Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
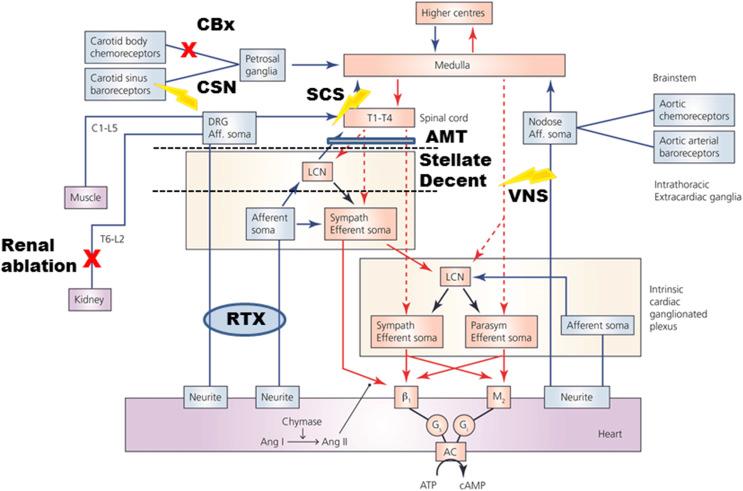
Neural control of the heart is dependent upon the dynamic interaction between nested feedback loops that involve peripheral and central aspects of the ANS. The primary structure/function organization of this neural network is summarized in Chapter 104 of this volume and titled, “Autonomic Control of the Heart.” The concept of hierarchical control (see Fig. 129.1 ) is predicated upon the fact that neuronal somata in the various intrathoracic extracardiac and intrinsic cardiac ganglia are in constant communication, not only with each other, but also with neurons in the spinal cord, medulla oblongata, and higher centers in the initiation of reflex control of regional cardiac indices ( ). Normally functioning in an interdependent fashion, imbalances in network function within this hierarchy for cardiac control can manifest itself in a deleterious fashion, sometimes with lethal consequences ( ). Mechanistically understanding these structural/functional changes is fundamental to evolving new therapeutic targets and technologies including neuromodulation therapies ( ).
Heart failure (HF) and arrhythmias are the leading cause of death in humans ( ), and considerable effort has been extended to find the optimal therapy for these cardiac diseases. Current therapies for HF include nonpharmacologic, pharmacologic, invasive therapies, and surgical treatments. Nonpharmacologic therapy includes sodium and fluid restriction and appropriate physical activity. Pharmacologic therapy involves one or more of the following agents, depending on etiology: angiotensin-converting-enzyme inhibitors, aldosterone receptor blockers, vasodilators, beta-blockers, aldosterone antagonists, and inotropic agents, along with diuretics and anticoagulants ( ). Cardiac resynchronization therapy, pacemakers, and implantable cardioverter-defibrillators are also indicated for subsets of these patients ( ). In some cases, coronary artery bypass or percutaneous revascularization, and as a last resort, heart transplants, are indicated for HF patients ( ). For arrhythmia management, current therapies include pharmacologic, surgical, cardioversion, catheter ablation and the use of implantable devices such as pacemaker or implantable cardioverter-defibrillator ( ). While each of these approaches has been utilized with varying degrees of efficacy, there remain large subsets of patients that are refractory to these treatment regimens.
Neuromodulation therapies have emerged as an alternative therapeutic option for the treatment of arrhythmias and HF ( ). In the setting of ischemic heart disease, both spinal cord stimulation (SCS) and vagus nerve stimulation (VNS) reduce the occurrence of sudden cardiac death and preserve myocyte viability ( ). Cardiac neuromodulation therapies suppress atrial ( ) or ventricular ( ) arrhythmias in part by stabilizing reflex processing within the cardiac nervous system ( ). Fig. 129.1 indicates the primary nexus points of the cardiac hierarchy against which various modes of autonomic regulation therapy are currently being deployed. Rather than one size fits all, the choice of optimum therapy is dictated by the characteristics of neural-cardiac remodeling for respective cardiac pathologies, both ischemic and nonischemic. In this chapter, we will discuss different modes of neuromodulation therapies for cardiac diseases, their efficacy, and current limitations.
Direct stimulation: In this method, regular electrical pulses are delivered directly to the vagus nerve by an implanted electrode interfaces (usually bipolar), attached to a programmable implantable pulse generator (IPG) ( ). The strength, duration, and frequency of the stimulation can be programmed wirelessly and by such means the degree of engagement of underlying afferent and efferent axonal projections is regulated ( ).
Indirect stimulation: To stimulate the vagus nerve indirectly and noninvasively, one method utilizes surface electrodes (cathode and anode) that are attached on to the tragus (the anterior protuberance of the outer ear) and its adjacent site ( ). Transcutaneous electrical stimulation of the auricular branch of the vagus nerve is performed by delivering electrical impulses to tragus via these two electrode interfaces. Although this represents noninvasive neuromodulation therapy, its long-term efficacy remains to be determined.
