Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The armamentarium of neuroimaging techniques includes: computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, x-ray, fluoroscopy, and nuclear medicine (NM). Of these, CT and MRI have found the most use in daily practice and are frequently used as first-line neuroimaging, with the remaining modalities providing important, often ancillary, functions. The strength of CT lies in its high spatial resolution, speed of acquisition, and lack of required patient prescreening, making it the ideal tool for rapid cross-sectional evaluation of the emergency patient. CT uses radiation to create images, whereas MRI depends on strong magnetic fields, radiofrequency waves, and field gradients. Individual sequences can probe the physiology of various common neurologic disease states, including stroke, hemorrhage, and tumors. Ultrasound is a cost-effective imaging technique that uses sound waves to produce both dynamic and static cross-sectional imaging, ideal for evaluating soft tissues and vessels. X-ray, with its unparalleled spatial resolution and ability to capture large areas, is ideal for investigating bony anatomy, the integrity of surgical hardware, and ventricular shunts. Fluoroscopy capitalizes on the high spatial resolution of x-ray but is used in real time to assess cerebrovascular anatomy and flow and to conduct various imaging-guided neurointerventional procedures. Finally, NM uses minute amounts of radiotracers to assess varying disease states ranging from infection and inflammation to neurodegenerative disease and neoplasia.
With such a variety of available imaging modalities, often with overlapping or complementary applications, knowing which exam to order becomes an important clinical skill. The ideal exam will answer the clinical question, provide potential alternative diagnoses, and keep detection of incidental, often distracting, findings to a minimum. For each modality, a fundamental understanding of image acquisition, strengths and limitations, and established clinical applications will help guide the clinician to the correct diagnostic exam and potentially aid in management and treatment.
