Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying slide presentation that has been prepared by the author: ![]() .
.
Movement disorders include hypokinetic-rigid and hyperkinetic or mixed forms mainly originating from dysfunction of the basal ganglia.
Disruption of the essential cortico–basal ganglia–thalamocortical and basal ganglia–brainstem-cerebellar connections are related to these disorders.
Major movement disorders concern parkinsonism, chorea, myoclonic syndromes, tremor syndromes, ballism, dystonias, and tics.
Most of the neurodegenerative movement disorders are proteinopathies caused by deposition of pathologic proteins in distinct brain areas representing diagnostic hallmarks.
“Prion-like” propagation and spreading of pathologic proteins suggested to cause progression of neurodegeneration/clinical disease is a matter of discussion.
Most neurodegenerative movement disorders are caused by genetic and environmental factors and their complex interaction.
The most frequent forms among α-synucleinopathies are Lewy body–associated disorders (Parkinson disease, Lewy body dementia) and multiple system atrophy (rather rare).
Tauopathies include progressive supranuclear palsy, corticobasal degeneration, frontotemporal dementia with parkinsonism, postencephalitic parkinsonism (rare), Pick disease, and others.
Polyglutamine repeat disorders include Huntington disease, choreoacanthocytosis, Machado-Joseph disease, and other rare forms.
Other neurodegenerative movement disorders include pantothenate kinase–associated neurodegeneration, Wilson disease, inherited dystonias, and others.
The etiopathogenesis of most neurodegenerative movement disorders is suggested to be a cascade of noxious factors (protein mishandling, mitochondrial dysfunction, oxidative stress, excitotoxicity, chronic neuroinflammation, energy failure) and their combination.
Current consensus criteria have increased the diagnostic accuracy of many movement disorders, although neuropathology is still necessary for definite diagnosis.
Recent molecular-pathologic, pathogenetic, pathophysiologic data, partly derived from animal models, will promote the correct diagnosis of movement disorders as a basis for prevention and treatment.
Movement disorders can be divided into four major groups according to clinical phenomenology ( Box 104.1 ); only the first two are discussed in this chapter. Most hypokinetic-rigid and hyperkinetic forms have their origin in dysfunction of the dorsal basal ganglia, which work in concert with the cortex via complex information circuits of the brain, although virtually the entire nervous system is engaged in motor control. The varied movement disorders result from dysfunction of the cortico–basal ganglia (BG)–thalamocortical circuits due to disruption of downstream network activities in the thalamus, cortex, and brainstem. Recent progress has provided insight into the anatomy, functional organization, and pathophysiology of basal ganglia in specific types of movement disorders, as well as the role of specific neuron subpopulations in mediating different aspects of motor control.
Hypokinetic-rigid forms
Parkinsonism: Parkinson disease, parkinsonian syndromes
Stiff man syndrome
Hyperkinetic forms
Chorea syndromes
Tremor syndromes
Dystonias
Myoclonus
Ballism
Athetosis
Tics
Atactic movement disorders (not discussed here)
Cerebellar ataxias
Spinocerebellar degeneration
Motor neuron disorders (not discussed here)
Motor neuron disease
Spinal muscular atrophy and related disorders
The basal ganglia are a highly interconnected set of subcortical nuclei that include (1) input nuclei (caudate nucleus, putamen, and nucleus accumbens); (2) output nuclei (internal segment of globus pallidus [GPi] and substantia nigra pars reticulata [SNr]); and (3) the intrinsic nuclei/external segment of the globus pallidus (GPe), the subthalamic nucleus (STN), and the substantia nigra pars compacta (SNc). The basal ganglia are viewed as a network of sets of multiple parallel loops, where cortical and subcortical projections interact with internal reentering circuits engaging motor, associative, and limbic territories in the control of movement, behavior, emotions, and planning, related to a basic architectural and functional organization. The interconnections of these nuclei are shown schematically in Fig. 104.1 .
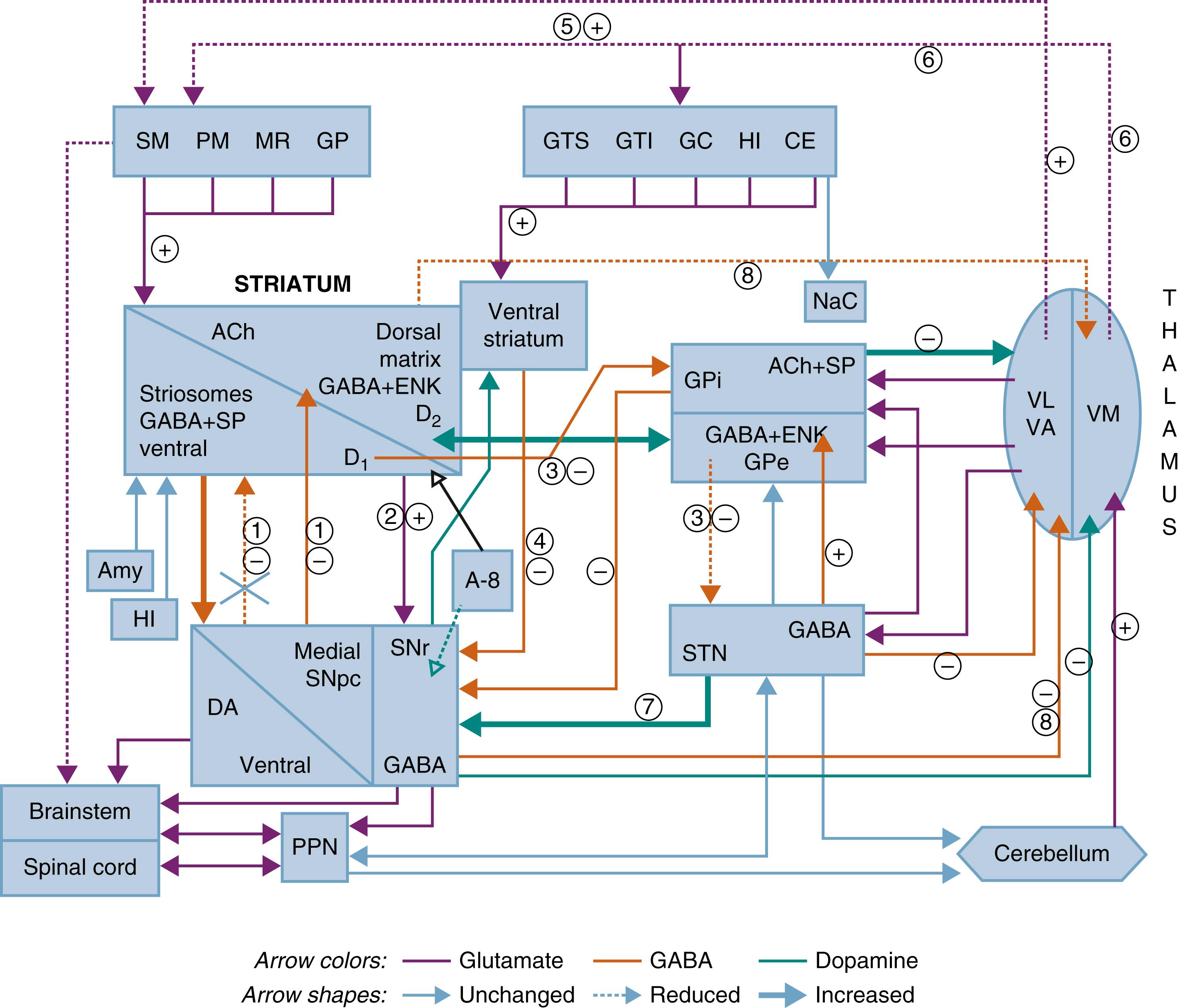
The three main transmitter systems involved in the integration of basal ganglia function are glutamate, γ-aminobutyric acid (GABA), and dopamine. Normal movement is controlled by cortico-BG-thalamocortical circuits: The striatum receives topographic glutamatergic input from the cerebral cortex, thalamus, and brainstem, primarily from dopaminergic cells. It sends GABAergic output to the SNc, SNr, GPe, and GPi, which release projections to specific thalamic nuclei and, to a lesser extent, to the deep layers of the superior colliculus and mesencephalic reticular formation. The respective thalamic nuclei have an excitatory glutamatergic input to specific regions of the motor cerebral cortex. In this circuit, the GABAergic output of the SNc and GPi diminishes the glutamatergic projections from the thalamus back to the cortex. Cortical regions send projections to the STN, the substantia nigra, the thalamus, and via pontine nuclei to the cerebellum. Projections of the GPe, dopaminergic SNc, and STN modulate the main flow of information through the basal ganglia. The functional specialization of striatum is closely related to its chemical heterogeneity. , The topography of cortico–basal ganglia (cortico-BG) projections has led to a model of their function based on parallel and segregated pathways operating through discrete functional channels that are represented in specific regions of each basal ganglia structure, indicating complex interactions between these channels. Similar to the distribution of body regions within the sensorimotor cortex, the basal ganglia nuclei are also somatotopically organized. ,
