Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neuromuscular disorders are a subset of neurology that affects the peripheral nervous system, including lower motor neuron projections from the spinal cord, spinal nerve root, peripheral nerve, neuromuscular junction, and muscle. A number of neuromuscular diseases have effects on the heart. Most often, diseases of muscles (myopathy), both acquired and hereditary, can affect cardiac muscle and therefore cause cardiomyopathy or conduction disease. Peripheral nerve disease can also affect the autonomic nervous system, causing arrhythmias or effects on blood pressure. Other genetic or acquired syndromes may have both neuromuscular and cardiac abnormalities. It is critical for the neurologist seeing neuromuscular patients to be aware of which conditions may be associated with cardiac complications, to ensure appropriate cardiac screening and monitoring. Cardiologists also need to be aware of when an underlying neurological disorder may be contributing to the cardiac condition of a patient. This chapter reviews many of the neuromuscular conditions associated with cardiac disease.
Myopathies include all diseases of muscle, including hereditary and acquired. Genetic myopathies, including muscular dystrophies, congenital myopathies, and metabolic myopathies, depending on specific genetic cause, can affect cardiac muscle. Some acquired myopathies can also have cardiac effects.
Traditionally categorized by mode of inheritance, age of onset, severity, and pattern of clinical presentation, the inherited muscular dystrophy disorders generally present with progressive muscle weakness in childhood or young adulthood.
Duchenne and Becker muscular dystrophies are X-linked recessive disorders that are caused by mutations in the dystrophin gene; therefore, they have reduced or absent expression of the dystrophin protein, which is an essential component of the cytoskeleton of skeletal and cardiac muscle. Progressive weakness and pseudohypertrophy of muscles, particularly the calves, are characteristic of both.
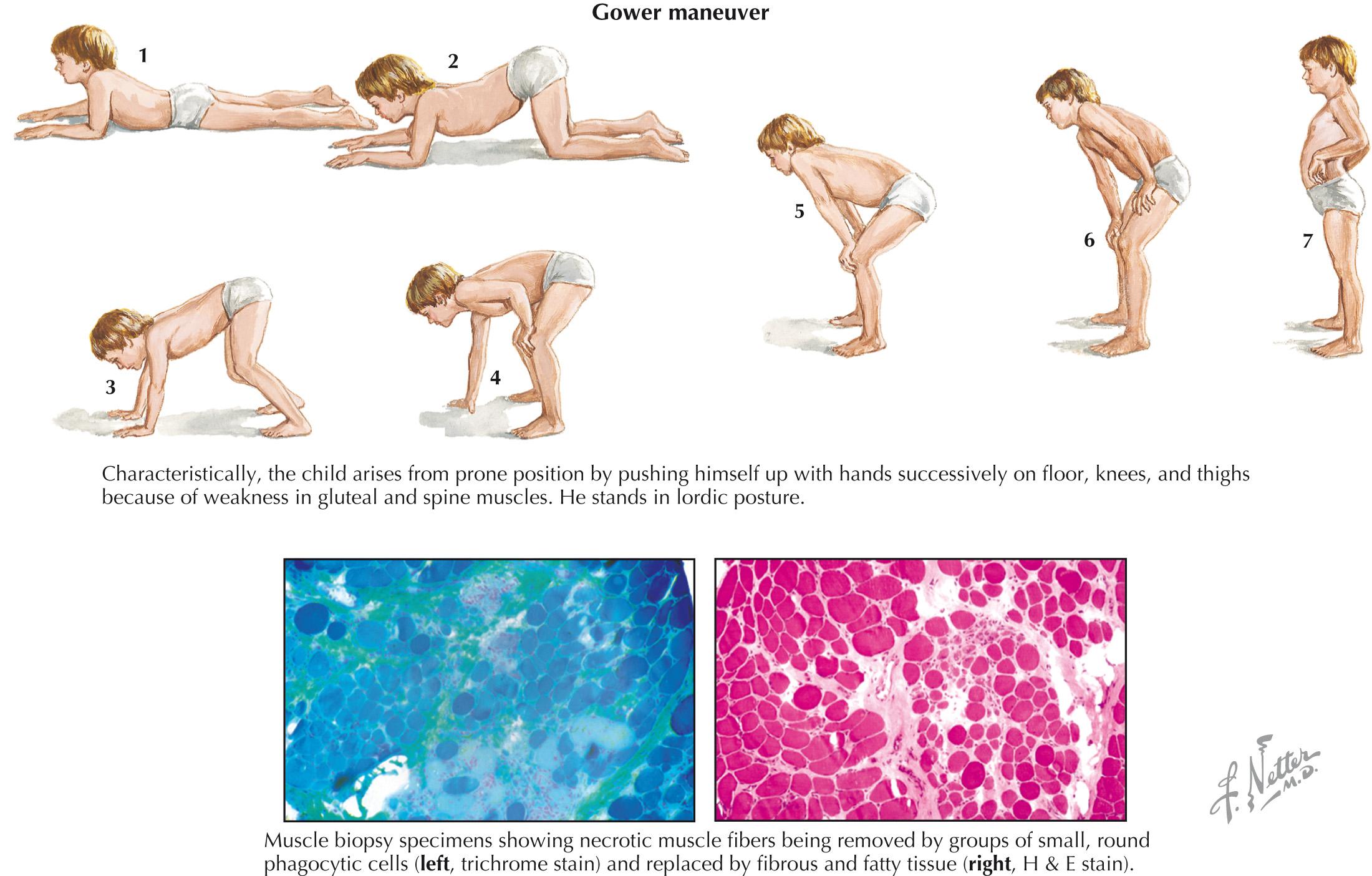
Clinical manifestations of Duchenne muscular dystrophy typically present in early childhood, with contractures and with proximal muscle weakness more than distal muscle weakness. The Gower maneuver, which is the use of the arms when trying to stand up from the floor, is a characteristic physical examination finding ( Fig. 65.1 ). Nonprogressive cognitive impairment or global development delay is common. There is steady progression to wheelchair use within 10 years, and death usually occurs in the second or early third decade of life from respiratory or cardiac failure, although use of corticosteroids may slow this progression.
Duchenne muscular dystrophy causes a dilated cardiomyopathy with left ventricular fibrosis, as well as arrhythmias and conduction abnormalities. Fibrosis of the posterobasal left ventricular wall can demonstrate the characteristic ECG changes of tall right precordial R waves with an increased R/S ratio and deep Q waves in leads I, aVL, and V 5 to V 6 . Mitral regurgitation may occur secondary to progression of the cardiomyopathy. The symptoms related to cardiomyopathy in Duchenne muscular dystrophy are often not appreciated until later in the disease course, but they can be detected by ultrasound before symptom onset.