A CT image constitutes a gray-scale map of the body's attenuation of x-rays. The images are produced by moving the patient through a gantry while a thin, fan-shaped x-ray beam rotates. On the opposite side of the x-ray beam, detectors measure the attenuated x-rays that are able to pass through the patient. Via the process of “filtered back projection,” computer software is then able to reconstruct an image based on the attenuation properties of the tissues imaged. The attenuation factor at each point in image space is a unitless number known as the Hounsfield unit (HU). For the purposes of image display, each HU is assigned a grayscale value which determines the brightness/darkness of each pixel within the display matrix. Therefore CT images are a map of tissue density or absorption at each voxel, expressed in HU, which ultimately represents the attenuation of x-rays at each point in space within the patient. CT's ability to discriminate small differences in tissue x-ray attenuation rapidly and with a high degree of spatial resolution is the basis of its diagnostic strength ( Fig. 3.1 ).
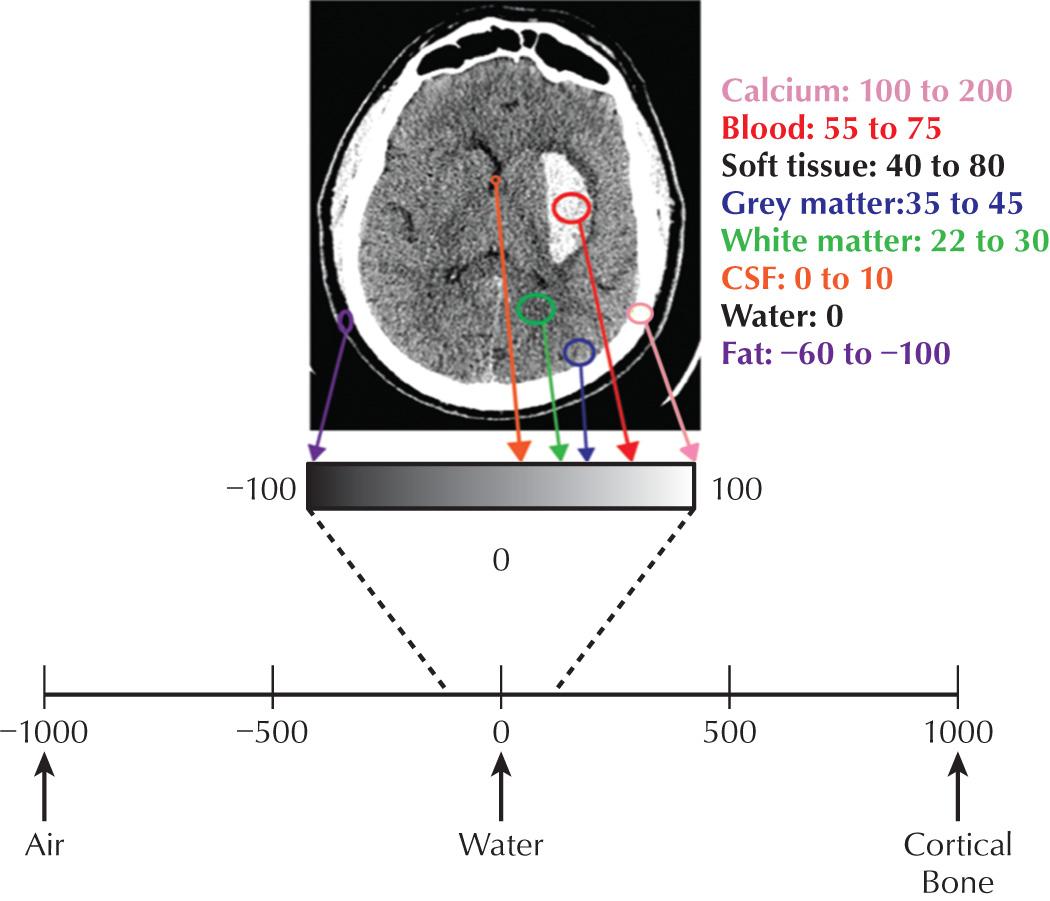
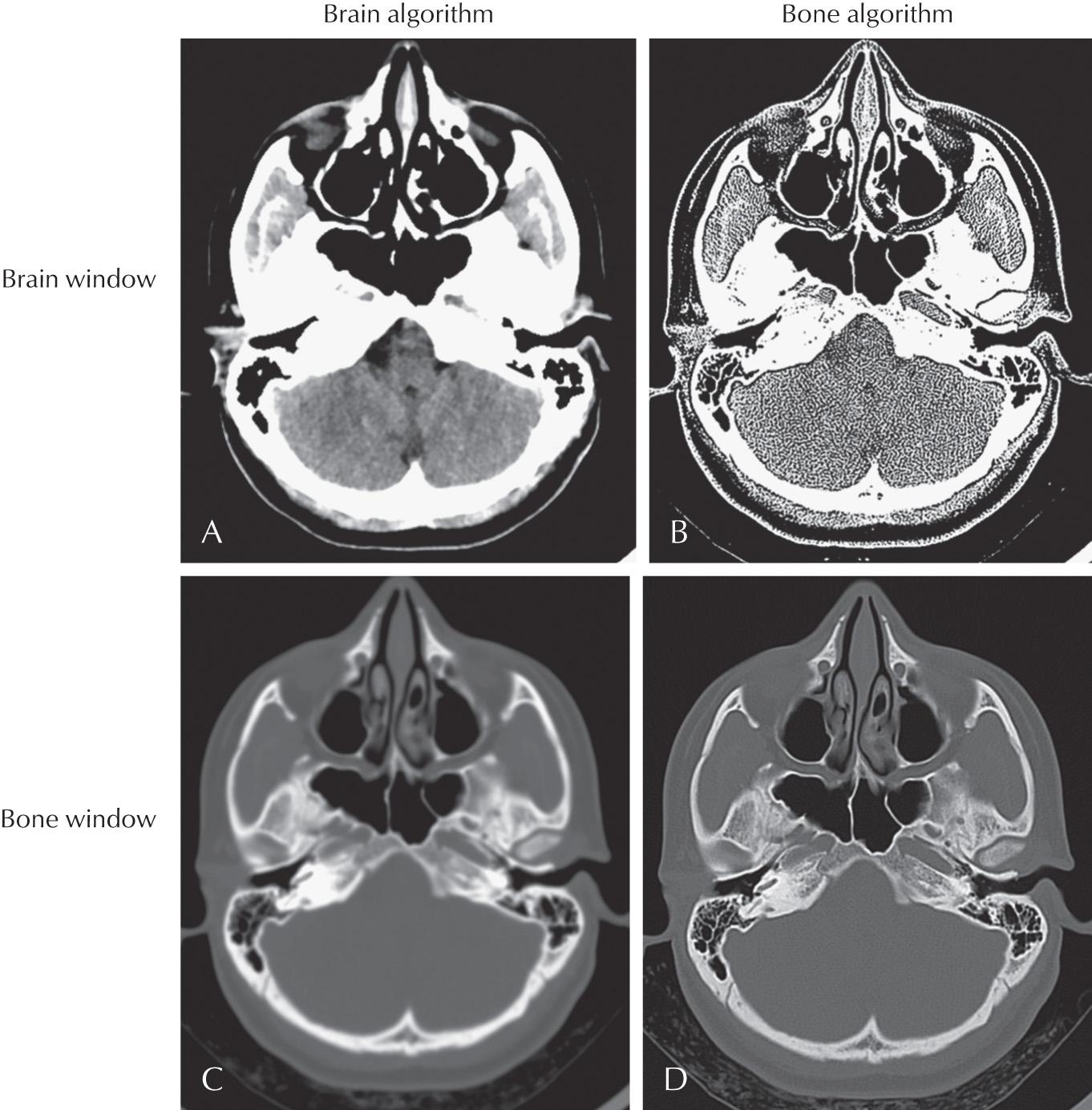
Once CT images have been acquired, several postprocessing tools can be used to improve and enhance the images for display and interpretation. A digital CT image has a dynamic range of 4096 shades of gray, which is well beyond what a monitor can display (256) and the human eye can distinguish (32). Therefore prior to interpretation, each image must be digitally manipulated to augment the gray scale of the tissue in question, whether this be air, fat, water, muscle, or bone. Common reconstruction algorithms include bone windows for accentuated visualization of bone, bone matrix, and osseous abnormalities; and soft tissue windows for accentuated visualization of soft tissues, muscle, water, and fat ( Fig. 3.2 ).
