Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neurological problems in the newborn infant can arise from innate processes such as genetic abnormalities or disorders of nervous system development or can be the result of acquired brain injury from external insults. Both innate and acquired brain injury in the newborn have lifelong important impact on the developing person and his or her family ( ). The increasing incidence of preterm delivery worldwide, currently estimated at 11%, and the improved survival of the sickest newborns require the neurologist to be aware of the neurological burden of perinatal and neonatal illness ( ). More than half of very preterm newborns will have developmental problems ranging from attention-deficit/hyperactive disorder to cerebral palsy ( ). Almost half of neonates with hypoxic-ischemic encephalopathy (HIE) have neurodevelopmental sequelae as well ( ). These developmental outcomes challenge the child, the family, and the community ( ).
The newborn, whether term or preterm, has a limited means of manifesting injury to the central nervous system (CNS). The adult brain manifests change in many domains with regional specificity, including cognition, behavior, speech, vision, and movement. In contrast, the neonatal brain is in a state of rapid development and most commonly reveals underlying brain dysfunction in two nonspecific ways: encephalopathy and seizures. In the preterm newborn, the neurological signs of significant injury can be even more subtle or not manifest at all.
Advances in neurodiagnostic testing, and neuroimaging in particular, have enhanced our understanding of the pathophysiology of brain injury in the newborn. Digital electroencephalography (EEG) with bedside trending, such as amplitude-integrated EEG (aEEG), and remote access availability grant the clinical team a real-time window into brain function ( Fig. 110.1 ). Neonatal neurology is a growing medical subspecialty with increasing numbers of dedicated multidisciplinary programs at academic institutions ( ). In addition to specific neuroprotective therapies, the increasing focus on the developing brain in the neonatal intensive care unit (NICU) may lead to improved outcomes via earlier recognition and treatment of neurological conditions ( ). A cooperative team effort often is the most effective approach to neurological problems in newborns. Many units also address fetal brain injury ( ).
This chapter reviews the practical aspects of diagnosis and management of neonatal neurological problems commonly encountered by practicing neurologists.
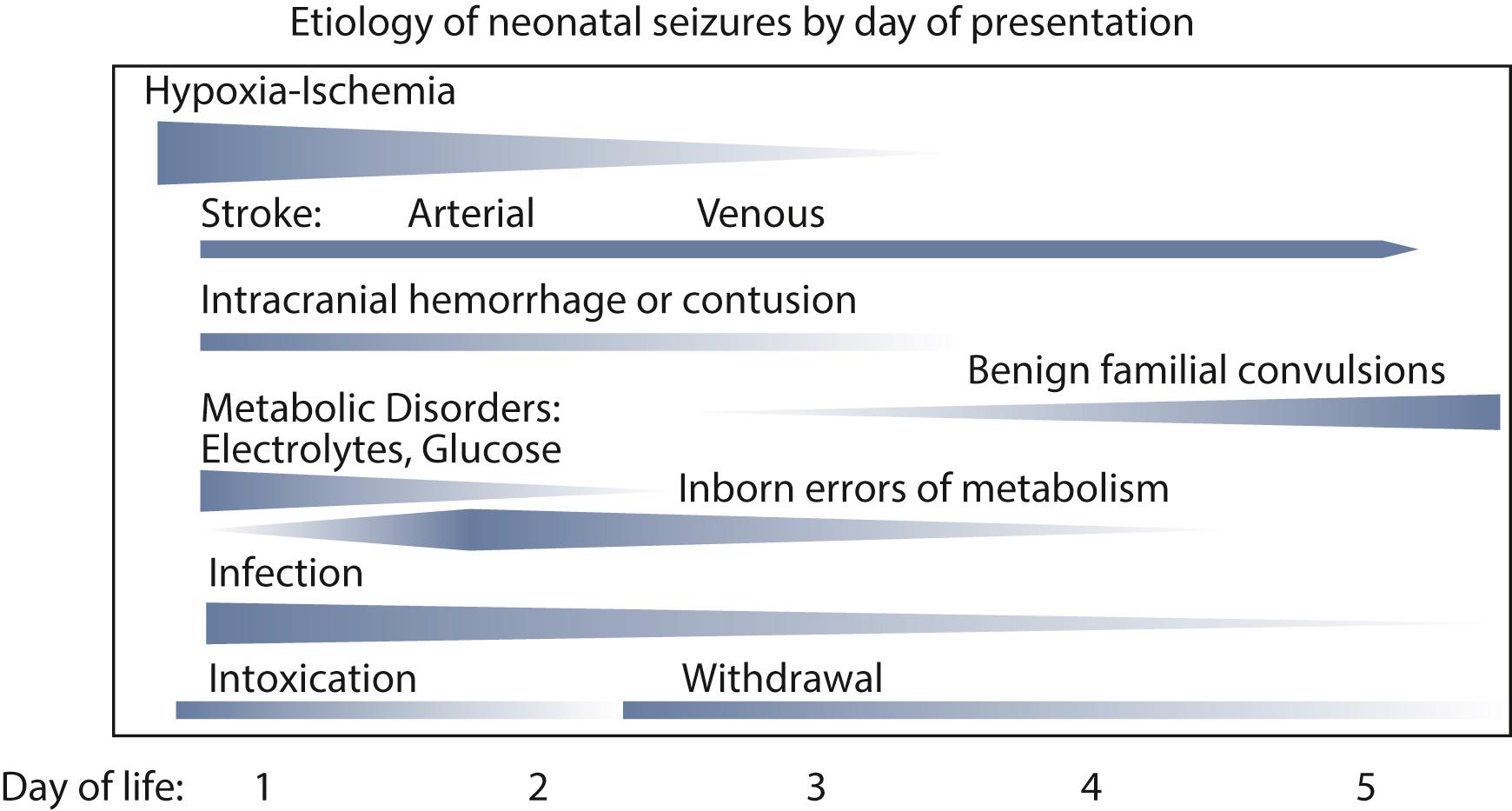
A seizure is defined clinically as a paroxysmal alteration in any neurological function accompanied by seizure activity identifiable on an EEG. Unlike the child and adult, in whom unprovoked seizures predominate, seizures in newborns are almost always acute symptomatic ( ). They are more common in the first 28 days of life than in any other time of life and represent one of the most common manifestations of neonatal brain injury ( ). The timing of onset of the seizures informs the etiology ( Fig. 110.2 ).
