Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Optogenetics is defined as the use of light-based technologies that are genetically targeted to certain cellular groups or proteins ( ). In practice, optogenetics most often refers to the use of genetically modified, microbial rhodopsins (pigmented light-sensitive membrane proteins) to manipulate the excitability of genetically targeted neurons. Given this high degree of temporal control over targeted neurons, with a well-understood mechanism of action, these techniques have resulted in a substantial contribution to basic neuroscience, as well as generating significant clinical translational interest, considering the nonspecificity of electrical stimulation. While optogenetics is presently an investigational technology, these tools have been used with translational promise in nonhuman primates. This chapter provides an overview of the development, mechanisms, diversity, and translatability of this technique. Other related genetically targeted techniques will also be described.
Pigmented rhodopsin photoreceptors were first described in the 19th century ( ) and a putative bacterial photoreceptor that binds retinal 1
1 Retinal is one of the many forms of vitamin A (the number of which varies from species to species). Retinal is a polyene chromophore, bound to proteins called opsins, and is the chemical basis of animal vision. Retinal allows certain microorganisms to convert light into metabolic energy. Taken from the internet: https://www.google.com/#q=what+is+retinal .
was found in the “purple membrane” fraction of Halobacterium halobium , in 1971 ( ), and named bacteriorhodopsin (BR). This was postulated to be a light-dependent proton transporter ( ); it was the earliest membrane protein for which the amino acid sequence was determined and was the first described seven–transmembrane domain receptor ( ). Unlike other G protein–coupled receptors (GPCRs), however, rhodopsins bind a retinal molecule, conferring light sensitivity, and microbial rhodopsins incorporate a transporter protein or ion channel. This simpler structure of microbial rhodopsin, comprising a single gene encoding both a light sensor and a membrane effector, resulted in the imagining of light-mediated neuronal manipulation ( ) and permitted genetic targeting of this protein to specific cells with the advent of recombinant and viral vector technologies. The principle was proved in 2002 ( ) with the nonselective light-dependent cation channel, channelrhodopsin-2, from Chlamydia rheinhardii ( ) (see Fig. 35.1 ). The development of optogenetic tools has depended mostly on microbial opsins, which, unlike animal rhodopsins ( ), have the advantage of spontaneous recovery from photobleaching ( ) and, relatedly, the ability to be functional with basal mammalian retinal concentrations ( ). Other major effector proteins were described soon afterward, including the halorhodopsins, which are chloride transporters ( ), and archaerhodopsin, a proton pump, similar to the originally described BR ( ).
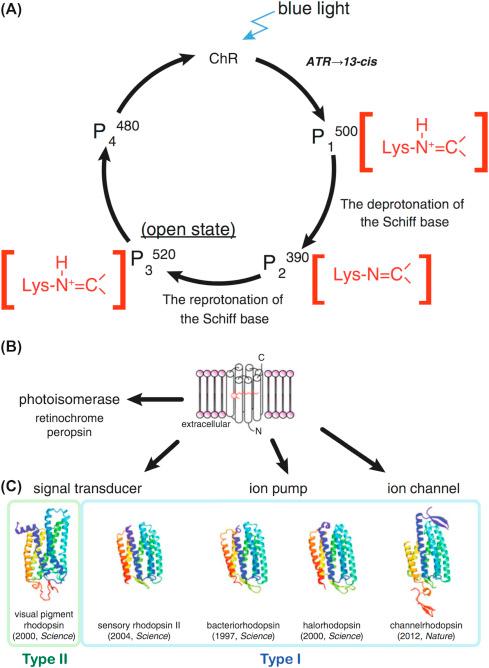
Since the development and proof of this technology, related channels have expanded substantially with different mechanisms, kinetics, light intensity, and wavelength sensitivities, as described later. Given the recent 10th anniversary of optogenetics, excellent reviews have been written about the history and state of optogenetics ( ), an even-handed look forward and back ( ), and limitations of this technique ( ).
Presently, optogenetic tools are widely used in basic applications, including in nonhuman primates. While vector technologies have been used in humans, and optogenetic activation has been shown in human retina, in vitro, optogenetics is presently not in use in humans.
After the appropriate optogenetic protein has been chosen, the means of transduction in the cell population of interest must be considered along with implantation of a selected light delivery device, as well as consideration of light intensity and the duty cycle. Considerations include equipment, light penetration, phototoxic effects, duty cycle (analogous stimulation parameters), and how to achieve the appropriate geometry to effectively deliver light to the tissue of interest.