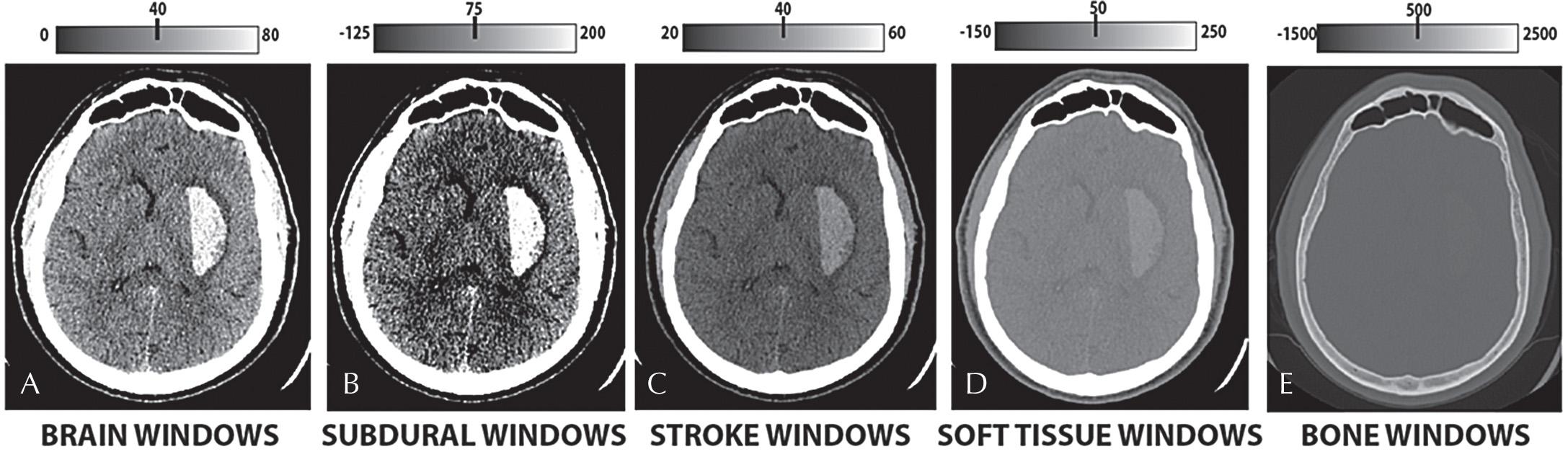
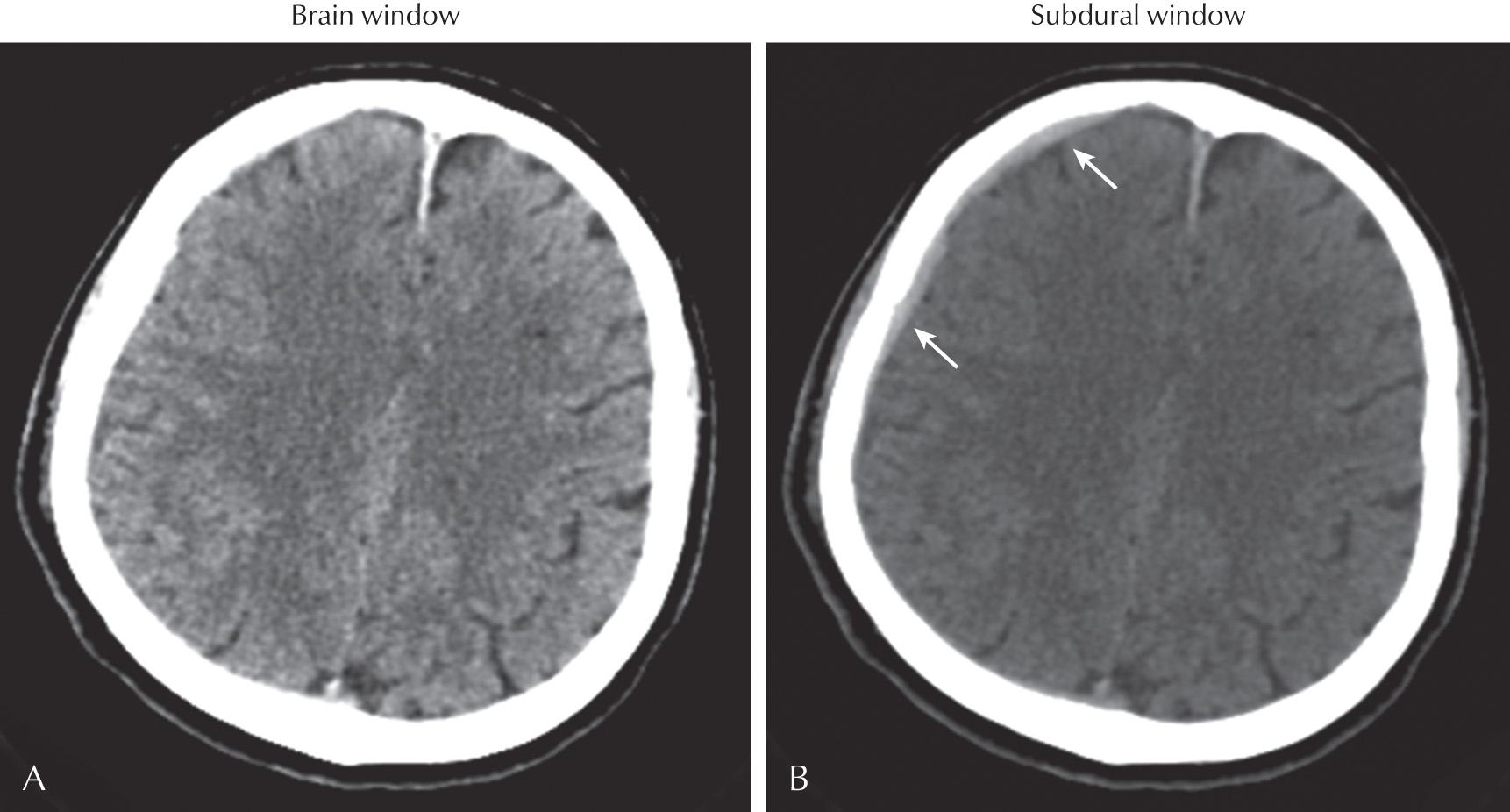
Each postprocessed series can then be “windowed and leveled,” which refers to window width and level selection. The window width is the range of HU displayed. The window level is the center point of the width. Only the tissues within the window width are assigned a variety of gray levels within the spectrum of display. Tissues displaying HU greater than the window level are assigned white pixels, and HU levels less than the lower range of the window level are assigned black pixels. Although the window width and level can be dynamically manipulated, commonly used “set points” include Brain Windows for accentuated visualization of the brain parenchyma, Narrow Brain Windows for accentuated visualization of gray-white matter differentiation, and Subdural Windows for accentuated visualization of hyperdense blood products adjacent to the inner table of the skull ( Figs. 3.3 and 3.4 ).
Postprocessing methods can also be used to reformat axially acquired data into two-dimensional (2D) images in other planes (coronal and sagittal) and three-dimensional (3D) data sets including volume-rendered and surface-rendered displays. The reformatting process does not alter the CT voxels; it simply displays the data in an orientation or configuration different than the way in which they were originally acquired ( Fig. 3.5 ).