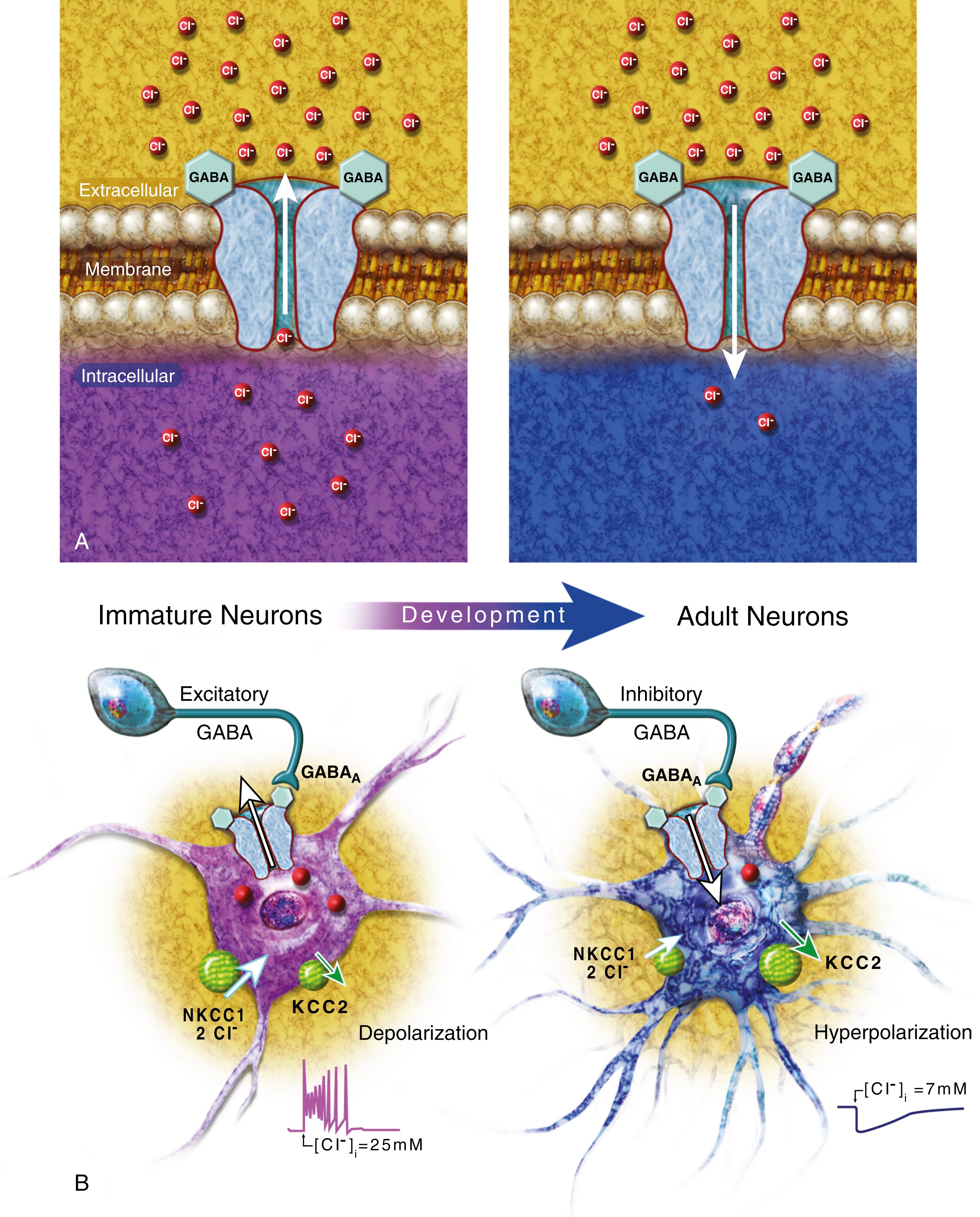
Several developmental factors leading to excess excitation and reduced inhibition influence the high rate of acute-symptomatic seizures in the neonatal period. In adult neurons, γ-aminobutyric acid A (GABA A ) receptor activation leads to chloride influx to produce membrane hyperpolarization and inhibition. In immature neurons, there is a net chloride efflux with GABA A receptor activation, which leads to membrane depolarization and excitability ( ). The developmental fluctuations in neuronal chloride gradients are mediated largely by membrane ion transporters: NKCC1 leads to a high intracellular chloride concentration, whereas KCC2 acts as an active chloride extrusion pathway ( Fig. 110.3 ). With increasing age, the expression of the KCC2 becomes dominant. These developmental changes in chloride influx/efflux may contribute to the often-disappointing response of neonatal seizures to GABA-agonist anticonvulsant medications such as phenobarbital.