Current concepts about the organization of the basal ganglia emphasize the existence of “internal” mechanisms that modulate input/output activity and sustain normal execution of movements. A bidirectional cortico-BG communication is differentially patterned across bands and during changes in movement. These circuits involve, in a sequential manner, specific parts of the prefrontal cortex, striatum, pallidonigral complex, and medial and ventral thalamus. Five such basal ganglia (BG)–thalamocortical circuits have been defined, forming a topographically organized integrated functional network: motor and oculomotor circuits, dorsolateral prefrontal circuits, lateral orbitofrontal circuits, and anterior cingulate or limbic circuits involving different parts of the striatum, pallidonigral complex, and thalamus.
A nigrostriatal circuit in which the SNc receives a GABAergic inhibitory projection from the striatum feeds back to the striatum with a modulating dopaminergic input; it provides the major source of dopaminergic innervation of the striatum. The retrorubral field (A8) and ventral tegmental area (VTA; A10) also participate in the mesostriatal and mesolimbic dopaminergic projections. Dopamine causes excitation of striatal neurons that project to the GPi and SNr (by dopamine 1 [D 1 ] receptors) and inhibits thalamic nuclei to maintain normal movements. Dopamine also inhibits neurons that project to the GPe or STN (by dopamine 2 [D 2 ] receptors) to check the normal negative effect on motor speed and tone associated with high output from the STN. Its outputs project to the GPi, SNr, GPe, striatum, and pedunculopontine nucleus (PPN). Dopamine, an important modulator of basal ganglia function, may also modulate it outside of the striatum, and these changes contribute to the symptoms of Parkinson disease (PD) and other disorders. The GPe receives GABAergic input from the striatum and sends glutamatergic projections to the STN, which in turn sends glutamatergic projections to the SNr, GPi, and GPe to inhibit glutamatergic excitation of the cortex. The STN-GPe system is considered a central pacemaker of the basal ganglia as a major input relay station receiving projections from various cortical and subcortical regions, including the hyperdirect cortico-subthalamo-GPi pathway, , with implications for their (dys)function.
Activity of basal ganglia output structures is controlled by two opposing striatal motor loops, originating from distinct populations of medium spiny projection neurons (MSNs) and projecting to different output structures. The striatal projecting neurons in the “direct” pathway express dopamine D 1 receptors, whereas D 2 -bearing neurons project to the globus pallidus and are part of the “indirect” pathway. The two receptors are associated with distinct G proteins and different intracellular signal pathways that support the dichotomous effect of their activation.
The direct pathway is a monosynaptic inhibitory projection from the glutamatergic cortex to the MSNs, which contains GABA, substance P, and dynorphin and expresses the dopamine D 1 receptor projecting to GABAergic neurons in the GPi and SNr. Activation of striatal MSNs leads to inhibition of the inhibitory GPi/SNr output and to disinhibition of basal ganglia target structures in the thalamus and midbrain. This facilitates thalamocortical activity and thereby promotes movement and behavior.
The indirect pathway includes isopolysynaptic disinhibitory projections from the glutamatergic cortex to striatal MSNs (containing enkephalin and GABA and expressing the dopamine D 2 receptor) along with sequential striatal projections to the GPe, GABAergic GPe projections to the STN, and glutamatergic STN projections to the GPi and SNr. Activation of striatal neurons leads to inhibition of GPe neurons, thereby reducing inhibition of the STN and its thalamic and mesencephalic targets, causing suppression of motor and behavioral output resulting in inhibition of movements. A signal through the indirect pathway (cortex-striatum-GPe-STN-GPi) terminates the movement. The SNr is an inhibitory GABAergic nucleus that works in conjunction with the GPi as the final output of the basal ganglia’s direct and indirect pathways. Excitatory glutamatergic drive of STN neurons along the corticosubthalamic pathway triggers GABAergic inhibition of pallidothalamic inputs. The STN is a critical component of complex networks controlling not only motor function but also emotion and cognition.
Balance between these two pathways at the level of the globus pallidus and substantia nigra is crucial for normal functioning of the BG-thalamocortical circuits, and in pathologic situations this equilibrium is disrupted. This core model explains some of the mechanisms for the major movement disorders, in which there is either increased inhibition of the thalamocortical pathway, resulting in hypokinetic disorders, or decreased inhibition of thalamocortical output, which induces hyperkinetic disorders. , The function of these networks is modulated by the release of dopamine in the striatum. When dopamine binds to D 2 receptors, the indirect pathway is inhibited to decrease GPi activity, thereby promoting movement. In contrast, dopamine depletion, as in PD, leads to an increase in basal ganglia output that inhibits their thalamic and midbrain targets, with reduced activity in the “direct” striatocortical-nigral-GPi projections, inducing the akinetic features in PD, , although abnormalities outside the motor and dopaminergic pathways may be associated with akinesia. Recent concepts explain how dopamine exerts modulatory signals on cortico-BG circuits to enable flexible motor and behavior control. There is differential distribution of D 1 and D 2 receptors on neurons in the striatopallidal pathways, and cholinergic interneurons acts as mediator of dopamine-mediated communication between the two pathways. , , They are not separate parallel systems, but are structurally and functionally intertwined inside and outside the striatum, where basal ganglia collaterals may bridge the two pathways. , According to other models, they are not alternatively but concomitantly active, and the pattern of coordinated activity across the two pathways regulates movement initiation and execution.
