Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
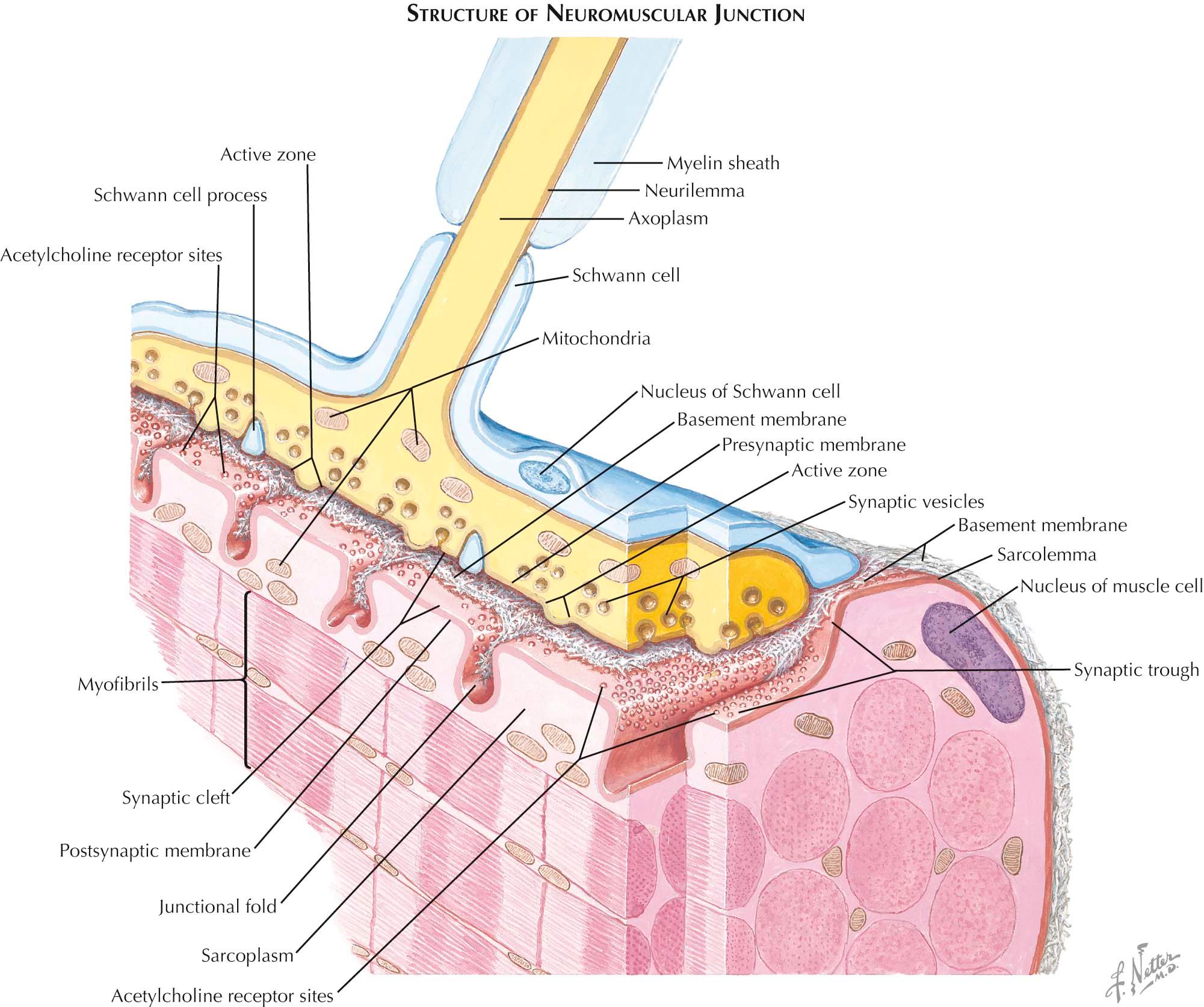
The outflow of nearly all behavior depends upon the neuromuscular system, where nerves emanating from the spinal cord and brainstem make connections with skeletal muscles that allow us to move, stand, and express ourselves. The numbers of skeletal muscles in the human body is daunting: somewhere between 500 and 1000. The face alone has enormous numbers of muscles that allow us to express our emotions, articulate our words, and eat our food. Our hands are the second most “muscular” parts, giving us the finesse to play musical instruments, communicate with sign language, and write. Some muscles are huge. The gluteus maximus, for example, has many thousands of muscle fibers and is essential for walking. Other muscles are miniscule, designed to cause the slightest movements of the eardrum or the larynx and have a few hundred muscle fibers or fewer. The set of muscle fibers innervated by an axon (a motor unit) is very small in muscles that require very fine control, such as the extraocular and finger muscles, where motor units can be fewer than 10, or very large (100s or 1000s) in postural (back musculature) and girdle muscles (gluteus maximus). Despite the functional and structural diversity of muscles, however, the communication between the nervous system and muscles is much the same throughout the body. In humans and other mammals, muscles are composed of many muscle fibers, and each muscle fiber is typically innervated by only one motor neuron, with innervation focused on a small region of the muscle fiber known as the neuromuscular junction (NMJ). A NMJ may occupy less than 0.1% of the muscle fiber's surface area and yet is sufficient, without fail, to cause the muscle fiber to twitch each time an electrical impulse travels from the motor neuron cell body in the central nervous system to the muscle via the peripheral nerve. Thus the NMJ is among the most reliable and powerful synapses within the body.
