Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An understanding of the development of the nervous system is essential for an understanding of fetal and neonatal neurology. An obvious reason for this contention is the wide variety of disturbances of neural development that are flagrantly apparent in the neonatal period and increasingly diagnosed in the fetal period. In addition, all of the insults that affect the fetus and newborn, which are the subject matter of most of this book, exert their characteristic effects in part because the brain is developing in many distinctive ways and at a very rapid rate. A strong likelihood exists that many of these common insults exert deleterious and far-reaching effects on certain aspects of neural development—effects that until now have escaped detection by available techniques.
In Chapters 1 and 2 , we emphasize the aspect of normal development that has been deranged, the structural characteristics of the abnormality, and the neurological consequences. It is least profitable to attempt to exhaustively characterize all of the presumed causes of these abnormalities of the developmental program. Although a few examples of environmental agents that insult the developing human nervous system at specific time periods and produce a defect are recognized, few of these agents leave an identifying stamp. This obtains particularly because, in the first two trimesters of gestation, the developing brain is not capable of generating the glial and other reactions to injury that serve as useful clues to environmental insults that occur at later time periods. The occasional example of a virus, chemical, drug, or other environmental agent that has been shown to produce a disorder of brain development is mentioned only in passing. However, we emphasize genetic considerations whenever possible because of their importance in parental counseling. Therefore the organizational framework is the chronology of normal development of the human nervous system. A brief review of the major developmental events that occur most prominently during each time period is presented, followed by a discussion of the disorders that result when such development is deranged.
This chapter is devoted to the first major process in human brain development: formation of the neural tube. These early events culminate in formation of the fundamental central neuro-axis. Development of the neural tube and the subsequent development of the prosencephalon (discussed in the next chapter) can be considered the neural components of embryogenesis . In subsequent chapters we discuss later fetal developmental events that lead to the intrinsic structural development of the central nervous system.
Developments in recent years have required a reevaluation of conventional paradigms for developmental disorders of the central neuro-axis. A variety of traditional classification systems have been unsatisfactory, and the persistent inconsistent use of terminology has compromised the diagnostic and prognostic accuracy in clinical practice. New insights from animal models, advanced fetal imaging, and fetal intervention trials for neural tube disorders have led to the notion of primary and secondary consequences of these conditions. For example, the unifying hypothesis for open neural tube defects proposes that the primary failure of spinal closure leads to cerebrospinal fluid (CSF) leakage, which in turn leads to the secondary consequences of posterior fossa underdevelopment, hindbrain herniation, and the development of hydrocephalus. More precise diagnostic paradigms consider the germ cell layers involved (i.e., ectodermal, neuroectodermal, or mesodermal) in the primary dysgenetic lesion, as well as the secondary effects of trauma, toxicity , and mechanical disruption of subsequent development. For example, it is now proposed that the primary defect in anencephaly is likely not failure of neurulation but rather failure of normal cutaneous and mesenchymal development (skin, bone, and dural coverings), with a secondary degeneration of the exposed neural tissue. Similarly, lesions such as meningoceles with no neural involvement are often still classified as closed neural tube defects although their etiology is unrelated to neurulation.
The major developmental events and their peak times of occurrence are shown in Table 1.1 . The time periods are those during which the most rapid progression of the developmental event occurs. Although some overlap exists among these time periods, it is valid and convenient to consider the overall maturational process in terms of a sequence of individual events. In a discussion of the timing of the disorders, the time periods shown in Table 1.1 are obviously of major importance. Nonetheless, it is necessary to recognize that an aberration of a developmental event need not be caused by an insult impinging at the time of the event . Thus a given malformation may not have its onset after the developmental event is completed, but the developmental program may be disturbed at any time before the event is under way. The concept of a termination stage refers to the time in the development of an organ after which a specific malformation cannot occur by any teratogenic mechanism. Thus in the discussion of timing of malformations, we state that the onset of a given defect could occur no later than a given time. Note that in this text the timing of events is based on the postconceptional (p/c) age, rather than the postmenstrual or gestational age.