Two “hyperdirect” pathways include a direct excitatory connection from the cortex to the STN, which has an excitatory connection to the GPi. Activation in this corticosubthalamic-pallidal pathway will increase GPi activity and inhibit thalamocortical targets by the action of the indirect pathway, causing suppression of all movements. The thalamostriatal system is a dual system, with one projection originating from midline and intralaminar nuclei and another one arising from ventral and relay nuclei using glutamate transporters. The reciprocity between basal ganglia structures is established by expanded circuitry of the GPe, which projects to the STN, and sends direct collaterals to the GPi and SNr and feedback projections to the striatum. The midbrain locomotor region, crucial for normal gait and posture, encompasses the cholinergic PPN that is interconnected with basal ganglia and delays basal ganglia activity to thalamic and brainstem nuclei, spinal effectors, and cerebellum, indicating their role in motor and cognitive control. Loss of the modulatory influence of STN on intracerebellar connectivity in PD may contribute to pathologic activities within the cerebellum.
Most movement disorders related to basal ganglia dysfunction are neurodegenerative diseases morphologically characterized by neuronal degeneration and loss accompanied by astrocytosis in various, often disparate parts of the nervous system. Conventional classification, based on pathophysiologic mechanisms involving the basal ganglia, distinguishes several movement disorders (see Box 104.1 ): (1) parkinsonian syndromes, characterized by rigidity, akinesia/bradykinesia, resting tremor, and postural instability; (2) chorea, in which fragments of movements flow irregularly from one body segment to another, to cause a dance-like appearance; (3) dystonia, characterized by prolonged muscle spasms and abnormal posture; (4) ballism, featuring high-amplitude movements of the proximal extremities; (5) myoclonus, with brief, quick movements; and (6) athetosis, which can be considered a “slow chorea” with lower amplitude.
Based on recent genetic and molecular-biologic data, movement disorders are classified into several groups ( Table 104.1 ). They are associated with cytoskeletal abnormalities, which represent important histologic signposts pointing to the diagnosis (see Box 104.1 ). Consensus criteria for their clinical and neuropathologic diagnoses have been established for most of these disorders.
| Neuropathologic features | Parkinson disease (Lewy body type) | Dementia with Lewy bodies | Multiple system atrophy | Progressive Supranuclear Palsy a | Corticobasal degeneration (4R-tau) | Pick disease (3R-tau) | Postencephalitic parkinsonism (3R- + 4R-tau) | FTDP-17 (3R- + 4R-tau) | Huntington disease | Neuronal intermediate filament inclusion disease | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSP-RS (typical, classic) | PSP-P (4R tau) | PSP-CBS | ||||||||||
| Lewy bodies Lewy neurites | Brainstem, NBM, amygdala, cerebral cortex, autonomic ganglia, olfactory bulb | Cerebral cortex (hippocampus), brainstem nuclei | Rare | None or rare | Rare | Rare | None | None | None | None | Lewy neurite–like in cerebral cortex | Rare |
| Neurofibrillary tangles and/or neuropil threads | Absent or age-related, none in brainstem | Only in Lewy body type of AD | None or extremely rare | BG/brainstem; STN, SN, pons | Similar to PSP-RS white matter | More cortical tau pathology | Numerous in substantia nigra | None or rare | Similar to PSP; minimal in oculomotor complex, cranial nerve IV nucleus, and pontine base | Neuronal pretangles, tangles, and globose tangles | None or extremely rare | None |
| Neuronal loss and astrogliosis | Severe in SNc, LC, many brainstem nuclei | Variable in SN or other brainstem nuclei | Striatum, SN, cerebellum, inferior, olives, pontine nuclei | Variable in affected areas, cortex | Similar but less BG | Variable in affected areas | Severe in SN, BG, cortex | Frontotemporal lobe | Severe in SN, less other brainstem nuclei | Severe in SN, cortex, hippocampus | Caudate > putamen, less pallidum | Frontal and temporal lobe, striatum |
| Atrophy | Cortex mild | Only if AD coexists | Striatum, pontine base, inferior olives | Brainstem; cortex mild | BG, brainstem, white matter | Mild | Parietal or frontoparietal asymmetric | Frontotemporal lobe severe | None | Frontal, temporal, parietal, SN | Striatum, caudate > putamen, cortex | Frontal, temporal cortex, putamen |
| Ballooned and achromatic neurons | None | None | None | Rare | Rare | Rare | In affected areas | Pick cells (ballooned) characteristic | None | In affected areas | None | Occasional |
| Pick argyrophilic inclusions (Pick bodies) | None | None | None | None | None | None | None | Many | None | In rare mutations | None | Hyaline conglomerate inclusions |
| Basophilic neuronal inclusions | None | None | None | None | None | None | SN | None | None | None | None | ? |
| Neuronal inclusions, αSyn+ | None | None | Isocortex, hippocampus | None | None | None | None | None | None | None | None | None |
| Oligodendroglial inclusions | None | None | αSyn+ in affected and other areas | Tau+ in affected areas | None | None | Tau+ in affected areas | None | Tau+ in affected areas | Tau+ in affected areas | None | Tau+ occasional |
| Astroglial inclusions | αSyn+ white matter | αSyn+ | None | Tau+ in affected areas (tufted) | Tau+ in affected areas (tufted) | Some | Astroglial plaques (white matter) | None | Tau+ in affected areas | Tau+ in affected areas (tufted) | None | Tau+ occasional |
| TDP-43 pathology | None | None | Rare | None | None | ? | In rare variants | ? | Frequent | None | None | None |
a The presence of Lewy bodies, oligodendroglial argyrophilic inclusions, changes diagnostic of Alzheimer’s disease (neuritic plaques), prion P–positive amyloid plaques, and larger or numerous infarcts exclude the diagnosis of any of these disorders, except combined progressive supranuclear palsy.
Movement disorders are also classified into morphologic and biochemical groups ( Box 104.2 ). The α-synucleinopathies are a heterogeneous group of neurodegenerative disorders caused by misfolded α-synuclein (αSyn) protein that forms amyloid-like filamentous inclusions. , They include Lewy body (LB) disorders—sporadic and rare familial forms of PD (brainstem type of Lewy body disease [LBD]), dementia with Lewy bodies (DLB), and pure autonomic failure—multiple system atrophy (MSA), and neurodegeneration with brain iron accumulation type 1 (NBIA 1) or pantothenate kinase–associated neurodegeneration (PKAN). Other major groups are tauopathies, featured by neurofibrillary tau pathology (progressive supranuclear palsy [PSP], corticobasal degeneration [CBD], frontotemporal lobe degeneration with tau pathology [FTLD-tau]); polyglutamine disorders linked to cytosine-adenine-guanine (CAG) trinucleotide repeats, such as Huntington disease (HD); and those associated with neuronal antibodies, paraneoplastic forms, or those without hitherto detected genetic or specific disease markers. The various phenotypes are related to the deposition of pathologic (misfolded) proteins in distinct brain areas and neuron populations. ,
Parkinson disease (brainstem type of Lewy body disease)
Sporadic
Familial with α-synuclein mutation
Familial with other mutations
Incidental Lewy body disease (subclinical Parkinson disease)
Pure autonomic failure
Lewy body dysphagia
Dementia with Lewy bodies; diffuse Lewy body disease
Multiple system atrophy
Striatonigral degeneration (MSA-P)
Olivopontocerebellar atrophy (MSA-C)
Pantothenate kinase–associated neurodegeneration (PKAN) (formerly Hallervorden-Spatz disease a
a No longer classified as an α-synucleinopathy.