VNS affects both afferent and efferent axons in the nerve ( ). Preganglionic parasympathetic axons contained within the 10th cranial nerve, the vagus nerve, project to distributed ganglionated plexi located on the heart; this neural network is referred to as the intrinsic cardiac nervous system (ICNS) ( ). VNS has direct and indirect influences on ICNS network function ( ). Activation of parasympathetic efferent projections to the heart decreases heart rate (negative chronotropic effect), slows conduction of electrical activity through the heart (negative dromotropic effect) and decreases contractile strength in atrial and ventricular tissues (negative inotropic effect) ( ). Activation of parasympathetic efferent projections by VNS can likewise impact intrinsic cardiac local circuit neuron function, thereby altering/stabilizing peripheral reflex function ( ) to exert cardio-protection. Modulating ICNS neurons by VNS causes an antiadrenergic effect as well as effects at the end-effectors and within the intrinsic cardiac ganglia themselves ( ). VNS also impacts the immune system response to cardiac disease, mediating, in part, cardio-protection ( ).
Reduced ejection HF is a clinical condition where cardiac output is insufficient to meet the flow requirements (metabolic needs) for the body. The prevalence of HF is high, with ∼5 million people currently diagnosed within the United States, alone, and whose prevalence is expected to increase to 8 million people by 2030 ( ). Different complex conditions can cause HF; including but not limited to coronary artery disease, cardiomyopathy, hypertension, valve dysfunction, thyroid, and kidney disease. HF remodels neural networks within the ICNS ( ), intrathoracic extracardiac neurons ( ), and central neural processing ( ). The end result of such remodeling is elevated sympathetic activity, reduced central parasympathetic drive, and reorganization of the neural and neural-myocyte interfaces ( ).
VNS can impact the cardiac ANS to restore a physiological balance between energy demands and energy supply of the failing myocardium ( ). VNS increases the level of acetylcholine (Ach) release via cholinergic, efferent postganglionic neurons that innervate the heart. Ach is a neurotransmitter that activates cardiomyocyte, M2, muscarinic receptors to induce negative chronotropic (heart rate), dromotropic (heart electrical conduction) and ionotropic (heart contraction) effects ( ). The ICNS is affected directly by the release of Ach from VNS ( ). VNS also causes a decrease in the sympathetic tone by affecting afferent transduction and peripheral reflex processing ( ). Multiple preclinical studies have shown the beneficial effect of VNS on HF ( ). These include improvement of autonomic control ( ), baroreflex function ( ), and left ventricular function ( ), with attenuation of the left ventricular remodeling ( ). In clinical trials, while some studies have shown feasibility and efficacy of VNS on HF ( ), others have shown marginal beneficial for HF ( ). Differences in results may be reflective of the different VNS protocols that were utilized in these studies ( ). All of these clinical trials for VNS, in the setting of reduced ejection HF, have demonstrated safety. See Chapter 108 in this book by Abraham for a more in-depth discussion of VNS for HF.
Myocardial infarction (MI) is a major cause of mortality and morbidity worldwide. Ultimately, the lack of adequate oxygen supply to cardiac regions downstream from the blockage results in an irreversible damage to the heart muscle. MI results in increase of sympathetic tone, which is a reflex response to altered afferent signaling ( ). Multiple preclinical studies have demonstrated cardio-protective effects of acute or chronic VNS in the setting of myocardial ischemia/infarction. These cardio-protective effects include attenuation of left ventricular remodeling ( ), stabilization of parasympathetic remodeling ( ), suppression of arrhythmias ( ), modulation of the myocardial redox state ( ), and inhibition of the expression of TNF-α (key component in inflammatory responses to acute MI) ( ). VNS has likewise demonstrated a mortality benefit ( ).
Ventricular arrhythmias: Abnormal ventricular rhythms, including ventricular tachycardia (VT) and ventricular fibrillation (VF) can be influenced by VNS ( ). Antiarrhythmic effects of VNS have been demonstrated for induced and spontaneous-occurring ventricular arrhythmias ( ). VNS reduces heart rate, increases the action potential (AP) duration of cardiac cells, mitigates cardiac AP dispersion, and affects the ventricular restitution/refractoriness ( ). These effects are manifest by direct parasympathetic efferent projections to cardiac tissues and by modulation of reflex function within different levels of the cardiac ANS ( ).
Atrial arrhythmias: More than three million people a year in the United States are affected by atrial fibrillation (AF) and it is estimated its prevalence will reach 5.6–12.1 million by 2050 ( ). Current treatments of AF include pharmacological therapies and cardiac catheter ablation ( ). As an emerging therapeutic alternative, VNS has the capability to reduce this arrhythmia potential ( ). There is a growing interest in VNS therapy for AF due to various complications, which are associated with ablation procedures ( ) and the minimal adverse effects that VNS impart ( ). VNS also targets select populations of intrinsic cardiac neurons to control neurally induced AF ( ). Together, these data indicate that a major target for neuromodulation based therapy is stabilization of peripheral reflexes. By limiting excessive excursions in network function by VNS, the potential for imbalances in efferent outflow is minimized.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here