Becker muscular dystrophy, in which there is altered or decreased dystrophin expression, but with some retained function, presents with a less severe phenotype, often at a later age. The severity of the Becker phenotype varies greatly. Cardiomyopathy also occurs with Becker muscular dystrophy and is sometimes more severe than the degree of generalized weakness might suggest. Right ventricular involvement occurs early in Becker patients, with later development of left ventricular dysfunction, which is related to a fibrotic process that results in heart failure. Conduction abnormalities may also be seen.
The diagnosis of Duchenne or Becker muscular dystrophy is often considered in a boy or young adult with the typical distribution of weakness, calf pseudohypertrophy, and high creatine kinase levels. Electrodiagnostic testing with electromyography confirms a myopathic process, and many patients now have diagnosis confirmed by genetic testing of the DMD gene, looking for deletions and/or duplications or point mutations. In atypical cases or when genetic testing is not readily available, muscle biopsy demonstrates a dystrophic process with reduced or absent dystrophin staining ( Fig. 65.1 ).
Treatment of Duchenne muscular dystrophy includes glucocorticoids, which have been demonstrated to slow progression of muscle weakness and scoliosis, and to improve pulmonary function. Several nonrandomized studies have described benefit in delaying the onset and progression of cardiomyopathy in Duchenne muscular dystrophy with steroid use, but further research is needed.
Aside from steroid use, treatment for Duchenne and Becker muscular dystrophy is supportive, with surgical management of scoliosis, noninvasive and invasive methods of ventilator support, and management of cardiac complications. In Becker muscular dystrophy, some patients with disproportionate cardiac involvement have successfully undergone cardiac transplantation.
Emery-Dreifuss muscular dystrophy (EDMD) is a genetically heterogeneous disorder characterized by early contractures of the elbows, ankles, and posterior cervical muscles, as well as slowly progressive muscle weakness in a scapulohumeroperoneal distribution, often accompanied by cardiac disease, including conduction defects, arrhythmias, and cardiomyopathy. Genes associated with EDMD phenotype include EMD (encoding emerin), FHL1 , LMNA , SYNE1 , SYNE2 , and TMEM43 . Dilated cardiomyopathy is common in these disorders, often with associated conduction abnormalities. Sudden cardiac death has been described in patients without overt muscle weakness.
Diagnosis of EDMD is suspected in any patient with scapulohumeroperoneal weakness with contractures and cardiac involvement, as well as studies suggestive of a myopathic process. Diagnosis is confirmed by genetic testing for the previously described genes associated with the EDMD phenotype and may preclude muscle biopsy in some cases.
Treatment for the muscle weakness is symptomatic. Cardiac monitoring is critical, and a pacemaker or implantable defibrillator may be indicated for patients with conduction disease or arrhythmias.