)
Parkinson disease with parkin- and LRRK2-linked mutations
Alzheimer disease (and other tauopathies)
Progressive supranuclear palsy (4R-tau doublet + exon 19)
Corticobasal degeneration (same)
Guamanian amyotrophic lateral sclerosis–parkinsonism-dementia complex (3R-tau + 4R-tau triplet)
Postencephalitic parkinsonism (3R-tau + 4R-tau triplet)
Chromosome 17–linked familial dementia (frontotemporal dementia and parkinsonism) (tau doublet) (FTLD-tau)
Pallidopontonigral degeneration (4R-tau)
Pick disease (3R-tau doublet without exon 10)
Advanced Alzheimer disease with subcortical neurofibrillary tangles
Perry syndrome
Frontotemporal lobe degeneration with MAPT mutation (FTLD-MAPT)
Frontotemporal lobe degeneration with ubiquitin inclusions
Frontotemporal lobe degeneration with fused in sarcoma ( FUS ) mutation and other mutations (e.g., FTLD, GRN, VCP )
Amyotrophic lateral sclerosis
Huntington disease—rigid type (CAG triplet repeat)
Choreoacanthocytosis (neuroacanthocytosis)
Machado-Joseph disease (spinocerebellar ataxia type 3 + type 2)
Dentatorubral-pallidoluysian atrophy
X-linked dystonia parkinsonism (Lubag disease)
Fragile X–associated tremor and ataxia syndrome (FXTAS)
Spinocerebellar ataxia
Hereditary striatal degeneration
Pallidal degeneration and related variants
Hallervorden-Spatz disease (without α-synucleinopathy)
Inherited metabolic disorders (e.g., Wilson disease, Menkes disease)
Neuronal intranuclear inclusion disease and basophilic inclusion disease
Inherited dystonias and dyskinesias
Hereditary ferritinopathies
αSyn is a partially unfolded, 140–amino acid presynaptic protein with potential for self-oligomerization and fibrillary aggregation under pathologic conditions. Its molecular basis, functions, interaction with dopamine metabolites, and relevant animal models have been extensively described. αSyn assembles into special oligomers and is prone to misfold, which may lead to dysfunction of axonal transport, , synaptic dysfunction, and neuronal death, It is a major component of LBs, dystrophic Lewy neurites (LNs), astroglia in PD and DLB, , and neuronal and glial inclusions in MSA. Elevated levels of soluble αSyn oligomers have been detected in postmortem brains of patients with PD and DLB, , mediating early synaptic pathology and cellular dysruption. αSyn toxicity is potentiated by glycation and lysosomal abnormalities.
Co-occurrence of αSyn, tau, β-amyloid (Aβ), and other proteins, and a strong interaction between their oligomeric forms, promotes their mutual aggregation, thereby amplifying neuronal damage. Modification of αSyn may induce both Lewy and tau pathologies, and enhances amyloid and tau accumulation, while tau and Aβ enhance αSyn aggregation and toxicity. At synaptic terminals, Aβ is associated with hyperphosphorylation of tau, whereas an interaction between Aβ and αSyn leads to inhibition of Aβ deposition. In the brains of patients with PD and DLB, concentrations of soluble serine 129–phosphorylated αSyn (pSer129-αSyn) correlated significantly with the levels of Aβ. Interaction of αSyn, tau, and Aβ (with metal ions) may represent a molecular mechanism in the overlapping pathology of different proteinopathies that represent a continuum depending upon genetic and environmental factors. , , ,
Distinct strains of αSyn are responsible for propagation and differences of regional distribution of lesions in various α-synucleinopathies and are involved in their heterogeneity, , , as has been observed following the injection of αSyn aggregates in animal models.
This group includes various α-synucleinopathies, tauopathies, and other hereditary degenerative disorders causing parkinsonian syndromes. , , , , Parkinsonism has many causes ( Box 104.3 ). Clinicopathologic studies have shown that α-synucleinopathies account for 73% to 83% of cases of parkinsonism, including 42% to 63% of PD cases, whereas other degenerative disorders masquerading as PD account for 9% to 33%. ,
Idiopathic Parkinson disease (sporadic, familial)
Multiple system atrophy
Dementia with Lewy bodies
Progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome)
Genetic Parkinson disease ( PINK1, PRKN, LRRK2 )
Vascular pseudoparkinsonism
Corticobasal degeneration
Alzheimer disease, Pick disease
Frontotemporal dementia with parkinsonism linked to chromosome 17
Guamanian parkinsonism-dementia complex
Metal storage disorder (Wilson disease, PKAN, HH, etc.)
Neuroacanthocytosis
Huntington disease, rigid type
Spinocerebellar ataxia type 3
Dentatorubral-pallidoluysian atrophy
Lubag disease (X-linked dystonia-parkinsonism)
Dopa-responsive dystonia
Pallidal degenerations, pallidonigroluysian atrophy
Neuronal intranuclear inclusion disease and neurofilament inclusion body disease
TDP-43 (Perry syndrome)
Vascular (pseudo-) parkinsonism (lacunar state, leukoaraiosis)
Drug-induced parkinsonism (dopamine receptor blockers, neuroleptics)
Toxin-induced disease (e.g., manganese, carbon monoxide, carbon disulfide, MPTP, rotenone)
Infections and postinfectious diseases (influenza virus, HIV encephalopathy, Creutzfeldt-Jakob disease, neurosyphilis, Japanese B encephalitis, herpes encephalitis, paraneoplastic limbic encephalitis)
Anoxic brain injury
Inherited metabolic disorders:
Lysosomal storage diseases: Gaucher disease, Niemann-Pick disease, GM1 gangliosidosis
Disorders of metal metabolism: Wilson disease hemochromatosis, PKAN
Disorders of amino acid metabolism: phenylketonuria, maple syrup urine disease, methylmalonicaciduria
Mitochondrial disorders
Other disorders:
Normal-pressure hydrocephalus
Space-occupying lesions (frontal lobe tumor, CNS lymphomas)
Posttraumatic parkinsonism (boxer’s encephalopathy/chronic traumatic encephalopathy)
Basal ganglia calcification (Fahr syndrome, hypoparathyroidism)
Brainstem tumors
Brainstem lesions due to increased intracranial pressure
The prominent cytoskeletal lesions are αSyn-positive LBs, cytoplasmic inclusions occurring in many regions of the nervous system and in multiple extraneural organs. , They are the morphologic hallmarks of PD and DLB but are also found in a variety of disorders—for example, in 7% to 71% of sporadic and familial forms of Alzheimer disease (AD) , and in 2% to 61% of elderly individuals with or without dementia.
LBs occur in two types: the classical brainstem type and the cortical type. Classical LBs are spherical cytoplasmic intraneuronal inclusions 8 to 30 μm in diameter with a hyaline eosinophilic core, concentric lamellar bands, and a narrow pale-stained halo. Some brain regions, such as the dorsal motor nucleus of the vagus nerve (dmX), contain intraneuritic LBs. Ultrastructurally, classical LBs are non–membrane-bound, granulofilamentous structures composed of radially arranged, 7- to 20-nm intermediate filaments associated with electron-dense granule material and vesicular structures, with the core showing densely packed filaments and dense granular material and the periphery having radially arranged 10-nm filaments. , Cortical LBs—eosinophilic, rounded, angular, or reniform structures without a halo—are ultrastructurally poorly organized, granulofibrillary structures with a felt-like arrangement composed of 7- to 27-nm-wide filaments. They are found in small nonpyramidal neurons in lower cortical layers, with accumulation in the insular and entorhinal cortex, amygdala, hippocampal sector CA2/3, and cingulate gyri. , Similar rounded areas of granular, pale-staining eosinophilic material displacing neuromelanin in brainstem neurons (“pale bodies”) are precursors of LBs. αSyn is the best marker to decorate LBs and LNs, selectively detected by antibodies that recognize N-terminal epitopes (synucleins 505, 506, and 514).