The structure of the neuromuscular junction explains why it is so powerful. An axonal branch terminates in a branched structure that is laden with mitochondria, synaptic vesicles, and a number of special features that ensure that when an action potential invades the terminal a sequence of events is set in motion, leading to the near-synchronous release of neurotransmitter as hundreds of synaptic vesicles fuse with the nerve's plasma membrane and release their contents into the synaptic cleft. The main constituent of these synaptic vesicles is acetylcholine (ACh), the neurotransmitter at all skeletal muscle neuromuscular junctions. The released acetylcholine diffuses across the synaptic cleft, which is a mere 10 to 20 nm wide. It takes roughly 1 µsec for an ACh molecule to traverse the cleft and reach the synaptically specialized membrane of the muscle fiber, known as the postsynaptic membrane.
However, at least half of the released neurotransmitter never reaches the postsynaptic membrane because there is a high concentration of an enzyme on the cleft that enzymatically inactivates the neurotransmitter, cleaving it into acetate and choline. It may seem strange that this enzyme, known as acetylcholinesterase, should be juxtaposed between the nerve's release site and the muscle fiber's receptive site. However, the large amount of ACh released from hundreds of synaptic vesicles means that there is far more ACh available than is normally required to cause the muscle fiber to twitch when an electrical impulse from the axon invades the nerve terminal. This “safety factor” means that, in normal use, it is very unlikely that the available neurotransmitter will fail to cause the muscle to contract. The muscle contraction is initiated by the binding of ACh to the acetylcholine receptors (AChR) in the postsynaptic membrane. The AChRs are packed into the postsynaptic membrane at as high a concentration as their size permits, about 10,000 receptors per square micron of membrane, guaranteeing that any ACh molecule that makes it through this gauntlet of esterases will find a receptor.
The AChR is a typical ligand-gated ion channel . Thus, when ACh (the ligand) binds to the AChR, the receptor becomes an ion channel that allows cations to pass through a central pore. The main cations are sodium (Na + ) and potassium (K + ) . The high concentration of Na + outside and the negative resting membrane potential drives Na + into the muscle fiber. The positive charges that enter the muscle fiber depolarize the muscle's membrane potential from a negative value to a much less negative value. This depolarization initiates a muscle fiber action potential that propagates away from the NMJ in both directions, rapidly causing the muscle fiber to contract.
The esterase in the synaptic cleft prevents the same ACh from rebinding multiple times to the receptors so that each single nerve impulse in the axon leads to exactly one action potential in the muscle fiber. The esterase plays a second essential role: the choline it creates from ACh is taken back (reuptaken) by the nerve terminal to make additional ACh via an intracellular enzyme (choline acetyltransferase). The NMJ is thus a highly regulated site where a nerve terminal, muscle fiber, and several supporting glial cells are juxtaposed. A wide range of pharmacologic agents, natural toxins, and electrolyte imbalances associated with disease have profound effects on the function of this synapse. For example, NMJ function can be blocked by agents that affect the muscle's AChRs, the nerve terminal vesicle release machinery, or even the synaptic cleft. Venom from certain poisonous snakes has a component (alpha bungarotoxin) that blocks the ability of ACh to bind to the AChR and is thus paralytic. Anaerobic Clostridia bacteria make a factor (botulinum toxin) that is also paralytic, by blocking the ability of synaptic vesicles to fuse with the nerve terminal membrane. Insecticides can block the function of the acetylcholinesterase, causing abnormally large amounts of neurotransmitter to reach the muscle fiber. Muscles protect themselves from excessive depolarization by inactivating their receptors, which also has the effect of paralysis.