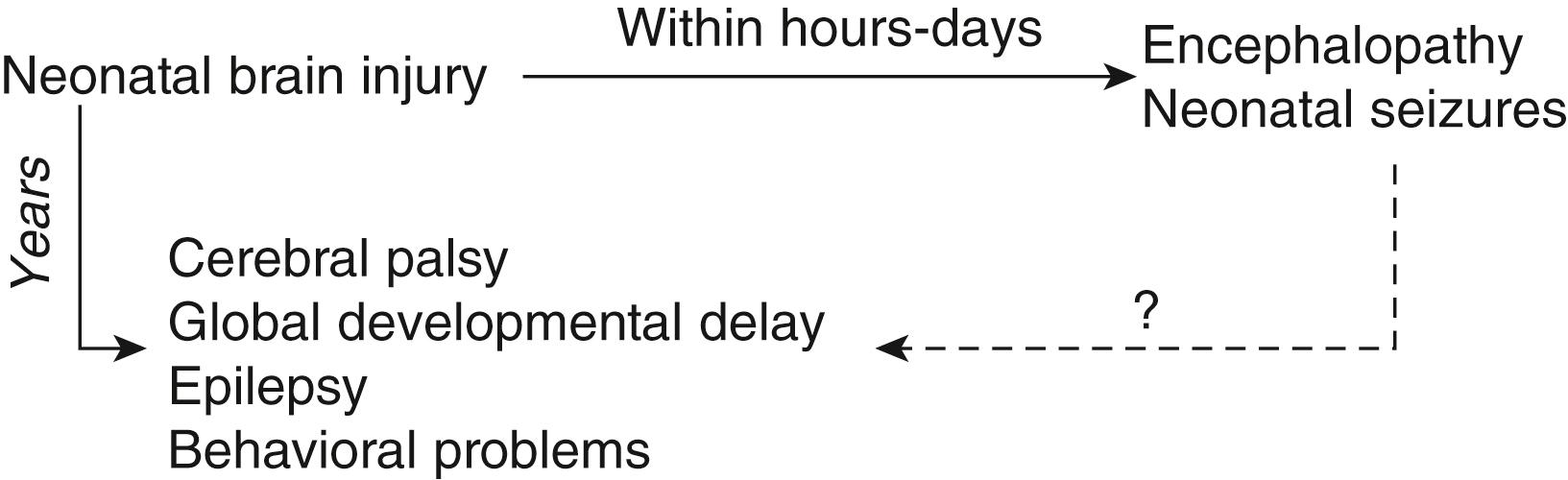
An understanding of the adverse consequences of neonatal seizures on the developing brain is emerging and informs the need to treat subclinical and refractory seizures ( Fig. 110.4 ). Prolonged seizures may cause neuronal injury principally via disturbances in cerebral energy metabolism, with eventual diminution of energy supplies, and excitotoxicity ( ). Recurrent, shorter seizures in animal models have not demonstrated neuronal injury but have been shown to mediate long-term morphological and physiological deficits, including suppression of neuronal stem cells ( ). There is also growing evidence that seizures in human newborns are associated with adverse neurobehavioral outcomes. Neonatal seizures, especially if frequent, intractable, or prolonged, are independently associated with further hypoxic-ischemic brain injury as measured by magnetic resonance (MR) spectroscopy and with later neurodevelopmental impairment ( ).
Most neonatal seizures are acute symptomatic and can follow a multitude of causes (see Fig. 110.2 ). The remainder relate to developmental brain abnormalities and neonatal epilepsy syndromes that are often of genetic origin. Determining the etiology, or etiologies, is important because it informs management and prognosis. HIE accounts for almost half of neonatal seizures in term newborns ( ). In preterm newborns, HIE and intracranial hemorrhage each account for approximately one-third of the seizures, although the clinician should be alert for multiple concurrent etiologies ( ).
Congenital CNS abnormalities account for 5%–10% of neonatal seizures and require neuroimaging for diagnosis. Hypoxic-ischemic brain injury in the second and third trimesters of gestation can result in malformation of brain development ( ). Seizures in the context of cerebral dysplasia are particularly refractory and usually progress towards epilepsy.
Neonatal epilepsy syndromes encompass genetic channelopathies and specific metabolic deficiencies that are increasingly recognized with next-generation genetic sequencing. Channelopathies can range from benign, with resolution of seizures and normal neurodevelopment, to severe early-onset epileptic encephalopathies.
Benign familial neonatal epilepsy is an inherited autosomal dominant condition. When familial seizures are not identified, then the condition is termed benign neonatal seizures. Several gene loci which encode for voltage-gated channels, including KCNQ2 , KCNQ3 , and SCN2A , have been identified ( ). Seizure onset is usually in the first week of life, and seizures classically remit within the first year of life.
In early infantile epileptic encephalopathy, seizures are often initially refractory. Some families retrospectively report rhythmic movements in utero. Some of the newborns are classified as having Ohtahara syndrome, a severe epileptic encephalopathy characterized clinically by intractable tonic seizures and burst suppression on EEG and caused by an increasing number of genetic abnormalities. Early myoclonic encephalopathy is another phenotype of early infantile epileptic encephalopathy featuring erratic focal myoclonus that shifts around the body in an asynchronous pattern, often with burst suppression pattern on EEG. Patients with early infantile epileptic encephalopathies may evolve into West or Lennox-Gastaut syndromes with age ( ). Sodium channel blockers, such as phenytoin and carbamazepine, are particularly effective in managing the channelopathies ( ).