Both types of LBs share immuno- and biochemical characteristics. , , The major components are αSyn, ubiquitin, and phosphorylated ubiquitin associated with many other substances: structural fibrillary elements; αSyn-binding proteins; proteins implicated in the ubiquitin-proteasome system; synphilin-1; aggresome- and mitochondria-related proteins; cytoskeletal, cytosolic, and cellular response proteins; and others. , LBs have a central parkin- and ubiquitin-positive domain with αSyn in the periphery. Colocalization of αSyn, synphilin, and parkin within LBs suggests that parkin plays a role in ubiquitination of αSyn, its oligomers inducing parkin nitrosylation. Synapsin III, a key component of αSyn fibrils, tyrosine hydoxylase (TH), and choline acetyltransferase (ChAT) are colocalized in cortical LBs. Brainstem LBs have TH and ChAT reactivity in the core, surrounded by a peripheral rim of αSyn. LBs and pale bodies are reactive for autophagic proteins p62 and NBR1, , and for the TIGAR protein that regulates tumor protein 53, which is absent in MSA inclusions. They contain 14-3-3 proteins that interact with αSyn, are involved in signal transduction pathways, and have multiple cellular functions. Leucine-rich repeat kinase 2 (LRRK2) is not a major component of LBs. αSyn aggregates in LBs are refractory to clearance and degradation. Purified inclusions contain approximately 50 isoforms of αSyn, including multiple truncated species. Regional levels of physiologic αSyn were directly associated with LB-related pathology (LBP). Proteomic analysis of cortical LBs has revealed 296 proteins related to multiple or unknown functions and 204 proteins in the brainstem of patients with PD, suggesting a complex formation process.
The formation of LBs runs through several stages. Classical LBs show an initial intraneuronal appearance of dust-like particles related to neuromelanin or lipofuscin that are cross-linked to αSyn, with homogeneous deposition of αSyn and ubiquitin in the center, showing diffuse, pale or fine granular cytoplasmic staining. The next step is condensation of dense filamentous inclusions, forming “early LBs” that later develop into classical LBs. Extraneuronal LBs, after disappearance of the involved neuron, are degraded by astroglia.
Cortical LBs show diffuse αSyn and ubiquitin labeling, whereas subcortical LBs have a distinct, central ubiquitin-positive domain with αSyn occurring in the periphery. Initial granular accumulation of αSyn in the neuronal cytoplasm is followed by stepwise accumulation of dense filaments, spreading to dendrites, later deformation of LBs, and final degradation by astrocytes.
LBs are associated with coarse LNs that also contain αSyn and ubiquitin as inclusions in axonal processes, which may evolve into LBs with ubiquitin at the core and neurofilaments at the outermost layer. LBs and LNs occur in virtually all brainstem nuclei and fiber tracts, with significant correlations between LBs and LNs, and LBs and coiled bodies, in both PD and DLB.
Intranuclear inclusions, referred to as Marinesco bodies, are frequently found in elderly individuals in pigmented neurons of the substantia nigra and locus ceruleus that contain LBs, and their frequency appears to have an inverse relationship with striatal concentrations of dopamine transporter (DAT) and TH. Their frequency decreases as duration of PD increases.
The biologic significance of LBs and their impact on neurodegeneration are poorly understood. As sequelae of frustraneous proteolytic degradation of abnormal cytoskeletal elements, they represent—similar to other inclusions such as neurofibrillary tangles (NFTs) or Pick bodies—end products or epiphenomena of unknown responses to cellular stress. Inhibition of complex I (reduced nicotinamide adenine dinucleotide ubiquitinone oxidoreductase) causes aggregation of αSyn due to impairment in protein handling and detoxification, whereas mitochondrial accumulated αSyn may interfere with complex I function. Small αSyn intermediates termed soluble oligomers lead to synaptic dysfunction, whereas insoluble aggregates may function as reservoirs for bioactive oligomers. Oligomerization of αSyn in the initial state of PD , may induce protein aggregation, disrupt cellular function, and lead to neuronal death due to mitochondrial energy deficit and oxidative stress. , , ,
The ubiquitin-proteasome system and the autophagy-liposome pathway that render damaged proteins less toxic than their soluble forms contribute to αSyn turnover, while alterations in these proteolytic pathways result in αSyn accumulation due to impaired clearance. The degree of the mitophagy marker phospho-ubiquitin increased in the early but decreased in late stages of LB or tangle aggregation, indicating mitochondrial damage. Proteinaceous inclusions also form as a consequence of fusion of autophagic vesicles in cells unable to degrade ruptured vesicles and their protein contents. Ubiquitinated proteins in LBs may be a manifestation of a cytoprotective response in order to eliminate damaged cellular components and to delay the onset of neuronal degeneration. LBs also interact with DNA to cause nuclear degeneration as a major cause of cell death. Mitochondrial DNA deletion was highest in LB-positive neurons, suggesting increased mitochondrial damage, whereas accumulation of mitochondrial DNA deletions within dopaminergic neurons triggers neuroprotective mechanisms. , , LBP is direct evidence of αSyn misfolding, but it is not yet clear whether LBs are an indicator of toxicity or neuronal protection. , ,
The uptake and clearance of misfolded/aggregated αSyn, reciprocally regulated by SUMOylation and ubiquitination, is a key process to control extracellular deposition of αSyn aggregates. All main brain cell types are able to internalize and degrade extracellular αSyn, but glial cells appear to be the most efficient scavengers. Impairment of clearance leads to accumulation of toxic species and dysfunctions of glia. αSyn-reactive astrocytes, the distribution of which parallels the spread of neuronal pathology in PD, , may be involved in the progressive neurodegeneration.
PD or the brainstem type of LBD, the most frequent neurodegenerative movement disorder (prevalence of 100 to 572 per 100,000; incidence of 4.5 to 21 per 100,000 person-years ), is manifested clinically by bradykinesia, rigidity, rest tremor, postural imbalance, and various nonmotor features. Subtle cognitive dysfunction and depression are often present early in the disease, dementia being common in later stages. PD is characterized by progressive degeneration of the dopaminergic nigrostriatal system and many cortical and subcortical networks associated with widespread αSyn pathology. The resultant striatal dopamine deficiency and other biochemical deficits produce a heterogeneous clinical phenotype. The accuracy of clinical diagnosis, according to a meta-analysis, was 73.8% to 79.6%. Using UK Parkinson’s Disease Society Brain Bank Research Center criteria, the pooled diagnostic accuracy was 82.7%. For the diagnosis of definite PD, histopathologic confirmation is required. Although LBs are not specific to PD and occur in a variety of conditions, a positive diagnosis of PD can usually be made by inspecting two unilateral sections from the midportion of the substantia nigra and finding LBs. If no LBs are found, two further sections should be examined. If LBs are not seen in either the substantia nigra or locus ceruleus, the diagnosis of PD of the LB type can be excluded. In case of cell loss in the substantia nigra and locus ceruleus in the absence of LBs or other αSyn-positive inclusions, an alternative cause of parkinsonism should be pursued.