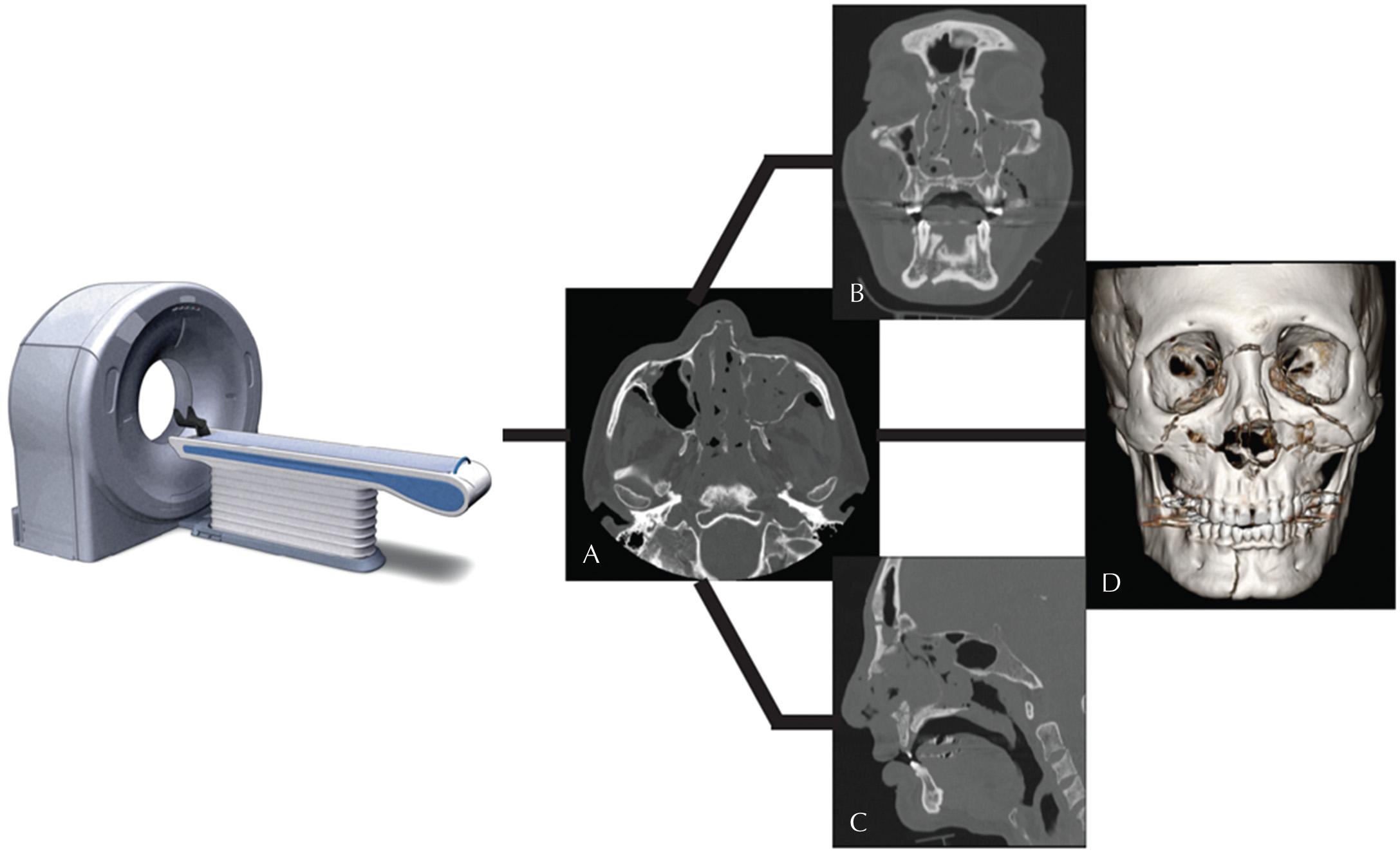
Once acquired and postprocessed, CT imaging of the brain allows for rapid exclusion or evaluation of intracranial hemorrhage, mass effect, midline shift, or hydrocephalus, making it the ideal first-line imaging tool to examine the neurologically compromised patient. Exclusion of hemorrhage and signs of completed infarction with CT are required prior to administration of tissue plasminogen activator (tPA) in the clinical setting of acute stroke. Although less sensitive than MRI in the detection of early infarction, subtle signs are often present on CT and can help confirm the diagnosis. The high spatial resolution of CT is also ideal for visualizing bony anatomy and detecting fractures, whether in the head or spine.
By administering intravenous iodinated contrast immediately prior to or during imaging, the capabilities of CT can be extended further. If given before imaging, contrast allows us to evaluate the integrity of the blood-brain barrier, thereby aiding in the detection of infection, inflammation, and neoplasia. Contrast administration during imaging can be used to visualize arterial (CT angiography [CTA]) and venous (CT venography [CTV]) anatomy. Axial, postprocessed 2D and 3D images can be used for the planning of aneurysm, arteriovenous malformation, and carotid stenosis intervention or surgery, or for the detection of vascular pathology, including stenosis, dissections, and vasculitis. Additional postprocessing techniques such as maximum intensity projections (MIPs) provide useful depictions of the often-tortuous vasculature. In addition, CT perfusion (CTP) allows for real-time dynamic data acquisition during contrast bolus administration and therefore provides quantitative and qualitative information in regard to cerebral perfusion ( Fig. 3.6 ).