Myotonic dystrophy includes two genetic muscular dystrophies that share the clinical phenotype of a dystrophic myopathy, with either the clinical or electromyographic finding of myotonia. The physical examination finding of myotonia is of delayed muscle relaxation after contraction or muscle percussion ( Fig. 65.2 ). Electrographic myotonia describes the waxing and waning high-frequency discharges seen on needle electromyography.
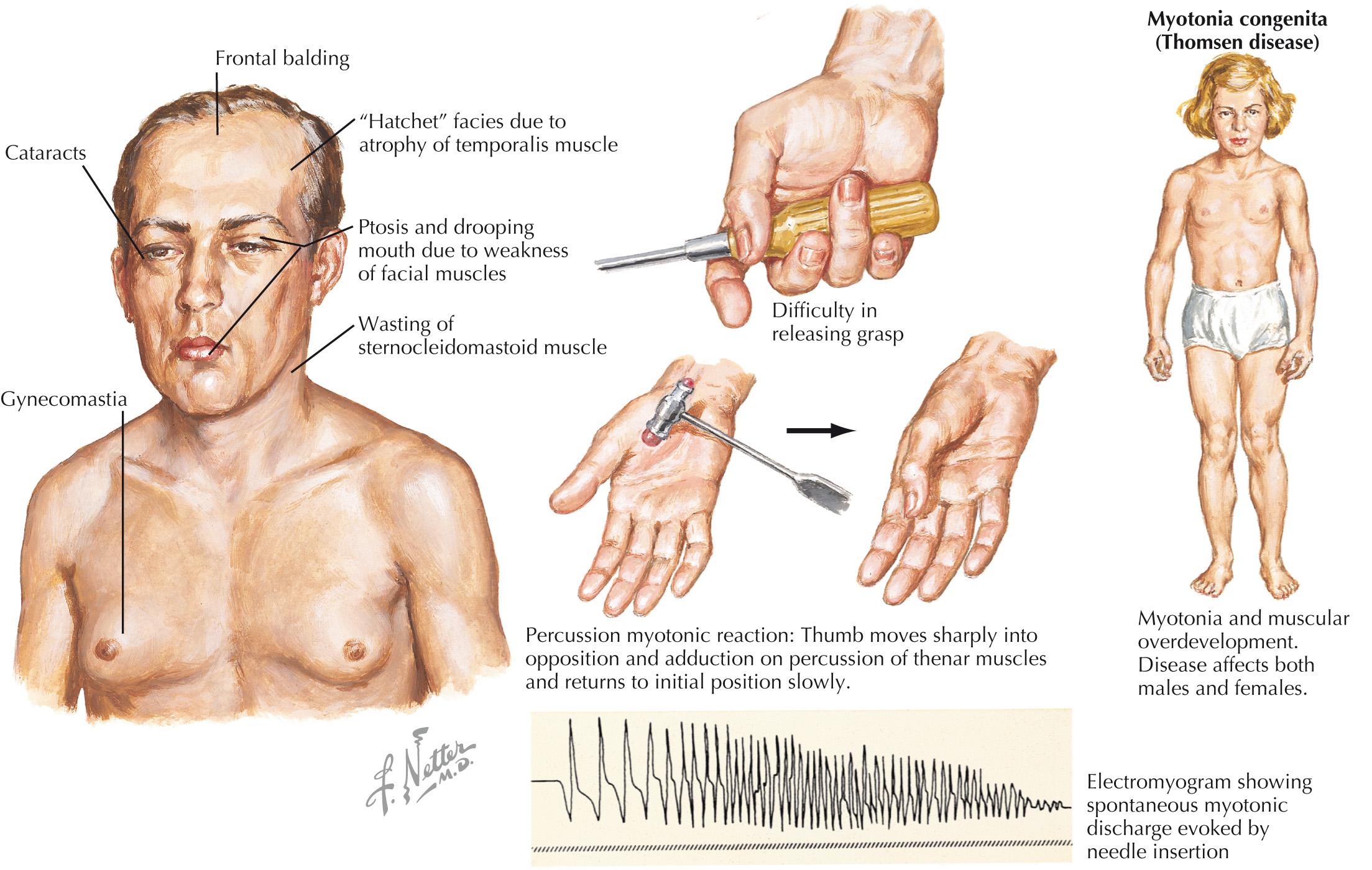
There are two genetic subtypes of myotonic dystrophy, both autosomal dominant, including type 1 (DM1), which is related to an expansion of the CTG triplet repeat of the dystrophia myotonica protein kinase (DMPK) gene, and type 2 (DM2), which is related to expansion of a CCTG repeat in the zinc finger protein (ZNF9) gene (also known as the CNBP gene).