The brain is usually grossly unremarkable or shows mild cortical atrophy, enlargement of the ventricles, and pallor of the substantia nigra and locus ceruleus. Histologic examination reveals widespread αSyn-immunoreactive deposits in neurons (LBs) and LNs throughout the nervous system, including all nuclei and fiber tracts in the brainstem and many visceral organs. , , , Recent reviews of the neuropathology of sporadic PD , , , , , and of genetic PD are available.
LBP is associated with variable neuronal loss in the midbrain and other subcortical nuclei, in particular the SNc, locus ceruleus, nucleus basalis of Meynert (NBM), VTA, dorsal raphe nucleus, dmX, and many other neuronal systems. Depletion of melanized neurons (45% to 66%) and dopaminergic neurons immunoreactive for TH, the key enzyme of dopamine synthesis (60% to 85%), affects the A9 group of the SNc, particularly in the ventrolateral tier (area A, 91% to 97%) projecting to the striatum. This corresponds to a somatotopic pattern of dopaminergic terminal loss that is more severe in the dorsal and caudal putamen with later involvement of the ventral putamen and caudate nucleus. Substantia nigra cell degeneration is preceded by loss of neurofilament protein, neuronal TH and DAT immunoreactivity, and cyclooxygenase indicative of functional neuronal damage. It is accompanied by extracellular release of neuromelanin with uptake by macrophages, rare neuronophagy, and astroglial response. Microglial activation in the affected nigrostriatal pathway in early PD suggests that neuroinflammation contributes to the degenerative process, even prior to nigral damage. Nigral neuronal loss is progressive during the first years after the onset of motor symptoms and more stable during later disease stages. The ventrolateral cell clusters are nearly wiped out at disease onset, while other dopaminergic and GABAergic neurons are spared at this time. As the disease processes, the nearby ventral and then dorsal substantia nigra cell clusters and their striatal projections are affected despite the diversity of both their neurochemistry and synaptic input.
In the substantia nigra, the proportion of LB-bearing neurons appears to be stable throughout the disease duration; between 3.6% and 15% of surviving substantia nigra neurons contain LBs. Their life span is around 6.2 months (15.9 months for any type of αSyn inclusion). The degree of A9 SNc cell loss and the reduction of TH and DAT immunoreactivity in the putamen, followed by the caudate nucleus and nucleus accumbens, indicate that the loss of dopaminergic neurons and terminals in striatum is correlated with the duration and severity of motor dysfunction. , At 4 years postdiagnosis and thereafter, PD patients showed virtually complete loss of DAT staining in the dorsal putamen with only an occasional dopaminergic fiber in the SNc, and a 50% to 90% loss of TH-positive neurons in the striatum. In end-stage PD, either a stable proportion of LB-bearing substantia nigra neurons , or a correlation between the number of neurons and LBs is seen. Despite a massive loss of substantia nigra neurons with atrophy of the remaining cells, degeneration of the striatonigral system is not total, even after many years of illness. A negative correlation between neuronal density and αSyn burden was observed in the substantia nigra, but no relationship with Hoehn and Yahr Scale stage or disease duration.
The A10 group of dopaminergic neurons—the VTA, nucleus parabrachialis, and nucleus parabrachialis pigmentosus—projecting to the striatal matrix, thalamus, and cortical and limbic areas (mesocorticolimbic system) shows less severe involvement (40% to 77%, average 53% cell loss ), whereas the periretrorubral A8 region, which contains only a few dopaminergic but calbindin-rich neurons, and the central periventricular gray matter show little or no involvement.
Degeneration of the nigrostriatal system causes denervation in the striatum with dopamine loss ranging from 44% to 98% and progressing from the ventrorostal to the posterior putamen and caudate nucleus. In earlier disease stages, dopamine depletion provokes an increased number of striatal dopaminergic neurons, a compensatory mechanism more efficient in younger PD patients. Higher nigrostriatal neuron loss occurs in early-onset rather than in late-onset PD. At the time of motor symptom onset, the extent of striatal dopamine marker loss exceeds that of dopaminergic substantia nigra neurons. It is more severe in the putamen (−98.4%) than in the caudate nucleus (−89%), whereas in GPi (−89%) and GPe (−51%) it is not related to the pattern of putamen dopamine loss. The concept that PD motor symptoms first appear when more that 50% of dopaminergic substantia nigra neurons are lost has been changed by the notion that at that time only around 30% of dopaminergic substantia nigra neurons, but 50% to 60% of their axon terminals, have been lost. This is preceded by loss of dopamine markers in the nigrostriatal terminals in early PD, while melanin-containing substantia nigra neurons more than TH-positive cells may persist for a longer time.
Biochemical increase of phospho-αSyn precedes αSyn aggregation followed by the formation of LBs and LNs, but it does not necessarily correlate with LBP, which shows an inconsistent relationship to clinical disease progression. Lower neuron densities in the substantia nigra occur before LB deposition, suggesting that cellular dysfunction precedes LBP and supporting a dying-back mechanism, in which dysfunction starts at the synapse, caused by accumulation of small αSyn aggregates at presynaptic terminals. Early intra-axonal aggregation of αSyn as premature “pale neurites” at axon collaterals extending centripetally into proximal segments damages the parental neurons by interfering with axonal transport. , Axonopathy in presymptomatic PD is followed by neuronal degeneration. Accumulation of αSyn is triggered by presynaptic dysfunction and mediates early synaptic pathology by disrupting synaptic vesicles. Hence the loss of dopaminergic neurons might be an epiphenomenon after the loss of synapses, defining PD as a “synaptopathy.” , , , The aberrant formation of DAT/αSyn complexes in the striatum due to synaptic alterations may contribute to dopaminergic neurodegeneration in PD.
LB/αSyn pathology in PD is not restricted to specific dopaminergic brainstem nuclei but involves nonnigral nuclei as well. It is associated with degenerative lesions affecting the central, autonomic, and peripheral systems, , , , , including the cholinergic basal forebrain, and multiple other neurotransmitter systems. The extranigral lesions correlate with early premotor symptoms (olfactory, autonomic, and sensory symptoms; sleep disturbances; pain; neuropsychiatric dysfunction), later nonmotor fluctuations, advanced non–dopamine-responsive nonmotor features, and neuroendocrine abnormalities. , LBP involves the spinal cord, , the autonomic and peripheral nervous systems, the sympathetic and parasympathetic ganglia and plexuses, the intramural enteric nervous system, and the skin, retina, uterus, submandibular gland, bladder, cardiac nervous system, and adrenals. , , , The musculoskeletal system and major parts of the sensory nervous system are spared, , whereas the involvement of cutaneous nerves occurs in later stages.