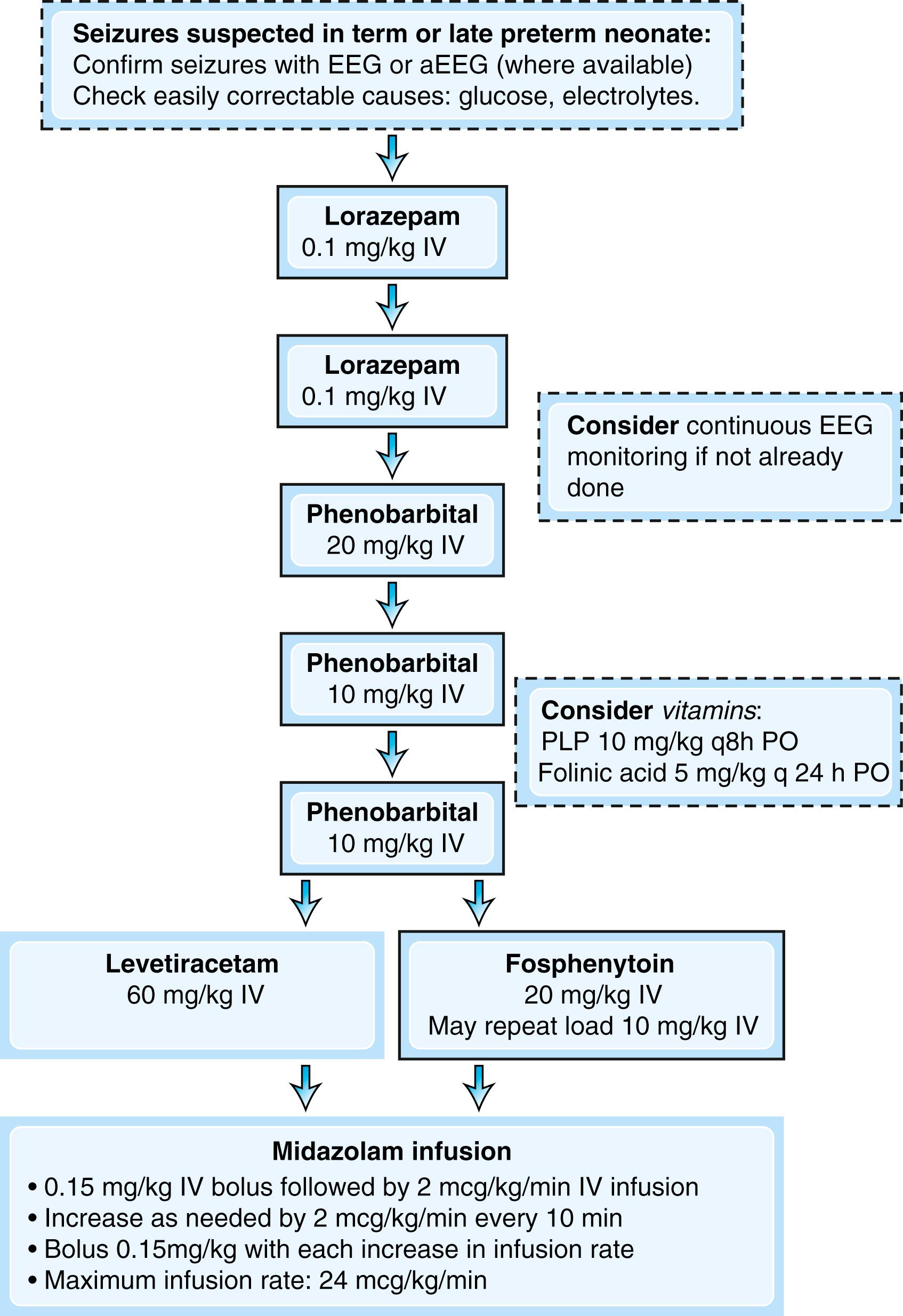
In the context of seizures of unknown etiology, treatable metabolic epileptic encephalopathies should be considered and appropriate therapy instituted pending diagnosis. These disorders include pyridoxine-responsive seizures and folinic acid–responsive seizures, which are both allelic to antiquitin (ALDH7A1) deficiency; pyridox(am)ine-5′-phosphate oxidase deficiency secondary to homozygous mutations of PNPO; and 3-phosphoglycerate dehydrogenase deficiency responsive to serine supplementation ( ). Classically, the diagnosis of pyridoxine-responsive seizures was based on a response to a single large dose (e.g., 100 mg) of intravenous pyridoxine with concurrent EEG recording. However, the pyridoxine challenge is not sensitive and can induce apnea. As such, one strategy for suspected vitamin-responsive early-onset epileptic encephalopathies is to initiate broad treatment with folinic acid and pyridoxal phosphate for 3 days and to continue the therapy if there is a clinical improvement pending genetic test results ( Fig. 110.5 ). Elevations of urinary α-aminoadipic semialdehyde (AASA) and serum or cerebrospinal fluid (CSF) pipecolic acid are nonspecific biomarkers for pyridoxine-dependent seizures ( ).
Many other inherited disorders can without specific treatments present with refractory neonatal seizures, such as nonketotic hyperglycinemia, peroxisomal biogenesis defects, respiratory chain disorders, sulfite oxidase deficiency, Menkes disease, and congenital disorders of glycosylation.