Alpha bungarotoxin has another use. Because it binds to tightly to the AChR, it provides a means of seeing each muscle fiber's postsynaptic site. This is accomplished by tagging bungarotoxin with a small organic fluorescent dye so that the alpha bungarotoxin (red in the figure) clearly delineates the postsynaptic site. Over the last several years, the ability to visualize nerves has become much easier thanks to the Nobel Prize–winning discovery (2003 chemistry prize) of the green fluorescent protein (GFP). This gene for a jellyfish protein that gives certain jellyfish their green bioluminescent glow has been modified so that it can be inserted into the genome of mammals, especially mice. The transgenic mice are engineered by molecular biologists to express the GFP in neurons selectively. In this way, a transgenic mouse can express GFP in its motor nerves, while the AChRs are labeled with red alpha bungarotoxin. The two distinct colors in the nerve (green) and the muscle membrane (red) show the remarkably precise alignment of the nerve's release sites and the muscle's AChRs. The fluorescent protein expression also allows visualization and identification of all the muscle fibers innervated by a single axon. The motor axon and all the muscle fibers it innervates are called a motor unit, and this is a critical aspect of neuromuscular function. Because an action potential impulse heading out an axon in a peripheral nerve will enter all the branches of the axon, the motor unit is the unitary muscle contraction from a single axon .
Motor units are recruited in a fixed order when muscles are used. Typically, the weakest motor units that cause the smallest muscle twitches are recruited first . If these are insufficient for the task, additional motor units are recruited so that each gives rise to progressively larger amounts of muscle tension. In this way, there is fine control of small muscle contractions and less control as the force of muscle contraction is increased. All the muscle fibers within a single motor unit have very similar contraction properties because they have the same subtype of the contraction protein myosin.