Among the earliest involved areas are the olfactory bulb and related olfactory nuclei in the brain (amygdala, perirhinal cortex), suggesting that olfactory dysfunction in PD is related to the involvement of central pathways rather than peripheral sensory nerve fibers. , αSyn aggregation in the olfactory system and its spreading to the brain may contribute to PD initiation by inducing pathologic changes in sensitive brain areas. Some structures are particularly susceptible for LBP and may potentially act as “sentinels” for its development: the olfactory bulb, dmX, and peripheral autonomic nervous system. , This is supported by an increase of phosphorylated αSyn restricted to the olfactory bulb and brainstem in early stages of PD. , Involvement of the autonomic nervous system and gastrointestinal tract before involvement of the central nervous system (CNS) has forwarded the hypothesis of a possible route for spreading αSyn via the vagus nerve to the brain, , confirmed by rotenone administration or αSyn inoculation into the mouse gastrointestinal tract. , Resection of the vagal nerve interrupted the disease progression to the CNS and was associated with reduced risk of PD, suggesting a possible role of the gut-brain axis in the pathogenesis of PD.
Three current major staging systems are in use for LB disorders, one for PD, , another for DLB, and revised guidelines for LBD. Based on semiquantitative assessment of LB distribution in a large autopsy series, a staging of the chronologic spread of LBP (the Braak stages) was proposed to designate the predictable sequence of lesions in the nervous system ( Fig. 104.2 ).
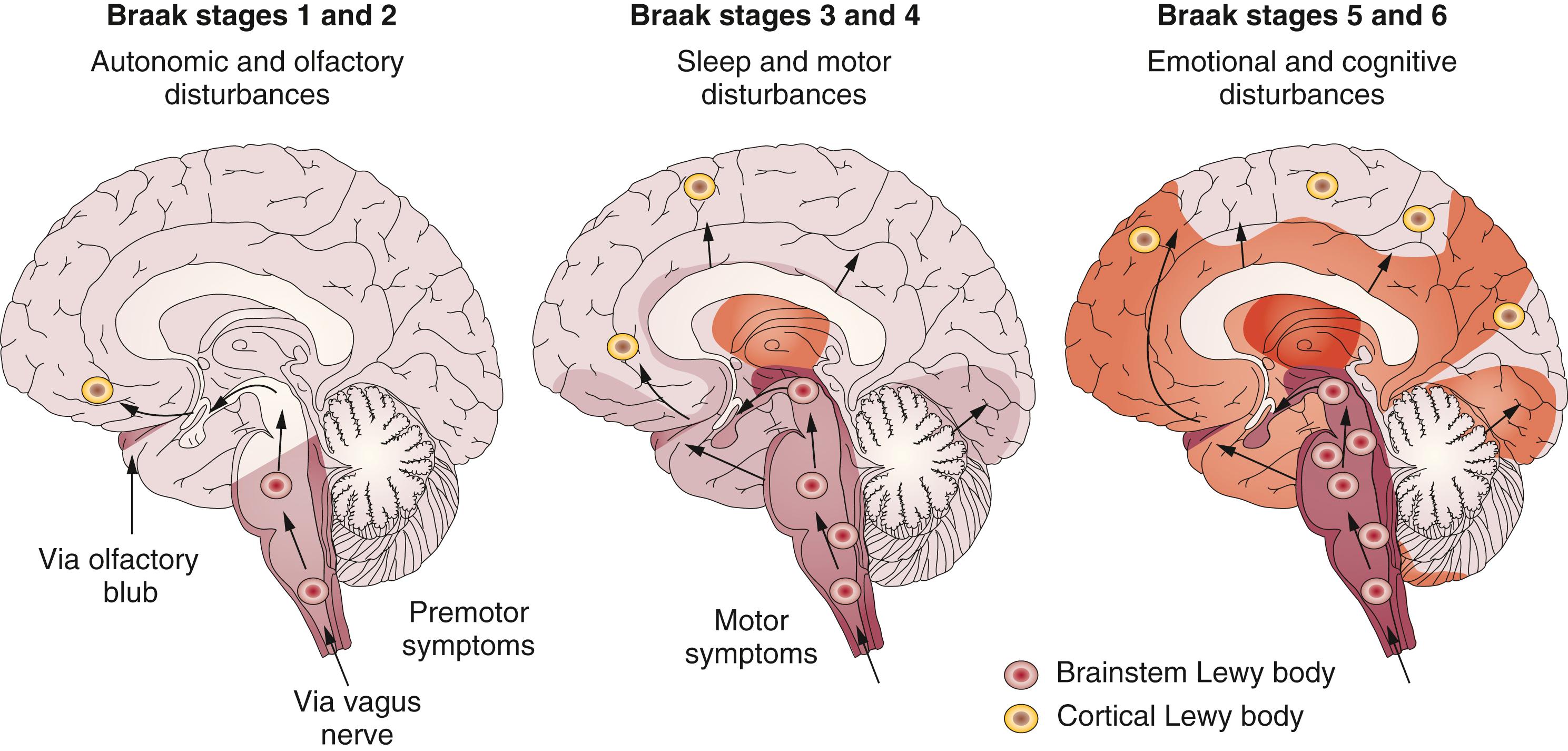
LBP initially involves the olfactory bulb and related olfactory nuclei of the brain, the peripheral autonomic system, and the adrenal medulla in neurologically unimpaired subjects, referred to as incidental Lewy body disease (iLBD). It involves the dmX and intermediate reticular zone, with the NBM and midbrain regions being preserved (stage 1). In stage 2, LNs occur in the enteric nervous system, in parasympathetic and sympathetic nerves, and in medullary nuclei of the level setting system (e.g., lower raphe nuclei, gigantocellular reticular nucleus, and ceruleus-subceruleus complex). These initial stages are considered asymptomatic or presymptomatic and may explain nonmotor (olfactory and autonomic [e.g., gastrointestinal and urinary]) symptoms that precede motor dysfunctions. , , , In stage 3, LNs and LBs occur in the PPN, locus ceruleus, amygdala, nuclei of the basal forebrain, upper raphe nuclei, magnocellular nuclei of the basal forebrain, hypothalamic tuberomammillary nucleus, posterolateral and posteromedial SNc, and spinal cord, whereas the allocortex and isocortex are preserved. This stage is associated with disturbed sleep, early motor dysfunction (asymmetrical tremor, rigidity, and hypokinesia), and several nonmotor symptoms. In stage 4, midline and intralaminar nuclei of the thalamus, anteromedial temporal limbic cortex (transentorhinal and entorhinal region), hippocampal formation, and second sector of the Ammon’s horn are involved, associated with severe motor dysfunction. In stage 5, LNs and LBs occur in the superordinate cortical areas for regulation of autonomic functions, in higher order sensory association areas and prefrontal fields. They are associated with late-phase motor disability and fluctuations. In terminal stage 6, the first-order sensory association areas and premotor fields, primary sensory, and motor areas or the entire neocortex are involved, , causing late motor disability, fluctuations, and cognitive impairment.
Nigral cell loss (12%) has been observed in stages 1 and 2 before the appearance of αSyn aggregates, and progression from stage 3 to stage 4 shows significant decrease in substantia nigra cell density (46%). An increase in the density of αSyn aggregates and LBs from stages 3 to 6 correlated negatively with the decrease in neuronal density. Metabolic and functional abnormalities in the brain occur in early stages of PD, not yet accompanied by LBP. , , Many of the regions containing LBP early in PD had no obvious cell loss even over time, while some became involved with increased disease severity. Stereologic studies showed no overall loss of neocortical neurons in end-stage PD, although there were many cortical LBs.