Accurate diagnosis of neonatal seizures requires electrographic corroboration due to the difficulty in accurately identifying clinical seizures. Seizure manifestations in newborns differ from those in older individuals in that newborns generally do not have well-organized, generalized tonic-clonic seizures due to the immaturity of their synaptic connections. In addition, seizures in the newborn are often clinically silent and detected only on EEG; these seizures are referred to as subclinical seizures ( Fig. 110.6 ; see later). In one study, only one-third of neonatal EEG seizures displayed clinical signs on simultaneous video recordings; only one-third of these clinical manifestations were recognized by experienced neonatal staff; and only one-quarter of clinically suspected seizures documented by staff had corresponding electrographic evidence of seizure activity ( ). As such, EEG monitoring to identify seizures is an essential tool to avoid missing and undertreating seizures and to avoid overdiagnosing and overtreating seizures ( ). If continuous EEG is not available, then continuous aEEG can be used. aEEG is a simplified trend monitor that displays one or two channels of time-compressed, processed EEG signal on a semi-logarithmic scale (see Fig. 110.1 ). Compared with EEG, it is less sensitive but readily interpreted by trained bedside practitioners. The use of these brain-monitoring tools is a cornerstone of modern neonatal neurocritical care ( ).
Clinical seizure semiologies have been classified by Volpe, and they are summarized in Table 110.1 ( ). Seizure types are not specific for etiology, but some are seen more often with certain underlying conditions. For instance, focal clonic seizures in the term “newborn” are most commonly associated with focal cerebral infarction. Subtle and generalized tonic seizures do not consistently show concomitant electrographic discharges. In these cases the abnormal movements may represent nonepileptic brainstem release phenomena or seizure discharges in deep cerebral structures which are not transmitted to surface EEG.
| Clinical Seizure | Manifestations and Key Points |
|---|---|
| Subtle | Eye deviation, blinking, fixed stare Repetitive mouth and tongue movements Apnea, other autonomic phenomena Bicycling, other rhythmic limb movements |
| Clonic: focal or multifocal | Rhythmic movements of muscle groups Often represents a focal pathology |
| Tonic: focal or generalized | Sustained flexion or extension of muscle groups |
| Myoclonic focal, multifocal, or generalized | Synchronous flexion jerks Must be distinguished from nonepileptic myoclonus |
Nonepileptic movements must be distinguished from seizures. Physiological myoclonus, which occurs in healthy newborns, differs from myoclonic seizures, which often have a dismal outcome: the seizures are not evoked by stimuli, are nonsuppressible by touch, and occur with concomitant encephalopathy. Jitteriness, which is an exaggerated startle response, is often confused with clonic seizures, especially because both jitteriness and clonic seizures occur in similar underlying conditions, such as HIE, hypoglycemia, and drug withdrawal. The absence of associated ocular movements and autonomic changes (e.g., tachycardia, hypertension, and apnea) and the presence of stimulus sensitivity distinguish jitteriness from seizures. The predominant movement in jitteriness is tremor, in which the alternating movements are rhythmic and of equal rate and amplitude ( ). In contrast, movements in clonic seizures have a fast and slow component. In addition, jitteriness stops when passively flexing the affected limb. Hyperekplexia is another abnormal movement that is nonepileptic. It is characterized by an exaggerated startle response and sustained tonic spasms in response to unexpected auditory, visual, and somesthetic stimuli and is caused by genetic mutations in genes involved in glycine neurotransmission ( ).
EEG and aEEG are necessary tools for the diagnosis of neonatal seizures. Continuous conventional EEG is currently recommended as the “gold standard” for diagnosis of neonatal seizures by the American Clinical Neurophysiology Society ( ). In most neonatal seizures, electrographic onset is focal or multifocal. Spread of the seizure within one hemisphere and secondary generalization to the contralateral hemisphere are uncommon, presumably due to the immature synapses in the newborn brain ( ).
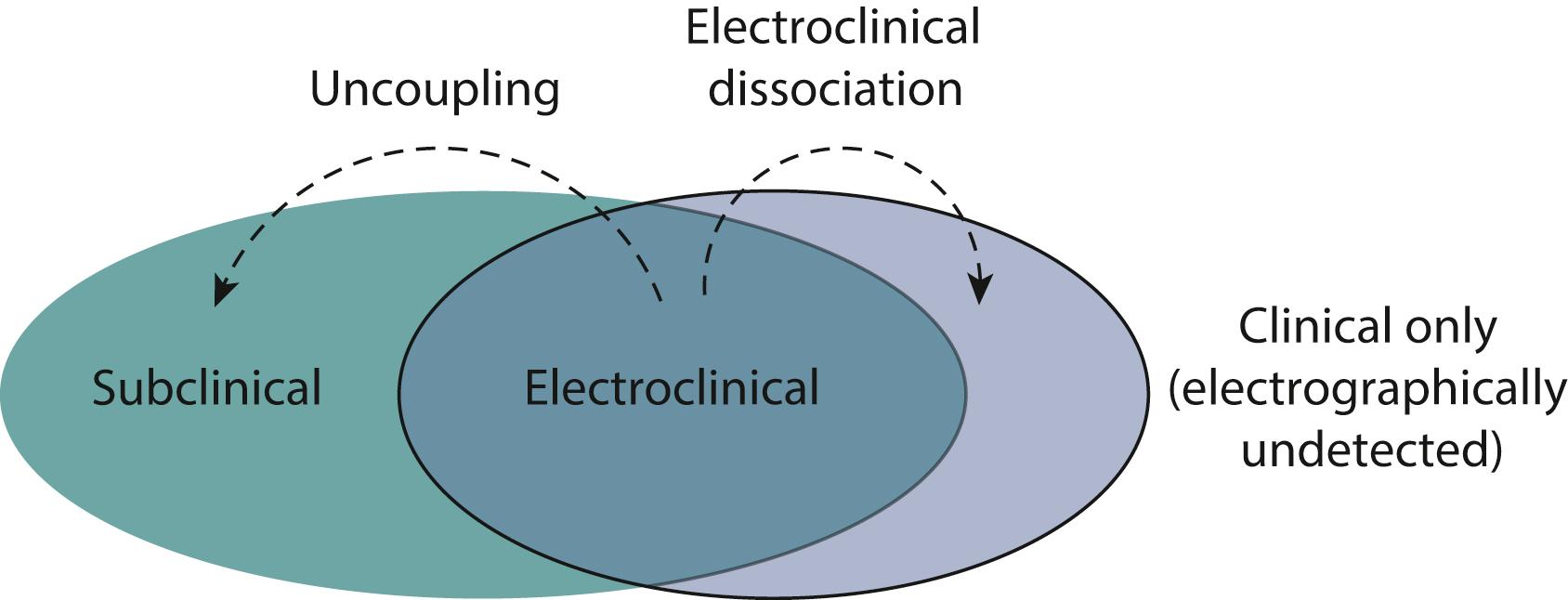
Neonatal EEG seizures have (1) a sudden electrographic change; (2) repetitive waveforms that evolve in morphology, frequency, and/or location; (3) an amplitude of at least 2 μV; and (4) a duration of at least 10 seconds ( ). Fig. 110.6 illustrates the terminology used to describe clinical, electrographic, and subclinical seizures. Uncoupling refers to the phenomenon by which the clinical manifestations terminate while electrographic seizures persist, and commonly occurs in neonates after the administration of anticonvulsant medications ( ). Electroclinical dissociation refers to the phenomenon by which the clinical expression of a seizure occurs without an electrical correlate ( ). Although electroclinical dissociation exists (e.g., seizures emanating from the mesial temporal lobe), it is not common.