The first motor units recruited comprise muscle fibers having “ slow” fatigue-resistant myosin that causes slow contractions. The last motor units to be recruited activate muscle fibers that have fast contractions, thanks to fast myosin, but are highly fatigable . It is possible to see positions of all muscle fibers in each of the motor units in one muscle. Such descriptions reveal “connectomes,” which are complete maps of all the positions of all the motor axons and their connections within a muscle.
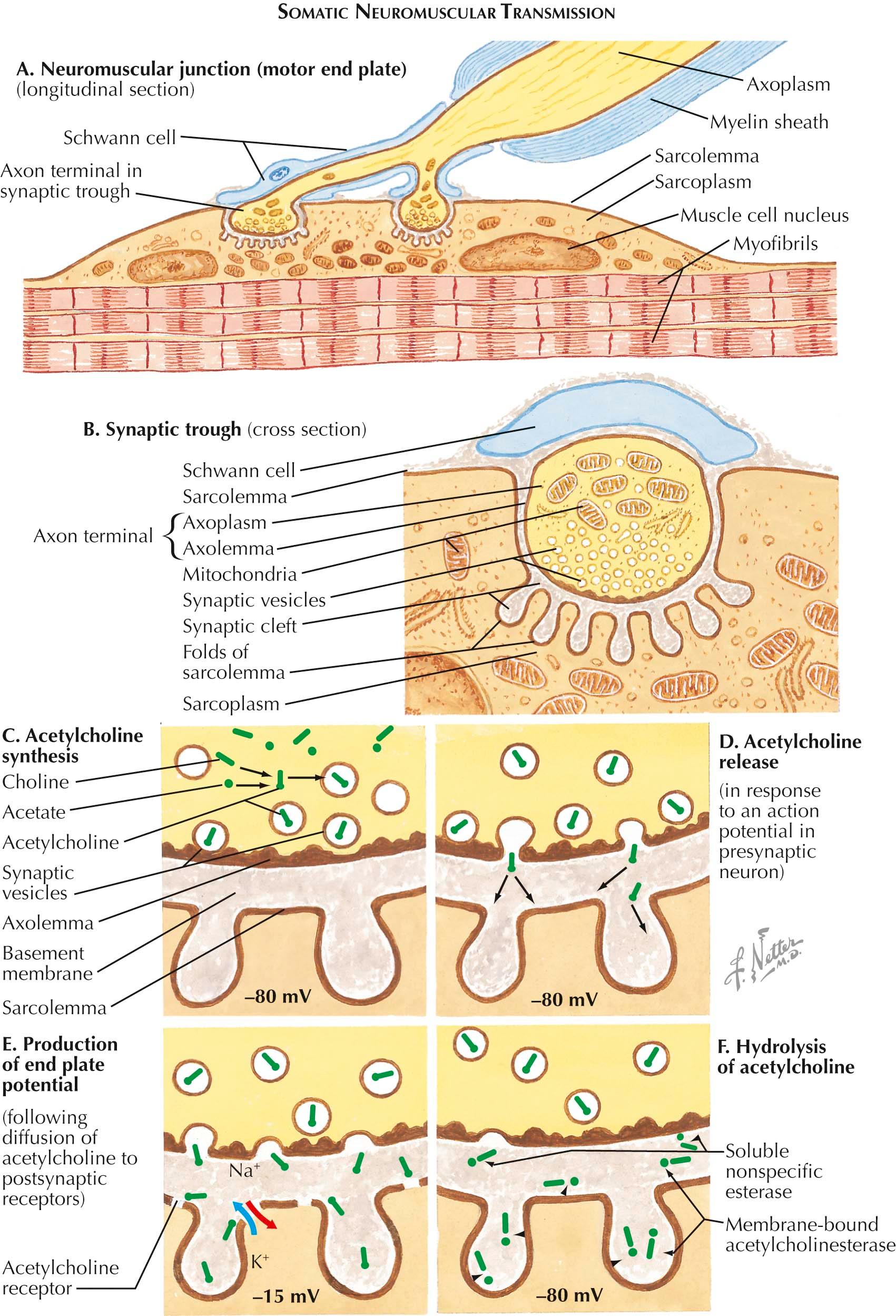
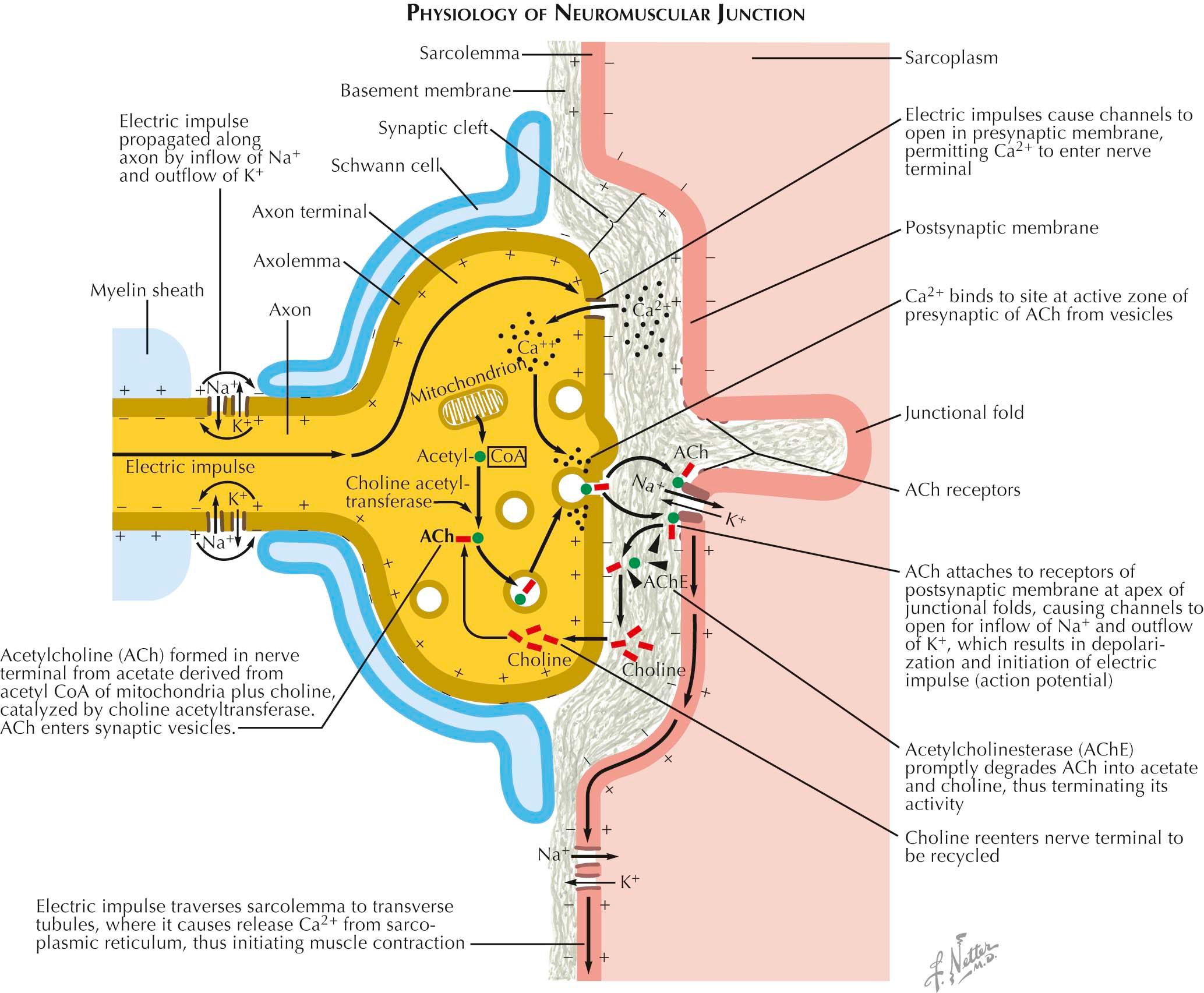
Normal somatic motor nervous system function requires rapid and efficient electrical impulse transmission. These initially propagate along peripheral nerves, releasing acetylcholine through a complex series of electrochemical processes at the nerve terminal to immediately bind at the postsynaptic neuromuscular junction (NMJ), generating electrical impulses that propagate along the muscle fiber. The subsequent muscle fiber action potentials couple with the muscle cell's inherent contractile mechanism, producing muscle contraction. The physiologic steps of synaptic transmission are divided into those that occur in the (1) presynaptic nerve terminal, (2) the synaptic cleft, and (3) the postsynaptic muscle membrane.
The presynaptic nerve terminal is the site of synthesis, release, and reuptake of the neurotransmitter acetylcholine (ACh), the chemical responsible for neuromuscular transmission. Acetylcholine is synthesized in the peripheral nerve terminal when acetate derived from acetyl CoA within the mitochondria and choline that has been recycled and taken up from the synaptic cleft is catalyzed by the enzyme choline acetyltransferase. The newly formed acetylcholine is then packaged into synaptic vesicles within the nerve terminal. Some ACh vesicles are located immediately adjacent to the nerve terminal membrane and are available for immediate release, whereas others are localized a short distance from the terminal nerve membrane and mobilized for rapid release.