The validity of the Braak staging scheme, which corresponds roughly to the original classification of LB disorders into three phenotypes—brainstem predominant, limbic/transitional, and diffuse neocortical —has gained acceptance, but has been a matter of debate. , , It often but not consistently shows correlations between morphologic and clinical data, mainly in a subgroup of patients with early onset and prolonged duration. Studies have shown that 51% to 83% of PD and DLB cases were compatible with this staging, , whereas between 6.3% and 47% of autopsy-proven PD cases did not conform to it. , , , , In 7% to 8.3% of PD cases, the dmX was not involved despite αSyn inclusions in higher brain stem or cortical regions, , , , whereas in large samples, 49% to 55% of individuals with widespread αSyn pathology lacked clinical symptoms or were unclassifiable. ,
The Braak hypothesis, suggesting predictable caudorostral spreading of LBP, is based on distribution of LBs but not on neuronal loss, which have no significant relationship, and is not identical with the spreading of αSyn. , It is valid for PD patients with young onset and long duration with motor symptoms, but not for others (e.g., late onset and rapid disease course). Between 10% and 15% of PD cases associated with genetic mutations show a pattern of LBP that is distinct from the Braak scheme. A recent theory proposed that corticostriatal activity may represent a critical somatotopic “stressor” for nigrostriatal terminals, driving retrograde degeneration and leading to focal motor onset and progression of PD. It may promote secretion of striatal extracellular αSyn, favoring its pathologic aggregation at vulnerable dopaminergic synapses. A similar process may occur at corticofugal projections to the medulla, contributing an integrative top-down perspective for the factors that impinge upon the vulnerability of dopaminergic cells in PD. A new staging system, the Lewy pathology consensus criteria (LPC) (based on the McKeith system but applying a dichotomic approach for the scoring of Lewy pathology [i.e., “absent” vs. “present”]), allows classification of all cases of LBD into distinct categories.
The term incidental Lewy body disease is used when LBs are found in the nervous system in subjects without clinical parkinsonism. The distribution of LBs is similar to that in PD, but some brains have sparing of LBs in the limbic cortex (average Braak stage of 2.7), whereas in definite PD cases, more LBs are found in all regions (Braak PD stage 4.4). A 70% substantia nigra cell loss and decreased TH immunoreactivity were shown in striatum and epicardial nerve fibers, but not to the same extent as in PD. , , Such cases must have reduced vulnerability to substantia nigra cell loss in the context of equal LBP load. Some cases of random eye movement sleep behavior disorder may represent iLBD, suggesting that it is a preclinical form of PD and that the lack of symptoms is due to subthreshold pathology.
Between 5% and 55% of neurologically unremarkable elderly people have shown abundant LBP with a distribution pattern similar to that seen PD, while the pigmented substantia nigra neurons were relatively well preserved , , or LBP was confined to the olfactory bulb. Others had sparse, but widespread LBP involving the cortex, , which would violate the theory of upward progression from the brainstem and perhaps fit better with a multicentric disease progression from the onset. LBP in the spinal cord and dorsal root ganglia in elderly persons was associated with that in the lower brainstem due to retrograde spread.
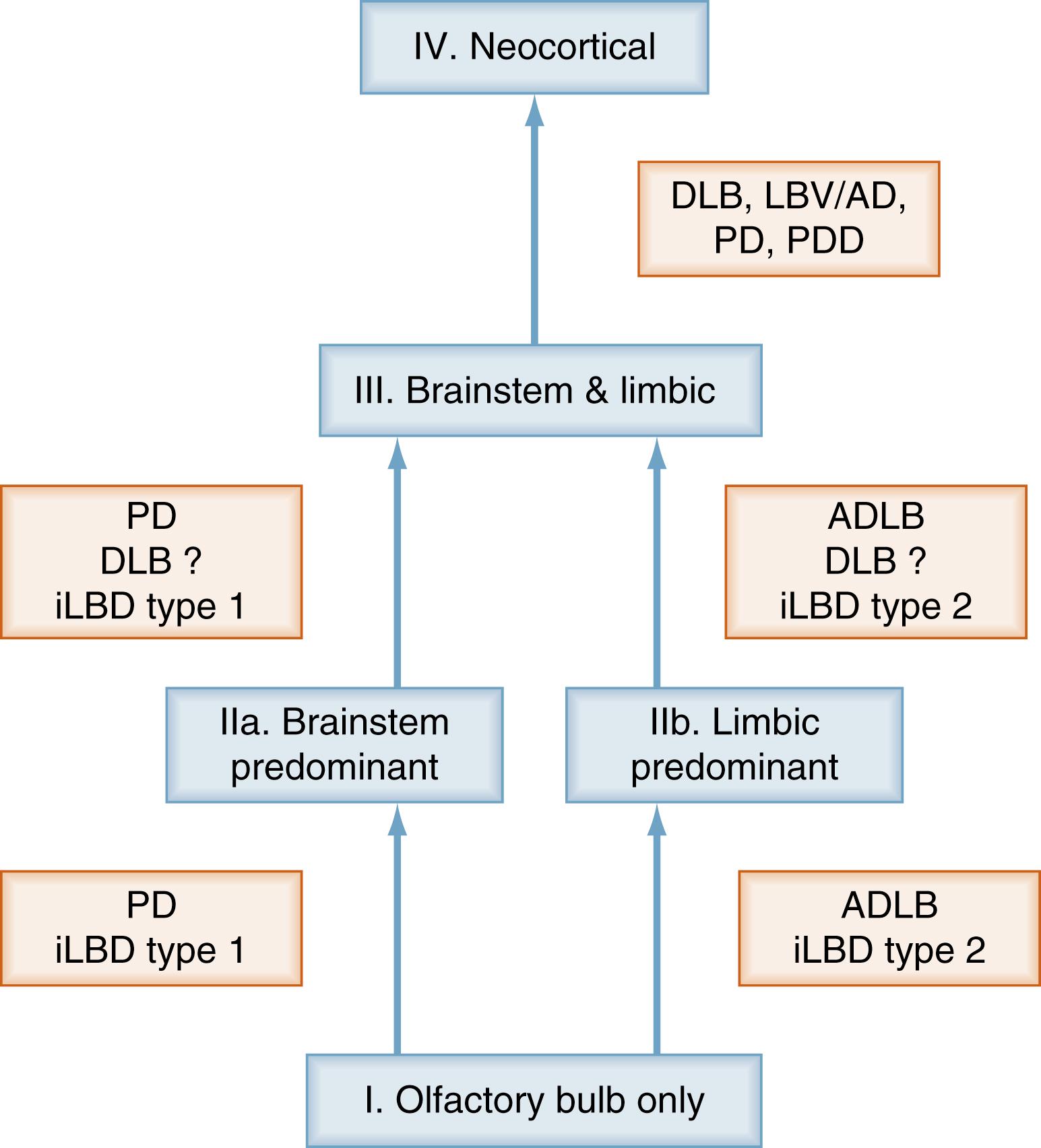
A new unifying system for LB disorders correlates with nigrostriatal degeneration, cognitive impairment, and motor dysfunction. Whereas the previous classifications left 42% to 50% of elderly individuals unclassified, this system allows all cases to be classifiable into one of the following stages: I, olfactory bulb only; IIa, brainstem predominant; IIb, limbic predominant; III, brainstem and limbic; and IV, neocortical ( Fig. 104.3 ). Progression through these stages was accompanied by stepwise deterioration of striatal TH concentration and substantia nigra pigmented cell loss, showing significant correlation with clinical and psychometric data.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here