Sharp transients are normal in premature newborns and should not be confused with seizure activity. Similarly, the trace-alternant pattern of quiet sleep in normal=term infants, in which normal low-amplitude activity is preserved between bursts, must be distinguished from the abnormal burst-suppression pattern, in which long periods of voltage suppression or absence of activity are recorded between bursts of high-voltage spikes and slow waves ( ). Interictal EEG has prognostic value: severe suppression of the background activity, whether interrupted by high-amplitude bursts or not, is associated with an abnormal outcome. Importantly, the severity of background disturbance is a more important predictor of seizure risk than are interictal epileptiform abnormalities in the neonate with brain injury ( ).
Suspected neonatal seizures require urgent investigation for acute-symptomatic etiologies and management because prolonged and recurrent seizures are likely independently associated with brain injury and abnormal brain development, as discussed earlier. Glucose and electrolyte levels should be measured and any abnormality corrected. A full sepsis evaluation, including cultures of blood and CSF, should also be undertaken if feasible and empiric antibiotics started. Neuroimaging studies should proceed according to institutional availability; ultrasound of the head is usually readily available and can give diagnostic clues ( ). Genetic epilepsy syndromes are diagnosed with appropriate gene panels or whole exome sequencing.
There is little in the way of evidence-based guidelines for the pharmacological management of neonatal seizures; nonetheless, treatment with anticonvulsant medications should be initiated once seizures are confirmed electrographically or if the clinical suspicion for seizures is sufficiently high. The World Health Organization (WHO) recommends phenobarbital as a first-line agent but other agents, such as phenytoin (or fosphenytoin) and benzodiazepines are acceptable alternatives and are all available intravenously ( ; ). Approximately half of seizures are controlled with a 20 mg/kg load of phenobarbital or phenytoin ( ). Benzodiazepines such as lorazepam or midazolam have the advantage of a short half-life and can be particularly useful when seizures are suspected based on clinical observation alone but unconfirmed by EEG or aEEG. The use of these medications as a first-line agent may obviate the need for further therapy in neonates in whom seizures do not recur or, are later shown, via neuromonitoring, not to have any epileptic correlate. Levetiracetam is increasingly being used as well despite limited safety and efficacy data ( ). A recent randomized controlled trial showed that levetiracetam was less effective than phenobarbital for the treatment of neonatal seizures ( ). If seizures are not controlled after repeated loading doses of standard medications, titration of a midazolam infusion may be indicated. An example of an abridged seizure management algorithm is provided in Fig. 110.5 , but individual institutions should develop their own.
The major determinant of prognosis following neonatal seizures is the underlying etiology (see Fig. 110.4 ). Of newborns with seizures who survive their acute injury, 25%–70% will go on to have subsequent neurodevelopmental impairments ( ). The frequency of epilepsy following neonatal seizures is between 10% and 30% ( ). A relatively high frequency of infantile spasms has been observed among survivors of neonatal seizures ( ).
The decision regarding maintenance antiepileptic medications after NICU discharge relates mostly to the likelihood of developing postneonatal epilepsy balanced with the potential long-term toxicity of the therapy. In most acute symptomatic seizures, the seizures remit within a few days of life. Currently, it is unknown if continuing antiepileptic treatment for up to several months is helpful or harmful. The rationale for using antiepileptic drugs at discharge is to decrease the likelihood of seizure recurrence. In the presence of risk factors for later epilepsy, antiepileptic medication may be warranted. These risk factors include status epilepticus, severe hypoxic-ischemic brain injury, and the use of more than a single antiepileptic medication to adequately control the neonatal seizures ( ). Importantly, there are theoretical concerns that some treatment strategies for neonatal seizures could be toxic to the developing brain, and, as such, it may be judicious to withhold maintenance antiepileptic medication in the absence of these risk factors for later epilepsy ( ).
The brain of the preterm newborn is in a state of rapid development and is susceptible to four overarching and overlapping forms of preterm brain injury: intraventricular hemorrhage (IVH), white matter injury (WMI), gray matter injury, and cerebellar hemorrhage.