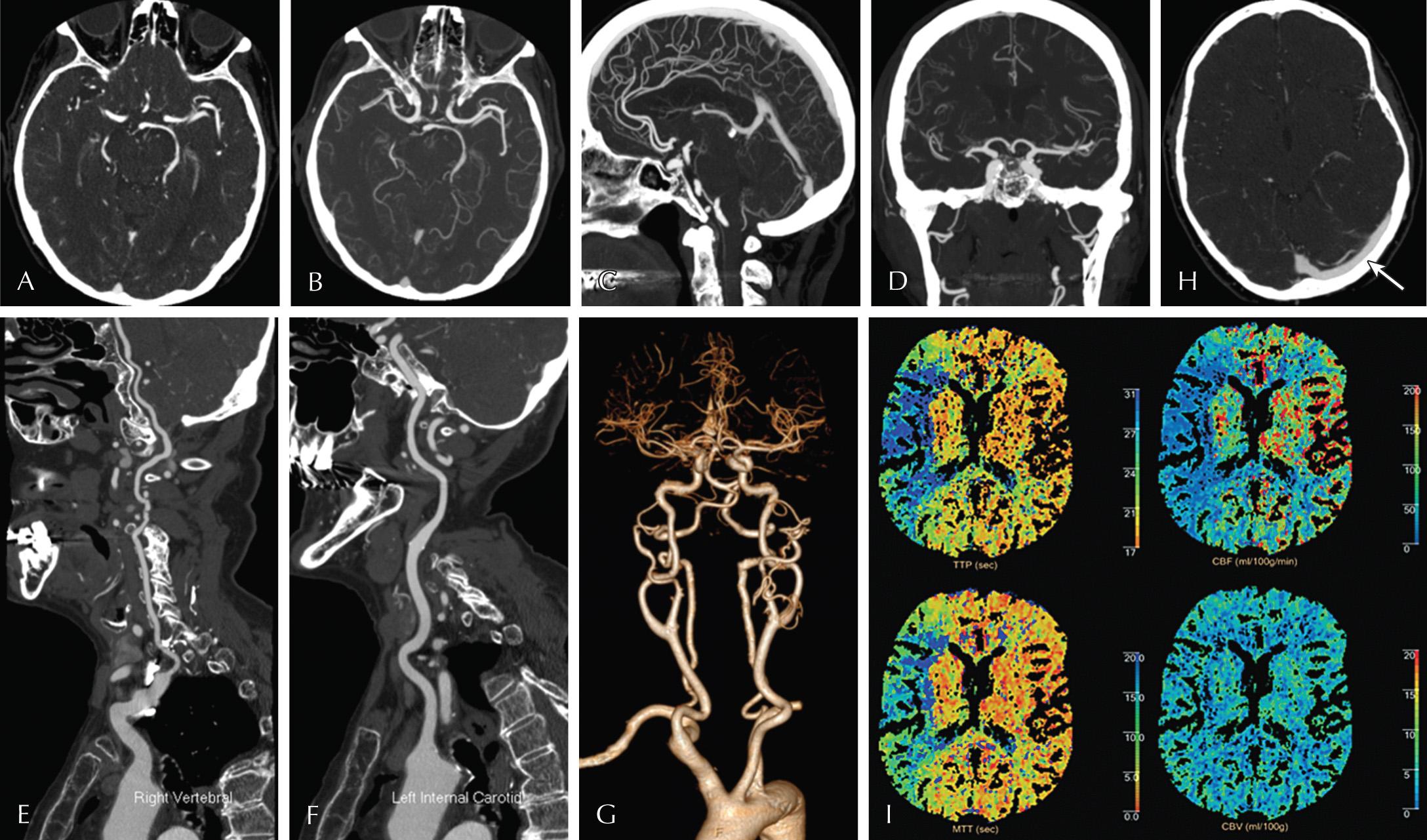
The speed of the CT image acquisition (just a few seconds for a head CT) not only expedites diagnosis, but it also reduces the likelihood of image degradation related to patient motion. The speed, combined with widespread availability and lack of required prescreening for implanted devices, makes CT extremely accessible for patients. The high spatial resolution of CT is particularly useful when imaging bone architecture or vasculature. Although catheter angiography remains the “gold standard” for evaluation of the vessels, the ability of CTA to rapidly and noninvasively image the vasculature has made this the modality of choice for initial assessment. Unfortunately, all the benefits of CT come at the expense of radiation, concerns about which are well reported in the medical and lay literature. The imaging industry is actively addressing these concerns with new and better dose-reduction techniques. Individual radiology departments endeavor to achieve the best imaging for the patient at the lowest possible radiation dose.