Typically, viral vectors are used, but transgenic techniques and the direct introduction of genetic material (e.g., electroporation) have been used in basic science applications. Most commonly, adeno-associated viral (AAV) vectors are used and are readily available (e.g., University of North Carolina Vector Core Facility) and include a variety of promoters or in a FLEX configuration ( ), so that expression is limited to Cre recombinase–expressing neurons. Cre recombinase and loxP constitute a bacteriophage recombination system, with the Cre recombinase enzyme recombining nucleotides flanking loxP restriction sites. This has been used in mammals and neuroscience research to knock-out genes of interest at a chosen age and location within the nervous system ( ). These latter approaches afford cell-type specificity, with anatomical specificity that is determined by stereotactic microinjection. Expression is often adequate at about 4 weeks after injection, with optical fiber insertion at the time of stereotactic injection preferred to facilitate anatomical colocalization of the transduced protein and light source. In the primate brain, vector delivery needs to include sufficient tissue for the desired effect as well as consideration of vector tropism (the tendency for the vector to be taken up or expressed more avidly in particular cell types), with active work on these issues under way ( ).
Light delivery in basic science applications typically relies on laser diodes and flexible optical fibers ( ), but activation of a large volume of tissue will likely require the development of alternate light delivery strategies, including flexible light emitting diode (LED) arrays ( ), waveguides ( ), or diffusing fibers ( ). If more than one site of light delivery is required, then multiple light sources or beam splitting is required. Light sources consist of either an LED or a laser source, with LEDs now becoming the most common and convenient, given the potential for miniaturization and both low-power consumption and minimal heating. The LED and laser are controlled by a current source and train generator that together determine light intensity, duration of illumination, frequency, and stimulus train characteristics. The onset and offset of maximal illumination must also be considered, given the delay in maximal illumination when some LEDs or lasers are turned on and off, with temporal precision improved by the use of a shutter to occlude or reveal the light source.
While light intensity from a point source falls exponentially, there are additional considerations when illuminating brain tissue. The degree of lensing of light exiting the fiber is an obvious consideration that can be deliberately manipulated. Light scattering varies by brain region, depending on tissue optical properties, with variation as much as 1 order of magnitude between different brain regions. Finally, the wavelength of light has a significant effect on the depth of penetrance, with longer wavelengths significantly less susceptible to scattering and therefore having deeper tissue penetration ( ). However, while longer wavelengths penetrate tissue to a greater distance, tissue heating is increased ( ).
There are four general untoward effects of stimulation that need to be considered: direct phototoxicity, toxicity of an excess of ion movement or exhaustion, the toxic effects of protein overexpression, and heating of tissue. While some have suggested that continued ability to activate or inhibit tissue is suggestive of the absence of toxicity ( ), it is possible that physiologic effects are due to cells outside of the area of potential toxicity that is immediately adjacent to the probe. Given the need for long-term stable stimulation, histologic verification of the absence of phototoxicity, in an appropriate, nonhuman model seems desirable. Phototoxicity is widely observed and appreciated in the setting of in vitro slice and cell culture work that requires fluorescent illumination but seems to have been neglected in in vivo optogenetic studies. Illumination below 10–20 mW/mm 2 (depending on wavelength) is likely safe and does not result in signs of photodamage ( ) – the temptation to increase illumination intensity to improve effect size and tissue penetration in larger brains needs to be avoided. While direct phototoxicity is likely related to the generation of oxidative radicals, activation-related toxicity may also include large ionic or pH shifts that are potentially metabolically overwhelming. The amount of expression of channel is difficult to control (requires optimization of delivery and vector, consideration of viral vector tropism), so these large ionic fluxes, produced by a channel that is not normally expressed, particularly in large amounts, represent one motivation for using alternative techniques such as d esigner r eceptors e xclusively a ctivated by d esigner d rugs (DREADDs, also referred to as “chemogenetics” or “pharmacogenetics,” see later) that rely on natively expressed G protein–coupled mechanisms. Overexpression of a single introduced protein or use of very high vector titers can be cytotoxic, which in turn depend on vector serotype and cellular tropism ( ). Adequate examination for cytotoxicity is presently not often performed in basic science applications. Finally, tissue temperature changes need to be considered, given time-dependent tissue damage above 44–45°C and thermal coagulation at 60°C. Lesser increases in temperature tend to plateau during a given intensity and duty cycle of stimulation but result in changes in neural activity – a significant, potential confound in preclinical studies ( ). In the case of translation, nonhuman primate or larger animal studies could be considered to further verify the absence of accumulating phototoxic effects, toxic effects of protein overexpression, and heating effects.
Ideally, one needs to choose an optogenetic protein that will permit the stimulation/inhibition characteristics that are desired while avoiding the toxic and heating effects of stimulation. This leads to the consideration of both the type of optogenetic protein used and the stimulation parameters used. The kinetics of optogenetic protein state changes determines the maximal reasonable stimulation frequency. For example, channelrhodopsin-2 (ChR2), owing to channel closing time, typically will not enable light-trigger neuronal firing (i.e., cannot “follow”) above frequencies of about 20 Hz ( ). In contrast, the modified ChR2 variant, ChETA, will follow at up to at least 200 Hz and result in less doublet firing and less associated ion movement ( ), given more rapid channel inactivation. Similarly, excess activation of archaerhodopsin results in large proton shifts that result in a refractory period for cellular activity, and there can be significant rebound firing at the end of halorhodipsin activation. An excellent resource for the selection of an appropriate optogenetic tool is the Stanford Online Optogenetics resource as well as a recent text ( ). This selection needs to be combined with an understanding of the physiologic characteristics and desired manipulation of the target neuronal population.