DM1 is more typical of a distal myopathy, and is accompanied by facial weakness, ptosis, bulbar weakness, temporal wasting, and frontal balding. DM2 shares many clinical features with DM1, but tends to be overall less severe, demonstrates more proximal rather than distal limb weakness, and shows less clinical myotonia. The diagnosis of myotonic dystrophy is often suspected based on clinical and electrodiagnostic features, and confirmed with genetic testing. In some cases, muscle biopsy is performed and typically shows myopathic changes with a notable increase of internalized nuclei.
Patients with DM1, and to a lesser degree, those with DM2, are at risk for cardiac conduction abnormalities, as well as atrial fibrillation and ventricular arrhythmias. Cardiomyopathy is less common in these disorders. Occasionally, sudden cardiac death is the presenting symptom of DM1. Therefore, patients with myotonic dystrophy are recommended for at least an annual cardiac screening with ECG, and in some cases, longer cardiac monitoring. Pacemakers or implantable defibrillators are occasionally warranted.
Limb girdle muscular dystrophy (LGMD) is a genetically heterogeneous group of autosomal-dominant (type 1) or autosomal-recessive (type 2) myopathies characterized by weakness and wasting of the shoulder and pelvic girdle muscles. The limb-girdle phenotype may overlap with other hereditary myopathies, and occasionally, acquired muscle diseases. There are currently 8 identified autosomal dominant LGMD subtypes (named LGMD1A-H) and 23 autosomal recessive subtypes (named LGMD2A-W).
Some genetic subtypes of LGMD are more likely to affect cardiac function, which is one primary rationale for establishing a genetic diagnosis in a patient suspected of having LGMD. The LGMD subtypes most commonly associated with cardiac involvement include LGMD1B, LGMD1E, LGMD2E, and LGMD2I. Others in which cardiac involvement may also be seen are summarized in Table 65.1 . Subtypes in which cardiac involvement is unusual include LGMD1C, LGMD2A, LGMD2B, and LGMD2L. Because cardiac involvement is generally common with LGMD, screening for associated cardiomyopathy is advised when exact genetic diagnosis cannot be established.
| Type | Locus | Gene | Protein |
|---|---|---|---|
| LGMD1A | 5q31 | MYOT | Myotilin |
| LGMD1B | 1q11-q21 | LMNA | Lamin A/C |
| LGMD1D(E) | 7q | DNAJB6 | DnaJ homolog subfamily B member 6 |
| LGMD2C | 13q12 | SGCG | Gamma-sarcoglycan |
| LGMD2D | 17q12-q21 | SGCA | Alpha-sarcoglycan |
| LGMD2E | 4q12 | SGCB | Beta-sarcoglycan |
| LGMD2F | 5q33-q34 | SGCD | Delta-sarcoglycan |
| LGMD2G | 17q11-q12 | TCAP | Telethonin |
| LGMD2H | 9q31-q34 | TRIM32 | Tripartite motif containing 32 |
| LGMD2I | 19q13.3 | FKRP | Fukutin-related protein |
| LGMD2J | 2q24.3 | TTN | Titin |
| LGMD2K | 9q34.1 | POMT1 | Protein-O-mannosyl transferase 1 |
| LGMD2M | 9q31 | FKTN | Fukutin |
| LGMD2N | 14q24 | POMT2 | Protein-O-mannosyl transferase 2 |
| LGMD2O | 1p34.1 | POMGnT1 | Protein O-linked mannose β1,2-N-acetylglucosaminyl transferase |
| LGMD2P | 3p21 | DAG1 | Dystroglycan |
In a patient clinically suspected of having LGMD based on history and physical examination, with confirmatory creatine kinase testing and electrodiagnostics suggestive of a myopathic process, diagnosis is often made directly with genetic testing.
Distal myopathies, or muscular dystrophies, are a genetically heterogeneous group of hereditary muscle diseases that clinically present with distal weakness greater and sooner than proximal weakness. The most common distal myopathy is Welander distal myopathy. Most of the genetic mutations categorized as distal myopathies do not have significant cardiac involvement, although conduction defects are occasionally noted. Myotonic dystrophy and myofibrillar myopathies may present with prominent distal weakness, but are more strongly associated with cardiomyopathy or cardiac conduction abnormalities.
Myofibrillar myopathies are another genetically heterogeneous group of hereditary muscle diseases associated with mutations of the Z-disk–related proteins. Myofibrillar myopathies are often associated with cardiomyopathy, conduction abnormalities, and arrhythmias, and in some cases, the cardiac involvement may be the most prominent or exclusive manifestation of disease. Gene mutations described to cause myofibrillar myopathies include desmin, αB-crystallin, ZASP , myotilin (allelic with LGMD1A), filamin C, BAG3 , SEPN1 , and FHL1 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here