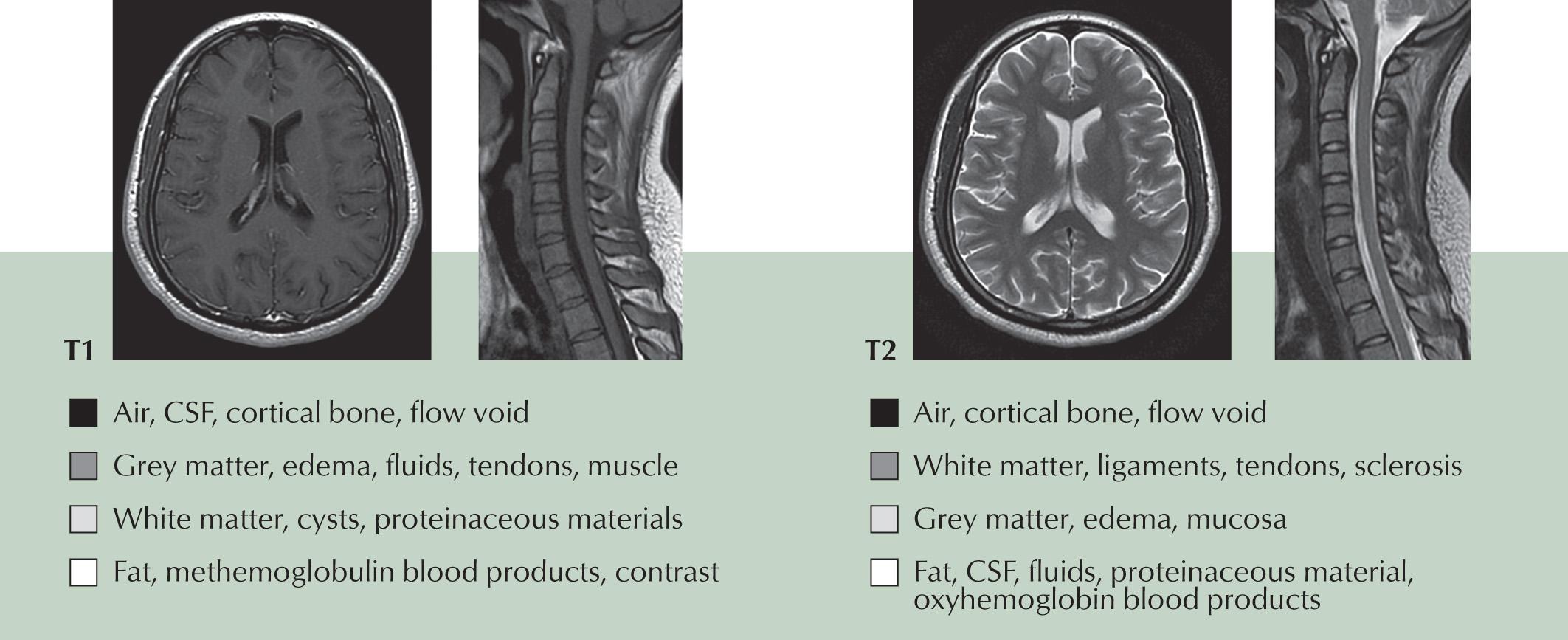
Instead of using radiation, MRI creates images of the human body based on hydrogen protons, which are abundantly present as water in the human body. Normally, these hydrogen nuclei are randomly aligned. However, when placed in a magnetic field, a small percentage of them align along the axis of that field. An applied external radiofrequency (RF) pulse disrupts this magnetization and creates two magnetization vector components: longitudinal magnetization (in the direction of the magnetic field) and transverse magnetization (perpendicular to the magnetic field). When the RF pulse is turned off, the net magnetization vector realigns with the MR magnetic field—therefore longitudinal magnetization starts to increase (T1 recovery), and transverse magnetization starts to decrease (also called T2 or T2* decay). Different tissues relax and decay at different rates, resulting in characteristic T1, T2, and T2* values that define their appearance on MR imaging ( Fig. 3.7 ).
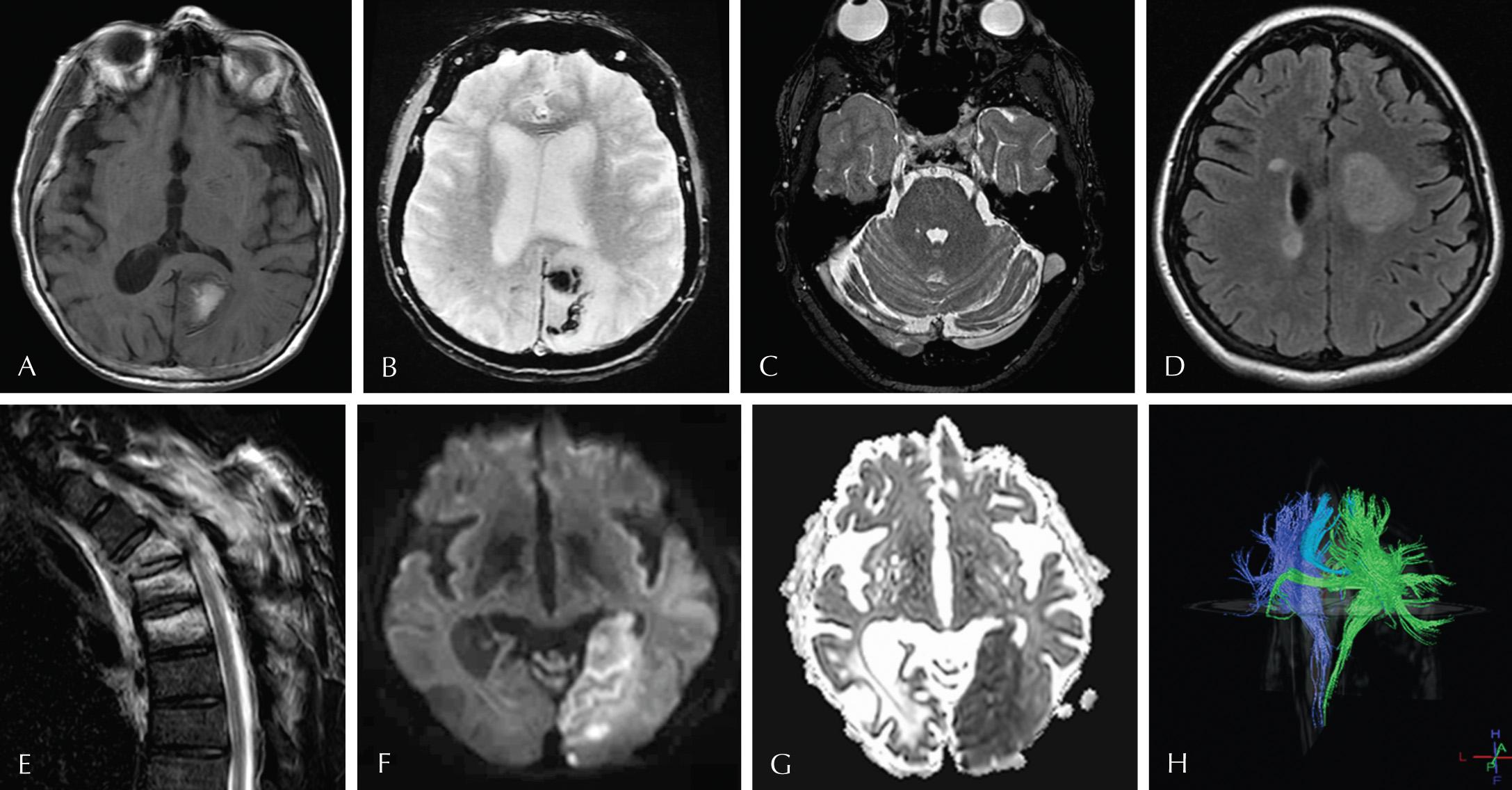
Although tissues and organs have a characteristic MR imaging appearance, tissue injury or disease, leading to even small differences in the biochemical composition, will result in often-detectable MR imaging changes. By adjusting MR imaging parameters (such as RF excitation, switching of spatial encoding gradients, relaxation waiting times, and signal measurements), different MR sequences are produced, which accentuate these differences. The most common sequences used currently for neurologic imaging include T1, T2, inversion recovery (IR), gradient echo (GRE), and diffusion-weighted imaging (DWI). T1 imaging is used to visualize anatomic detail and highlight specific disease entities, such as fat, protein, melanin, hemorrhage, and MR contrast (gadolinium) that are hyperintense on MR imaging. On T2-weighted imaging, fluid is hyperintense, enabling visualization of the cerebrospinal fluid (CSF)-containing structures, and highlighting smaller entities, such as vessels, cranial nerves, and spinal nerve roots that traverse that fluid. IR sequences alter these basic T1 and T2 images by suppressing signal from fat (called short tau inversion recovery [STIR]) or fluid (fluid attenuation inversion recovery [FLAIR]), respectively. STIR is excellent at highlighting disease states