ACh release is triggered by calcium influx into the nerve terminal. As a propagating nerve action potential reaches the nerve terminal, the depolarization activates voltage-gated calcium channels in the active zones of the terminal membrane, resulting in an influx of calcium (Ca 2+ ) ions into the axon terminal. The Ca 2+ binds to active zones within the nerve terminal lying in juxtaposition to the muscle postsynaptic ACh receptors. This allows for synaptic vesicle membrane fusion to the nerve terminal membrane, with ACh release into the synaptic cleft.
The synaptic cleft is the neuromuscular junction site where ACh released from the nerve terminal crosses and eventually binds to postsynaptic muscle membrane ACh receptors. The time required for ACh to move across the synaptic cleft is slower than electrical impulse transmission along the axon or muscle fiber membrane. Unlike action potential transmission, movement of ACh across the cleft is unidirectional. ACh remaining within the cleft, either before or after attachment to the ACh receptors, is rapidly degraded within the synaptic cleft into acetate and choline by acetylcholinesterase (AChE), thus terminating its activity. Subsequently, choline is redirected into the nerve terminal and recycled to form new ACh transmitters.
The final step of neuromuscular transmission occurs at the postsynaptic muscle fiber membrane, composed of junctional folds within the membrane; AChRs are concentrated at the apex of the folds. When ACh is released from the nerve terminal and crosses the synaptic cleft binding to ACh receptors, muscle fiber membrane sodium channels open, resulting in Na + influx into the muscle fiber with generation of muscle fiber action potentials.