While optogenetic techniques have been very useful in rodent studies, the range of scientific questions and ease of use in therapies for experimental animals and humans with larger brains are limited by scale. Only a small area can be illuminated (1 mm 3 ) from existing light-delivery devices. Nontoxic levels of illumination, such as 3 mW/mm 2 , result in limited tissue heating but illuminate only about 1–1.5 mm of tissue immediately surrounding the optical fiber, depending on the wavelength ( ). Nuclei of this size that would be an effective functional neurosurgical target are few, with possible exceptions of the basal forebrain (improving level of arousal, attention), nucleus accumbens (for obsessive compulsive disorder and addictions), amygdala subnuclei (including treatment of anxiety), thalamic subnuclei (for potential seizure termination, indirect cortical modulation, tremor) and other diencephalic targets, including the approximately 5-mm subthalamic nucleus (treatment of Parkinson disease [PD]). Even for these smaller human brain targets, a conventional single fiber seems unlikely to provide adequate coverage (see earlier for alternatives). Moving beyond available technologies, while one can imagine a diffuser device or array for the cortical surface, this would not easily access sulci, given significant vasculature in the sulcus (and thus only covers about the one third of cortex at the gyral crest), or complicated subcortical targets, such as the cerebellum. Understanding the involvement of smaller targets in human functional disorders will aid in the employment of optogenetic vector technologies for treatment, but obtaining safe stable and not excessive construct expression remains an important consideration.
In addition to these potential toxic effects of this technology are unexpected physiologic effects, including a dissociation between neuronal firing and transmitter release, as well as rebound firing. Interestingly, transmitter release has been shown to increase after activation of putatively inhibitory opsins that are expressed in presynaptic terminals, including increased release on light onset with a modified channelrhodopsin chloride channel and increased transmitter release after sustained archaerhodopsin activation ( ). Rebound phenomena are widely appreciated with halorhodopsin and archaerhodopsin but can be attenuated by gradually tapering light offset ( ), while determination of this effect in vivo requires simultaneous and often technically difficult measurement of firing of manipulated neurons.
Given the large diversity of engineered, microbially derived opsins, this section focuses on the major classes of opsins that are further divided into groups by the protein from which they were derived or by functional characteristics. Given the large number of available proteins, the continued development of new constructs and excellent accessible resources, we will provide an overview and refer to other resources for a more exhaustive listing of proteins and describe how to obtain associated constructs and vectors. Although there are other possible ways to organize the diversity of optogenetic tools, here we organize these microbial opsins into those incorporating a transporter versus those that incorporate an ion channel. We will then discussing light-sensitive GPCRs in the next section. Fig. 35.2 schematizes these general types of light-sensitive proteins, and common example proteins discussed here are listed in Table 35.1 .
| Example | Mechanism | Agonist | |
|---|---|---|---|
| Optogenetic Effectors | |||
| Light-Sensitive Transporters | |||
| Bacteriorhodopsin | eBR | H + pump | |
| Archaerhodopsins | ArchT3.0 | H + pump | |
| Halorhodopsins | eNpHR3.0 | Cl − pump | |
| Light-Sensitive Ion Channels | |||
| Channelrhodopsins | ChR2, ChETA | Nonselective cation conductance | |
| Chloride channel | C1C2 | Chloride channel | |
| Potassium channel | Potassium channel | ||
| OptoXRs | |||
| G protein binding chimeric opsins | |||
| Bacteriorhodopsin/bovine rhodopsin/adrenergic receptor chimera | Opto-β 2 -AR, Opto-α1-AR | ||
| Combined transporter/G protein binding | |||
| Microbial/bovine rhodopsin chimera | GR-E132Q | Combined H + pump and G protein coupling | |
| Chemogenetic Proteins | |||
| Inhibitory | |||
| Modified human glycine receptor | hGlyR | Chloride channel | Ivermectin |
| Nematome glutamate-gated chloride channel | GluClR | Chloride channel | Ivermectin |
| Modified muscarinic M4 | G protein coupled (Gi) | Clozapine- n -oxide | |
| κ opiate | KOR | G protein coupled (Gi) | Salvinorin-B |
| Excitatory | |||
| Modified muscarinic M3 | hM3Ds, hM3Dq | Clozapine- n -oxide | |
| Luminopsins | |||
| Inhibitory | |||
| Luciferase-halorhodopsin | RLuc-NpHR (iLMO) | Cl − pump | Coelenterazine |
| Excitatory | |||
| Luciferase red—shifted ChR1 | sbGluc-VCHR1 (LMO3) | Nonselective cation conductance | Coelenterazine |
The major opsin transporter proteins, archaerhodopsin and halorhodopsin, derive from the halophilic bacteria, Halobacterium sp. and Natronomonas pharaonis ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here