The neurodevelopmental impact of preterm brain injury is substantial and lifelong. Among contemporary cohorts of children born very preterm, (i.e., at <32 weeks’ gestation) 5%–10% have major motor deficits, including cerebral palsy, and more than half have significant cognitive, behavioral, and/or sensory deficits ( ). The principal major motor deficit is spastic diplegia, because the periventricular lesions traverse descending fibers from the motor cortex subserving lower extremity function. Although most very preterm-born children have normal intelligence and no cerebral palsy, careful assessment at school age reveals an important burden of motor dyspraxia, processing deficits in attention and executive functions, anxiety, and visually based challenges with information processing and language ( ). These less overt neurodevelopmental challenges are likely linked to impairments in cerebral growth and disturbances in cerebral maturation rather than injuries that are appreciable on diagnostic brain imaging ( ).
IVH is a common injury in the preterm brain, and its incidence is inversely proportional to gestational age. The bleeding originates in the subependymal germinal matrix, from which cortical neuronal and glial cell precursors develop during the late second and early third trimesters. The highly vascularized germinal matrix involutes with advancing gestation ( ).
The predisposition of the preterm infant to germinal matrix hemorrhage is due to several factors: arterioles lack autoregulation and exist in a pressure-passive state; blood vessels lack a supporting basement membrane; and extravascular tissue pressure is low in the first few days of extrauterine life. Thus IVH may occur in the setting of elevated venous pressure or fluctuations in cerebral blood flow triggered by factors that include respiratory distress, pneumothorax, asphyxia, left ventricular dysfunction, patent ductus arteriosus, hypotension, hypothermia, and hyperosmolarity ( ). In addition, individual NICU characteristics are critical determinants of severe intraventricular hemorrhage ( ).
The risk period for IVH is highest in the first 3 or 4 days of life, with 50% of hemorrhages detectable by 24 hours and its clinical presentation is usually clinically silent . If large enough, it may manifest as sudden deterioration with neurological signs such as stupor, seizures, decerebrate posturing, or apnea. A tense fontanelle, together with a sudden anemia, hyperglycemia, hyperkalemia, or bradycardia, may herald an IVH; hyponatremia from inappropriate secretion of antidiuretic hormone may occur as well.
Cranial ultrasound examinations are typically performed in the first week of life to detect IVH, and the images are used to classify IVH according to the algorithm adapted from ( ) ( Fig. 110.7 ). Grade I IVH is limited to the subependymal region; grade II IVH has blood in the ventricles without ventricular distension; and grade III IVH shows enlargement of the ventricles secondary to distention. The classic grade IV IVH arises from venous infarction of the periventricular white matter rather than from a direct extension of the IVH into the parenchyma.
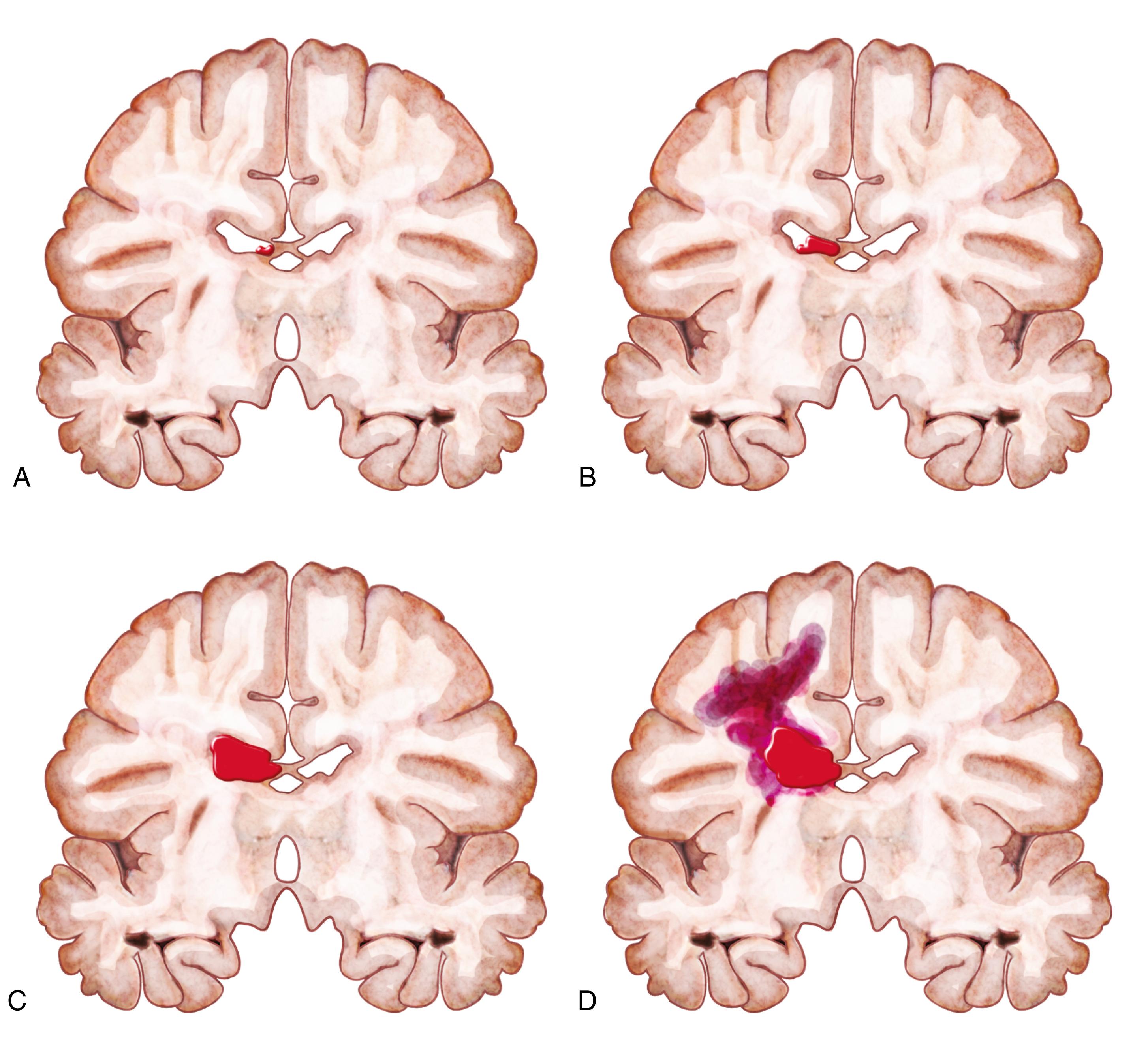
A larger IVH may produce an inflammatory reaction that obliterates the subarachnoid space or cerebral aqueduct with subsequent ventricular dilatation and hydrocephalus ( ). Symptoms of progressive hydrocephalus, such as increasing head circumference, a full anterior fontanelle, separation of cranial sutures, or autonomic symptoms of high intracranial pressure, such as apneas, bradycardia, or hypertension, are not sensitive or timely markers of hydrocephalus because they often appear days or weeks after the onset of ventricular dilatation.
