Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Prostate cancer (PCa) is the most commonly diagnosed solid tumor among men in the United States and Europe. Even without treatment, PCa-specific mortality rates at 5 and 10 years remain low. Improvements in optimizing screening, diagnosis, and treatment have resulted in decreasing PCa mortality. Nevertheless, certain challenges still remain for men facing important disease state decisions and there is a need for biomarkers that can help
Increase the probability of an initial positive biopsy,
Reduce the number of unnecessary repeat biopsies by better distinguishing benign from malignant disease,
Stratify low risk from higher risk tumors,
Stage the disease or classify the extent of disease, and
Predict and monitor clinical response to an intervention.
Improved prognostic biomarkers that can provide individualized patient risk assessment are needed to assist informing treatment decisions for patients and physicians. Additionally, biomarkers, which can improve the precision of risk assessment, are needed to enhance decision-making for physicians and patients, especially when the traditional clinical parameters (prostate-specific antigen (PSA), digital rectal examination (DRE), pathology) do not provide an accurate assessment of risk.
Patients with early-stage PCa can benefit from a more precise, personalized assessment of their tumor biology given that current clinical risk assessment tools may be less than adequate.
For those with CRPC, the increasing complexity of treatment decisions has led to research efforts to define predictive biomarkers that identify men most likely to benefit from a given therapy.
A new generation of biomarkers ( Table 66.1 ) has emerged to help improve risk assessment, guide diagnostic strategies, and ultimately enhance treatment outcomes through more targeted screening, more accurate diagnosis, improved risk stratification, which should lead to improved treatment recommendations and subsequent selection of therapy ( Figure 66.1 ).
| Clinical scenarios addressed by currently available biomarkers | |||
|---|---|---|---|
| Who should be biopsied? | Who should be rebiopsied? | Who should be treated vs monitored? | Therapeutic response assessment |
|
|
|
|
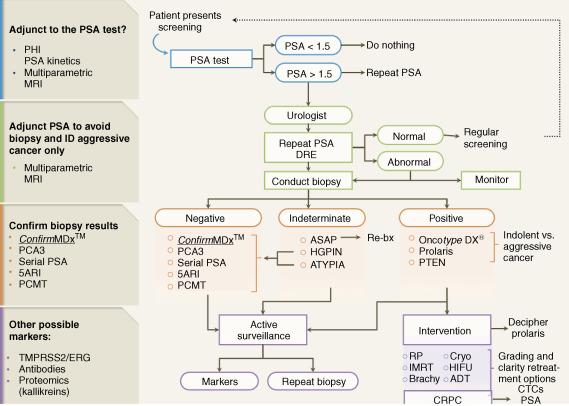
PSA testing became the cornerstone of early PCa detection after its approval over 20 years ago. However, due to the low disease mortality rate, controversies have emerged with early detection strategies.
New biomarker assays have been developed to help reduce the burden of biopsies in men with a low probability of PCa.
After its approval by the Food and Drug Administration (FDA) in 1986, the availability of PSA dramatically influenced PCa early diagnosis. In the United States, approximately 19 million men receive annual PSA testing, which results in more than 1.3 million biopsy procedures and a resultant 240,890 newly diagnosed findings of PCa cases.
Nonetheless, reliance on PSA testing alone for the detection of PCa has inherent limitations. First, the test is prostate specific but not PCa specific, and it may give false-positive or false-negative results. Most men with an elevated PSA level (above 4.0 ng/mL) are not found to have PCa; only approximately 25% of men undergoing biopsy for an elevated PSA level actually have PCa. Conversely, a negative result may give false assurances that PCa is not detected, when, in fact, a cancer may still exist. Second, the test does not always differentiate indolent from aggressive cancer and thus early detection of PCa may not impact eventual mortality from the disease and potentially lead to overtreatment. This limitation of PSA testing was largely responsible for the recent recommendation of the USPTF against continued routine screening.