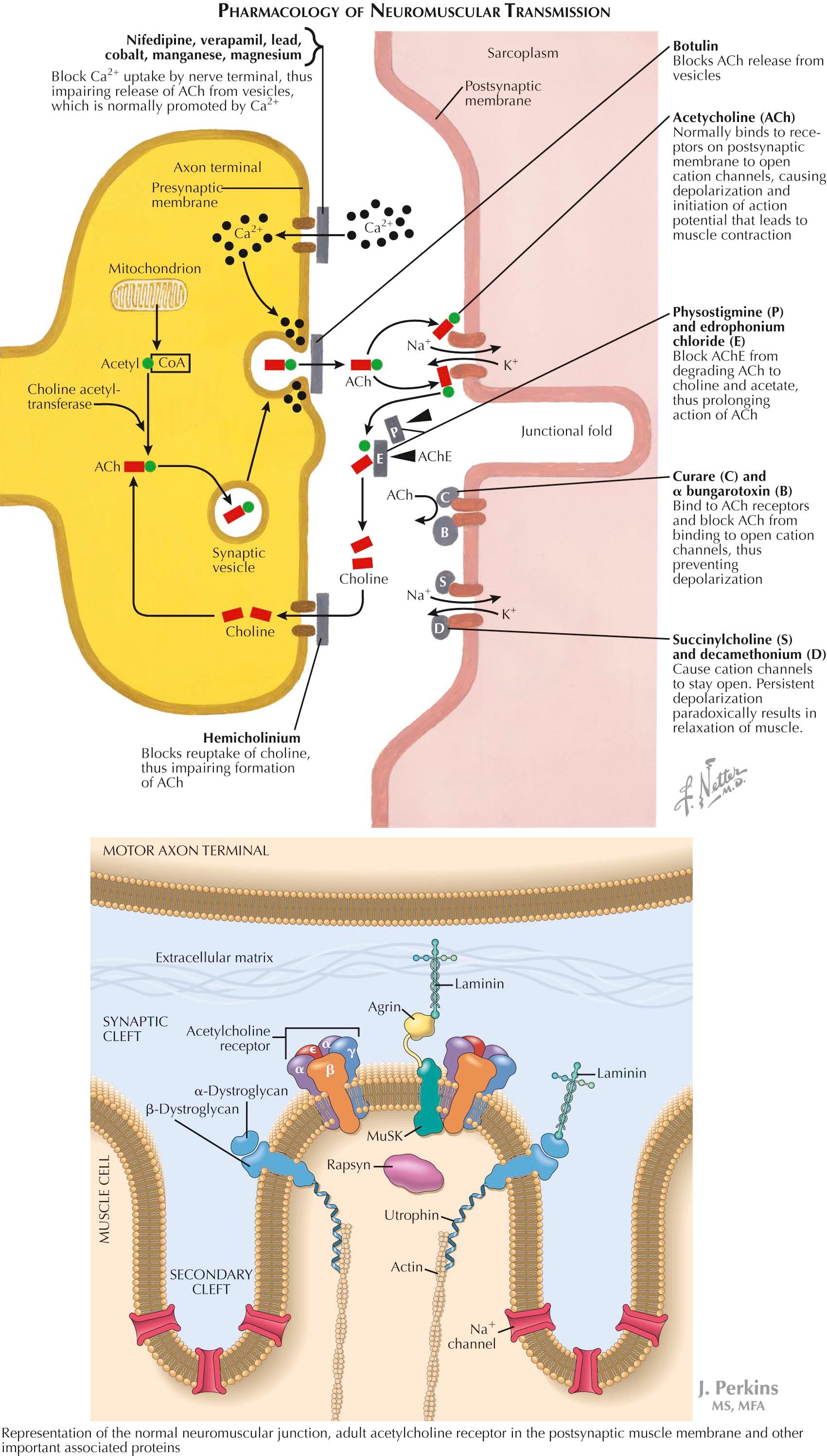
The nicotinic AChR contains five subunits arranged radially around a transmembrane ion channel. Antibodies generated in myasthenia gravis are primarily directed against the AChR alpha subunit. These antibodies may bind at or near the acetylcholine binding site, directly preventing acetylcholine binding, or may alter receptor function through other mechanisms, such as increased receptor degradation or complement-mediated receptor lysis. The receptor that is required for NMJ formation is called the MuSK receptor (muscle-specific kinase). MuSK, agrin, and rapsyn are important for clustering of acetylcholine receptors during neuromuscular junction development by allowing binding to the receptor's skeletal muscle cytoplasmic domain. Agrin binds several other proteins on the surface of muscle, including dystroglycan and laminin. Rapsyn anchors or stabilizes the AChR at synaptic sites linking the receptor to the underlying postsynaptic cytoskeleton.