Prenatal administration of steroids and maternal transfer to a tertiary-level hospital are the best means of preventing IVH. Practice bundles that limit fluctuations in cerebral blood flow via maintenance of partial pressure of carbon dioxide (P co 2 ) and metabolic status within a normal range, avoidance of excessive suctioning and handling, and avoidance of rapid volume infusions and infusions of sodium bicarbonate are increasingly used ( ).
Serial head ultrasound examinations are necessary in the setting of significant IVH to detect progressive ventricular dilatation. Clinical guidelines for the management of posthemorrhagic hydrocephalus are locale specific and accumulating evidence suggests that early removal of CSF in the setting of posthemorrhagic ventricular dilatation may reduce the need for ventriculoperitoneal shunt placement and attenuate neurodevelopmental impairment ( ). The commonest methods of CSF removal are serial lumbar punctures and insertion of a ventricular access device with a reservoir (e.g., Ommaya reservoir). Placement of a ventriculoperitoneal shunt or endoscopic third ventriculostomy are options for long-term CSF diversion for persistent hydrocephalus ( ).
Grade I and II IVH are associated with a statistically higher likelihood of later moderate or severe impairment, but most neonates with mild IVH do not have such impairments ( ). Neonates with grade III IVH, and especially those with associated periventricular hemorrhagic infarction or posthemorrhagic ventricular dilatation, are at higher risk for poor neurodevelopmental outcome.
WMI is the commonest form of brain injury in preterm neonates and is linked in experimental models and clinical studies to hypoxia-ischemia, infection, and inflammation ( ). The susceptibility to WMI is critically related to the timing of the insult, as, over the third trimester, distinct cell types are selectively more vulnerable to injury. Moreover, a wide variety of additional factors may synergistically sensitize the brain to injury. Such factors include nutritional status, systemic illnesses, painful procedures and exposure to glucocorticoids, sedatives, and prenatal exposure to drugs of abuse ( ).
WMI encompasses two major groups of pathology: focal necrosis, which itself ranges from cystic to microscopic, and diffuse nonnecrotic lesions ( ) ( Fig. 110.8 ). Focal cystic necrosis, of which periventricular leukomalacia (PVL) is the most severe, occurs in the white matter adjacent to the ventricles and pathologically involves degenerative axons and phagocytic macrophages. The large necrotic lesions of PVL have become uncommon in contemporary cohorts of preterm neonates. Punctate and diffuse WMIs are currently the predominant lesions in most preterm neonates imaged with magnetic resonance imaging (MRI). Punctate WMI appears as areas of abnormal signal intensity with a characteristic topology, with most lesions occurring in the periventricular regions ( ). Pathologically, diffuse WMI features selective degeneration and regeneration of preoligodendrocytes, a mitotically active progenitor cell line that peaks as a cell line between 23 and 32 weeks’ gestation. In preterm diffuse WMI, the preoligodendrocytes that degenerate then fail to mature to myelin-forming oligodendrocytes; however, axons are spared ( ). Over the past two decades, the prevalence of WMI seems to be decreasing in some centers ( ).
Although WMI is the most commonly observed abnormality in preterm neonates on diagnostic MRI, it does not fully account for the burden of neurodevelopmental disability in this population ( ). Impaired development of the white matter contributes more to neurodevelopmental disability following preterm birth. In other words, white matter dysmaturation, secondary to developmental arrest of preoligodendrocytes rather than injury, is the primary brain abnormality in contemporary cohorts of preterm neonates ( ).
Importantly, WMI is the “tip of the iceberg” following hypoxia-ischemia in the third trimester of gestation because it is most readily identified on MRI. Experimental models of preterm hypoxia-ischemia demonstrate concurrent impairments of neuronal development in the cerebral cortex and basal ganglia, as well as in transient cell populations such as the subplate ( ). Neuronal degeneration may occur either as a primary process or via retrograde degeneration secondary to axonal injury in foci of white matter necrosis. In contemporary cohorts studied with MRI, overt evidence of neuronal necrosis is uncommon. Rather, reduced cortical and subcortical growth is accompanied by a reduction in the complexity of the dendritic arborization ( ). This process is likely reflected in reduced volumetric growth of gray matter structures on MRI ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here