that replace fat, such as metastases or infection in fatty bone marrow. FLAIR is excellent at highlighting brain parenchymal disease which may be isointense to CSF on routine T2, such as tumor, infection, inflammation, and hemorrhage in certain stages. GRE sequences are more sensitive to magnetic susceptibility effects, which result in faster T2* relaxation and, in turn, reduction in signal. It is this prominent reduction in signal which makes GRE sequences particularly sensitive for the detection of blood products, iron deposition, and calcification. DWI sequences are based on the detection of the random motion of water molecules within tissue. Pathologic processes such as infarction or abnormal viscous fluids such as pus will exhibit restricted diffusion and appear bright on DWI and dark on ADC images. Diffusion tensor imaging (DTI) is an extension of DWI which allows for the depiction of white matter tracts. A 3D representation of white matter tracts is referred to as diffusion tractography ( Fig. 3.8 ).
Similar to iodinated contrast with CT, the administration of paramagnetic gadolinium as intravenous contrast markedly improves our sensitivity to pathologic areas of breakdown of the blood-brain barrier, including tumors, infection, and inflammation. Imaging of vessels with MR can be performed without intravenous contrast by using pulse sequences to “label” flowing blood while at the same time suppressing the background tissue signal. These time-of-flight (TOF) techniques can be performed in both 2D and 3D and are particularly useful in patients requiring vessel imaging who cannot receive contrast due to renal insufficiency or allergy. Postcontrast vessel imaging is also used and is usually preferred because is it less prone to flow-related artifact than the TOF techniques.
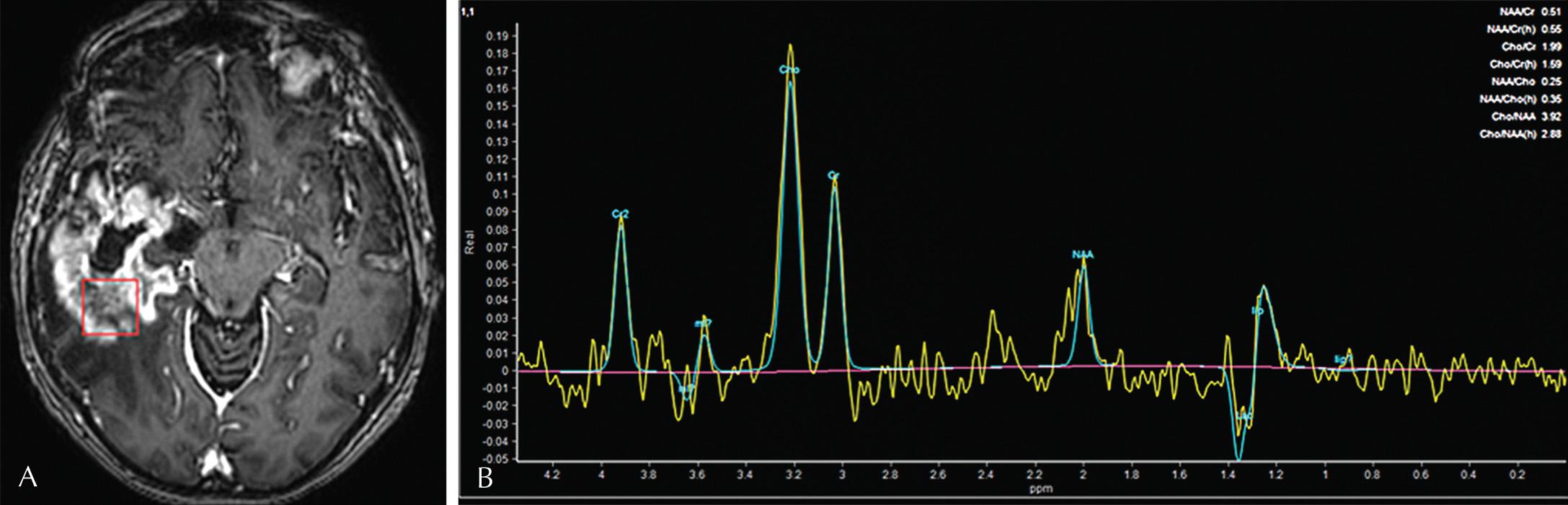
MR spectroscopy (MRS) is used to determine the concentration of metabolites within the tissue in question. The main central nervous system (CNS) metabolites include N-acetyl aspartate (NAA), choline (Cho), creatine (Cr), and lactate. NAA is a marker of neuronal integrity and therefore is the tallest peak in normal brain. Cho is a marker of membrane turnover. Cr is a marker of cellular metabolism. Neoplastic processes with high cellular turnover frequently exhibit elevated Cho peaks ( Fig. 3.9 ).