Acquired MG develops as a result of formation of antibodies primarily to the alpha-1 postsynaptic NMJ immunogenic regions (epitopes). AChR antibodies trigger immune-mediated AChR degradation. The loss of large numbers of functional AChRs decreases the pool of muscle fibers available for depolarization during motor nerve terminal activation. This results in decreased generation of muscle fiber action potentials and subsequent muscle contraction, leading to clinical weakness if large numbers of NMJs are affected.
Many medications have their pharmacologic site of action at the NMJ, subsequently affecting neuromuscular transmission. Several block Ca 2+ uptake by the nerve terminal, resulting in impaired mobilization of the ACh vesicles and subsequent ACh release. These include calcium channel blockers and heavy metals. Although these agents do not often produce clinically evident NMJ failure in healthy persons, exposure to these medications in patients with a NMJ disease (i.e., myasthenia gravis or Lambert Eaton myasthenic syndrome) may cause clinical exacerbations.
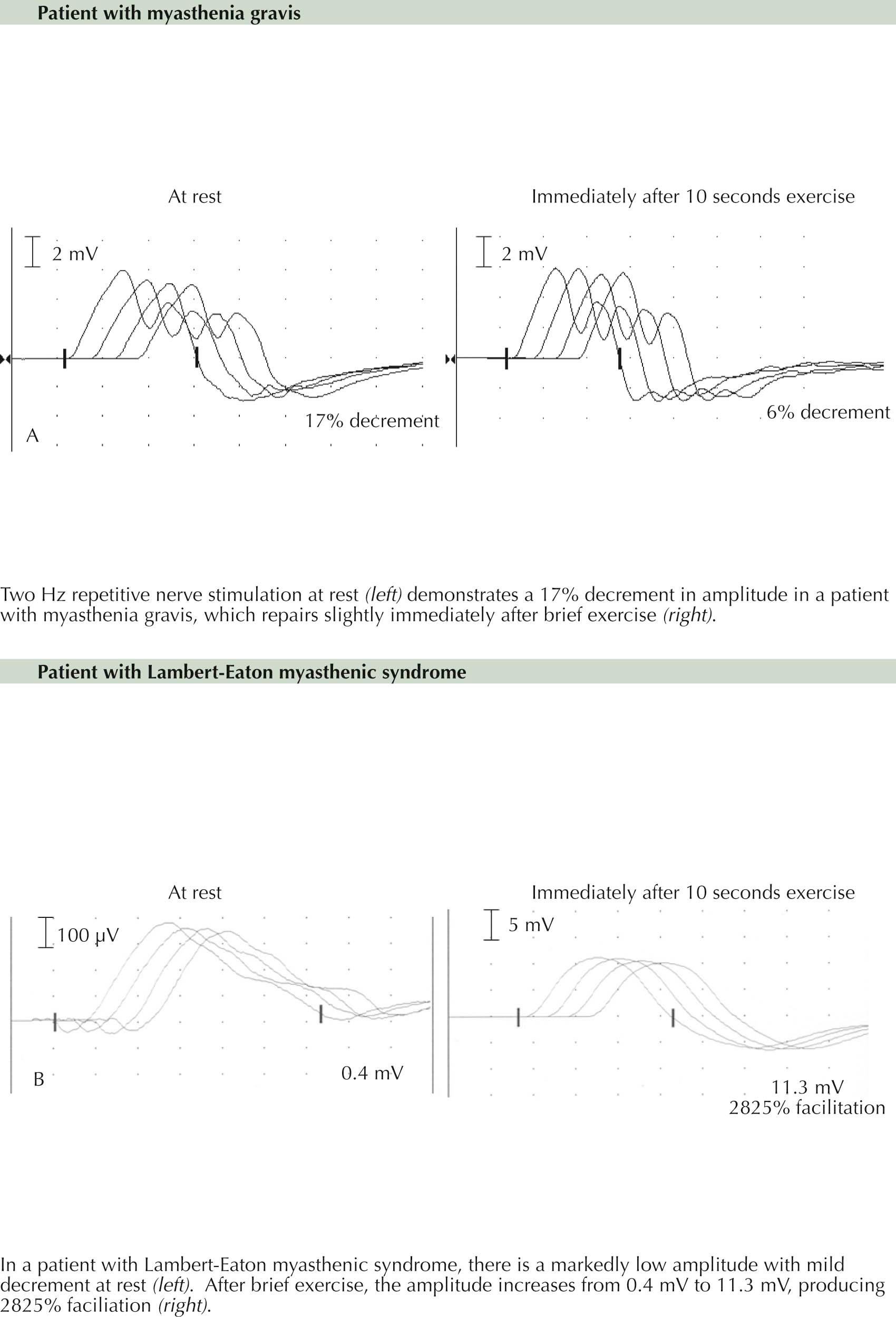
The integrity of neuromuscular transmission can be assessed by repetitive nerve stimulation studies during clinical neurophysiologic testing. When an action potential propagates along the nerve and reaches the nerve terminal, multiple quanta of ACh are released from the presynaptic nerve ending. When the ACh binds to the AChR, an end-plate potential (EPP) is generated. When the difference between the actual end-plate potential and the threshold for muscle fiber action potential (known as the safety factor ) is large, small reductions in the EPP do not have a significant effect on neuromuscular transmission, and a muscle fiber action potential is generated; however, when there is either a presynaptic or postsynaptic NMJ disorder, this safety factor is no longer operational, and clinical symptoms occur. Repetitive motor nerve stimulation (RMNS) provides a neurophysiologic means to assess neuromuscular transmission.
