling pathway | Negative |
| Structural Proteins and Adhesion Molecules | ||
| Vimentin | Intermediate filament | Negative |
| Fascin | Actin-bundling protein, dendritic cell marker | Negative |
| CD44H | Mediates adhesion of leukocytes | Negative |
| EMA | Epithelial cells, plasma cells | Variably positive |

CD30 staining is usually negative. In a few cases, weak, usually cytoplasmic staining of LP cells can be discernible. The NLPHL-derived cell line DEV also expresses CD30, albeit less intensely than the CHL-derived cell lines. Thus, expression of CD30 should not totally exclude a diagnosis of NLPHL. In contrast, strongly CD30-positive parafollicular immunoblasts located outside the B-cell nodules are more commonly identified and represent a potential diagnostic pitfall. LP cells are typically negative for CD15, but CD15 may be expressed in a subset of neoplastic cells in otherwise typical cases.
LP cells, in contrast to classical HRS cells, produce J chain, a 15-kD polypeptide essential for linking to the tailpieces of multimeric immunoglobulin molecules (see Fig. 27-6, B ). Because J chain is not present in serum, the demonstration of J chain in LP cells cannot be the result of phagocytosis or endocytosis but indicates immunoglobulin production, providing the first definitive proof of the B-cell origin of LP cells. In paraffin sections, LP cells infrequently express demonstrable cytoplasmic IgG, IgM, and IgA. However, strong expression for only IgD is identified in a subset of cases, most often young males with cervical lymph node involvement. Fc receptor homologue expressed in B cells (FREB), a member of the family of Fc receptors for IgG, is expressed in normal GC B cells, mantle zone cells, and most NLPHLs. Activation-induced cytidine deaminase (AID) is indispensable for class-switch recombination and somatic hypermutation of immunoglobulin genes. In keeping with the notion that LP cells represent transformed GC B cells showing evidence of somatic hypermutation, AID is consistently expressed in LP cells. New markers of GC derivation, such as GC B-cell expressed transcript 1 (GCET1), human GC-associated lymphoma protein (HGAL-GCET2), and switch-associated protein-70 (SWAP70), are also expressed in most NLPHLs. However, another GC B-cell marker, CD10, is negative.
Among transmembrane adapter proteins known to date, LP cells express only the non–T-cell activation linker (NTAL) that is also expressed in most B cells and B-cell neoplasms. This linker functions as a negative regulator of early stages of B-cell receptor signaling. Syndecans (SDCs) are transmembrane proteoglycans that play an important role in cell-matrix and cell-cell interactions, as well as modulating receptor activation. In hematopoietic cells, SDC1 (CD138) is expressed only in B cells at pre-B and plasma cell differentiation stages. LP cells are SDC1 negative, in accordance with their derivation from GC B cells.
In most CHL cases, several receptor tyrosine kinases are expressed, whereas none are detected in 50% of NLPHL cases. Receptor tyrosine kinase A, which is essential for the survival of memory B cells, was expressed in only 30% of NLPHLs in one study. JAK2, an intracellular non–receptor tyrosine kinase that transduces cytokine-mediated signals via the JAK2/STAT pathway, is expressed in most NLPHLs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here