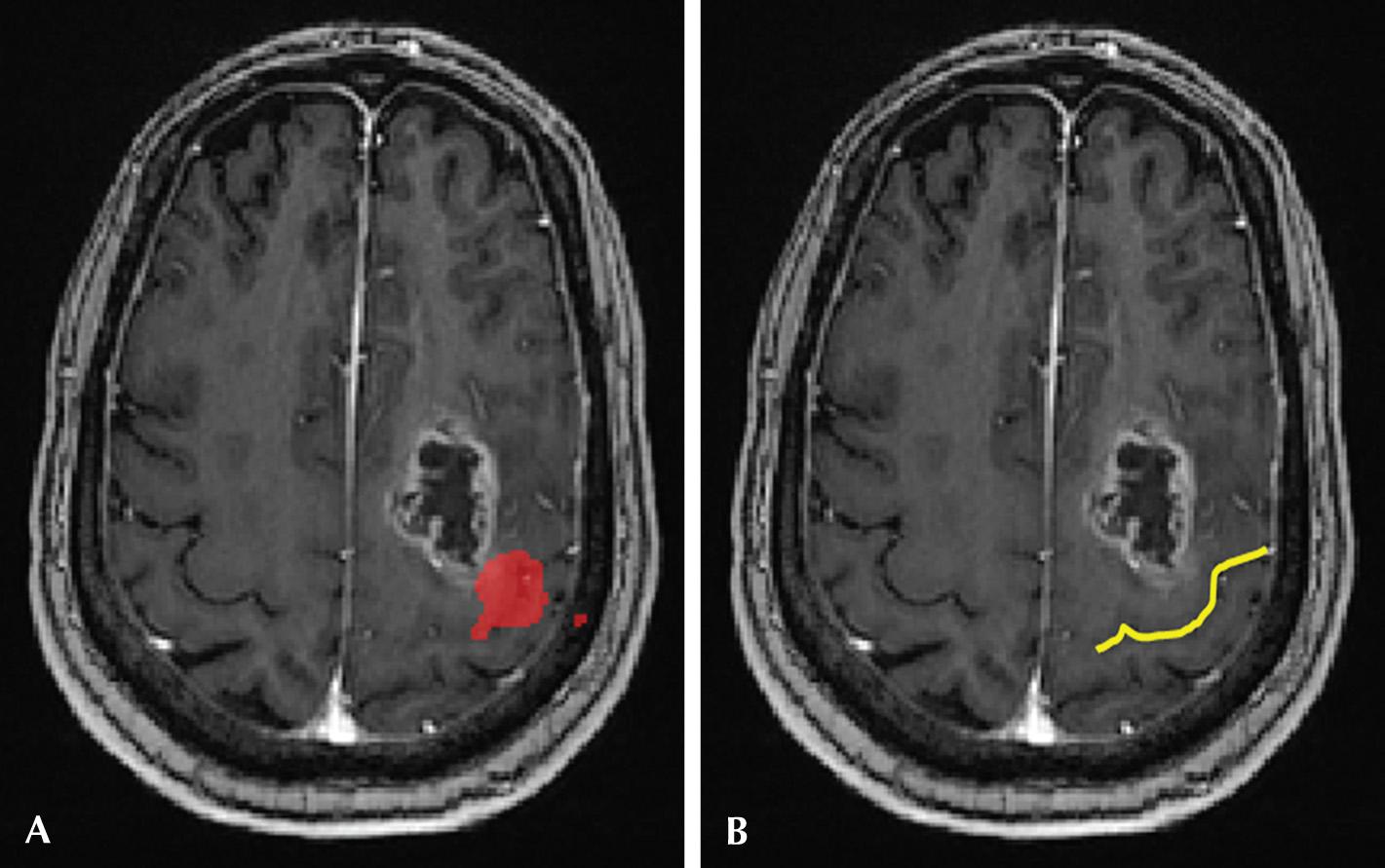
Functional MRI (fMRI) measures brain activity by detecting minute changes in blood flow by exploiting the blood oxygen level–dependent (BOLD) contrast method. Detecting transient changes in blood flow related to neuronal activity helps localize cerebral function and therefore noninvasive assessment of brain function. fMRI is performed while the patient is engaged in neurocognitive tasks which in turn places increased metabolic demands on the activated parts of the brain. This allows us to measure the minute differences in local perfusion and therefore map activity. fMRI has found particular clinical utility in preoperative planning, to allow the surgeon to preserve eloquent cortex while still maximizing tumor resection ( Fig. 3.10 ).
Although the superior contrast resolution of MRI is invaluable in the detection of neurologic abnormalities, the comparatively limited availability, in addition to the time-intensive imaging protocols and degradation due to patient motion, are important considerations for clinicians and patients. In addition, contraindications to scanning such as noncompatible cardiac pacemakers, metallic foreign bodies, noncompatible surgical clips, and monitoring systems are important considerations. The strong magnetic field of an MRI scanner can cause implanted medical devices with metal to malfunction (e.g., pacemakers). Implanted medical devices that are ferromagnetic (such as some aneurysm clips or skin staples) may be dislodged or cause burns secondary to heating.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here