This technique is performed to specifically stress the safety factor by rapidly mobilizing and releasing multiple stores of ACh. With a 2-to 5-Hz RMNS, fewer stores of ACh are released, and less ACh is available to bind to the AchR with each stimulus, up to approximately 5 to 6 stimuli. By assessing the change in the recorded compound muscle action potential (CMAP) after depolarization of all of the axons, and therefore all of the muscle fibers within a muscle, defects of neuromuscular transmission can be identified.
When evaluating normal neuromuscular junctions (NMJs), the EPP is much larger than the threshold required to initiate an action potential along the muscle fiber. As a result, the reduction in ACh release after repetitive stimulation at slow rates does not reduce the EPP below the threshold for depolarization, and action potentials are initiated in all muscle fibers innervated by the nerve. The resulting CMAP amplitude and area after each stimulus is therefore identical, and no reduction (decrement) of the responses occur.
When patients have a presynaptic dysfunction , such as in Lambert-Eaton myasthenic syndrome (LEMS) or infantile botulism, the resting EPP is markedly reduced as a result of a reduction in release of ACh from the presynaptic nerve. The ACh release is diminished at the peripheral nerve terminal at the NMJ because of either an autoimmune disorder blocking presynaptic uptake of calcium (Ca 2+ ) ions or a specific effect of the botulinum toxin having a similar effect. This EPP is often lower than the threshold for depolarization of the muscle fiber, and therefore a single stimulus will not produce a muscle fiber action potential in many fibers. With standard motor nerve conduction studies, the CMAP amplitude is often low as a result. With slow rates of stimulation during RNS, there is an additional reduction in the release of ACh stores with each stimulus and a decrement in the CMAP amplitude and area (similar to that occurring in a postsynaptic neuromuscular junction disorder) is seen. However, after brief isometric exercise for 10 seconds, the influx of Ca 2+ and mobilization and release of additional ACh stores results in a significant increase (increment or facilitation) of the CMAP amplitude ( Plate 11-5 , upper panel). This facilitation is a characteristic and diagnostic finding in Lambert-Eaton myasthenic syndrome.
In patients with a much more common postsynaptic dysfunction, as is typical in myasthenia gravis (MG), there is an autoimmune disorder leading to accelerated breakdown of the ACh receptors at the muscle side of the NMJ as well as a blockade of the ACh at the postsynaptic end plate. This affects the ability of ACh to produce a normal EPP. Therefore the resting EPP may be lower than normal as the safety factor for neuromuscular transmission is reduced.
Therefore RMNS results in a reduction of both the recorded CMAP amplitude and area with each stimulus for the first 5 to 6 stimuli ( Plate 11-5 , lower panel). This decrement results from a loss of summated muscle fiber action potentials from those fibers in which the EPP does not reach the threshold for depolarization. Brief isometric exercise for 10 seconds leads to increased Ca 2+ permeability in the presynaptic nerve terminal, causing mobilization and a release of additional stores of ACh. As a result, the degree of decrement immediately after brief exercise is less than at rest. However, with repeat testing between 1 to 4 minutes later, there is progressive NMJ fatigue, and the degree of deficit defined with RMNS increases up to a maximum at this time and then begins to improve with sequential testing.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here