The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial is a large population-based randomized trial designed and sponsored by the National Cancer Institute to determine the effects of screening on cancer-related mortality and secondary endpoints in men and women aged 55–74. Regarding the PCa arm of the trial, after 13 years of follow-up, there was no evidence of a survival benefit for planned annual PCa screening compared with mandated screening. Additionally, there was no clinical impact with benefit for scheduled versus unplanned screening as it related to age, baseline comorbidity, or pretrial PSA testing. PLCO had high rate of screening (∼50%) in the control arm, thus limiting its conclusions. However, Crawford et al. have reported a survival benefit for screening in men without significant comorbidities.
Follow-up results of 11 years from the European Randomized Study of Screening for Prostate Cancer study demonstrated that screening does significantly reduce death from PCa. A potential reason for these differing results is that in the US-based PLCO Cancer Screening Trial, at least 44% of participants in the control arm were already PSA-tested prior to being randomized into the study, confounding interpretation of the results.
Roobol et al. stated that there was “poor compliance with biopsy recommendations” in PLCO, as the trial did not mandate biopsies. Screening test results were sent to the participant and his physician, and they decided upon subsequent biopsy per shared decision-making.
In order to improve the sensitivity and specificity of serum PSA, several PSA derivatives and isoforms (e.g., PSA Isoforms and PSA Density) have been used. The National Comprehensive Cancer Network (NCCN) recommends PSA density when assessing for very low risk PCa patients.
Of note, the Goteborg trial, a prospective randomized trial of 20,000 men born between 1930 and 1944, showed that the benefit of PCa screening compared favorably to other cancer screening programs. PCa mortality was reduced by almost half over 14 years of follow-up.
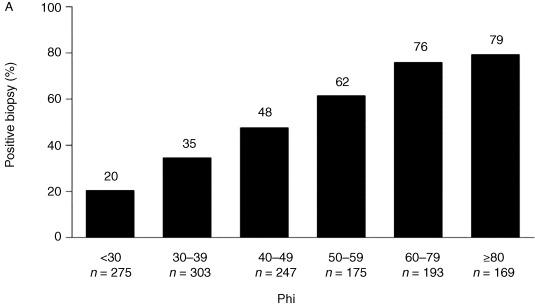
Efforts have been made to reduce PSA-associated over-biopsying, which may lead to overtreatment in very low and low-risk patients. The PHI was approved by the FDA for use in 2012 in those with serum PSA values between 4 ng/mL and 10 ng/mL in an effort to reduce the burden of biopsies in men with a low probability of PCa.
The PHI (PHI = [−2] proPSA/fPSA × PSA1/2; proPSA is a PSA subtype and fPSA is free PSA) was initially developed as an additional diagnostic biomarker in men with a serum PSA level of 2–10 ng/mL in European trials; an elevated proPSA/fPSA ratio is associated with PCa. Percent-free PSA, PHI score, and PCA3 have been described as markers of specificity within the 2014 NCCN guidelines for early detection of prostate cancer.
PHI score has a high diagnostic accuracy rate and can be used in PCa diagnosis. PHI score may be useful as a tumor marker in predicting patients harboring more aggressive disease and guiding biopsy decisions.
PHI also predicts the likelihood of progression during active surveillance. Tosoian et al. showed that baseline and longitudinal values of PHI predicted which men would have reclassification to higher-risk disease on repeat biopsy during a median follow up of 4.3 years after diagnosis. Baseline and longitudinal measurements of PHI had C-indices of 0.788 and 0.820 for upgrading on repeat surveillance biopsy, respectively. In contrast, an earlier study in the Johns Hopkins active surveillance, PCA3 did not reliably predict short-term biopsy progression during active surveillance.
In patients with persistent suspicion of PCa and a negative biopsy, testing with PCA3 and PHI has been proposed as a way to reduce the number of unnecessary repeat biopsies ( Figure 66.2 ).
4KScore is a newly available commercial assay panel that is designed to help predict which men with an elevated PSA will have high-grade disease upon tumor biopsy. By combining measures of total, free, and intact PSA with human kallikrein 2 (hK2) and other clinical parameters, the 4KScore was shown to be better than PCPT at predicting the occurrence of high-grade disease on biopsy.
For patients with negative biopsies who are believed to be at high risk for PCa, biomarker tests (such as PCA3 and PHI) should be consideration to improve specificity of the diagnosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here