| MAJOR DEVELOPMENTAL EVENT | PEAK TIME OF OCCURRENCE |
|---|---|
| Primary neurulation | 3–4 weeks of gestation |
| Prosencephalic development | 2–3 months of gestation |
| Neuronal proliferation | 3–4 months of gestation |
| Neuronal migration | 3–5 months of gestation |
| Organization | 5 months of gestation to years postnatally |
| Myelination | Birth to years postnatally |
Formation of the neural tube and its coverings proceeds through four phases ( Table 1.2 ), namely, gastrulation, primary neurulation, secondary neurulation, and dorsal midline closure of the mesodermal-cutaneous ectodermal layers. Gastrulation is the fundamental formation of the trilaminar plate between days 16 and 18 p/c during which the endodermal, mesodermal, and ectodermal layers of the embryo become distinct. The ectodermal layer differentiates into cutaneous and neural ectoderm. The neural ectoderm develops in the longitudinal plane along the dorsal surface of the embryo as the neural plate. Neurulation refers to the inductive events that occur in the neural plate that result in formation of the neural tube, which ultimately gives rise to the entire central nervous system (CNS). Neurulation can be divided into primary neurulation —that is, formation of brain and spinal cord down to the sacral level—and secondary neurulation; that is, events related to the later formation of the sacrococcygeal segments of the spinal cord. With fusion of the neural folds there is separation of the cutaneous and neural ectoderm, with the ingrowth of the mesodermal layers between them; the timing of these events follows closely upon the progressive closure of the neural tube. Primary neurulation and secondary neurulation (and related junctional neurulation) are discussed separately.
| MAJOR DEVELOPMENTAL PHASE | PEAK TIME OF OCCURRENCE |
|---|---|
|
|
By the end of gastrulation, the most anterior part of the axial mesoderm is the prechordal plate, a critical ventral patterning center for the developing forebrain, and the more posterior parts of the axial mesoderm are made up of the notochord. Primary neurulation is a series of events in the dorsal neural ectoderm of the embryo culminating in formation of the neural tube—rostral to the upper sacral level—and its separation from the other germ cell layers. The critical events are summarized in Box 1.1 . By 18 days p/c the neural plate has been formed by differentiation of the dorsal neural ectoderm from the original ectodermal layer. Next the lateral edges of the neural plate become elevated into neural folds, and the midline of the neural plate invaginates ( Fig. 1.1 ). The neural folds continue to elevate in a dorso-medial direction, until the edges meet in the midline to begin closure of the neural tube. In the human embryo, the first fusion of the neural folds is at the level of the future hindbrain-cervical junction (foramen magnum) and occurs at 22 days p/c. Closure generally proceeds rostrally to form the anterior neural tube (and then the brain) and caudally to form the posterior neural tube (and then the spinal cord), although it is not a simple, zipper-like process. The anterior neuropore of the neural tube closes at approximately 24 days, and the posterior neuropore closes at approximately 26 days, at which point primary neurulation is complete. During closure of the neural tube, cells from the dorsal-most region become separated from the neural tube to form the neural crest, which in turn gives rise to the future craniofacial bony structures, dorsal root ganglia, sensory ganglia of the cranial nerves, autonomic ganglia, Schwann cells, and cells of the pia and arachnoid (as well as melanocytes, cells of the adrenal medulla, and certain skeletal elements of the head and face). Finally, the neural tube becomes physically separated from the meso-endoderm and cutaneous ectoderm, a process called disjunction (see Table 1.2 ). Ongoing interaction between the neural tube and surrounding mesoderm gives rise to the dura and axial skeleton (i.e., the skull and the vertebrae). Understanding this sequence of normal differentiation of the different germ cell layers is critical to an understanding of the different features of cranial and spinal dysraphism.
3–4 weeks of gestation
Notochord, chordal mesoderm → neural plate → neural tube, neural crest cells
Neural tube → brain and spinal cord → dura, axial skeleton (cranium, vertebrae), dermal covering
Neural crest → dorsal root ganglia, sensory ganglia of cranial nerves, autonomic ganglia, and so forth
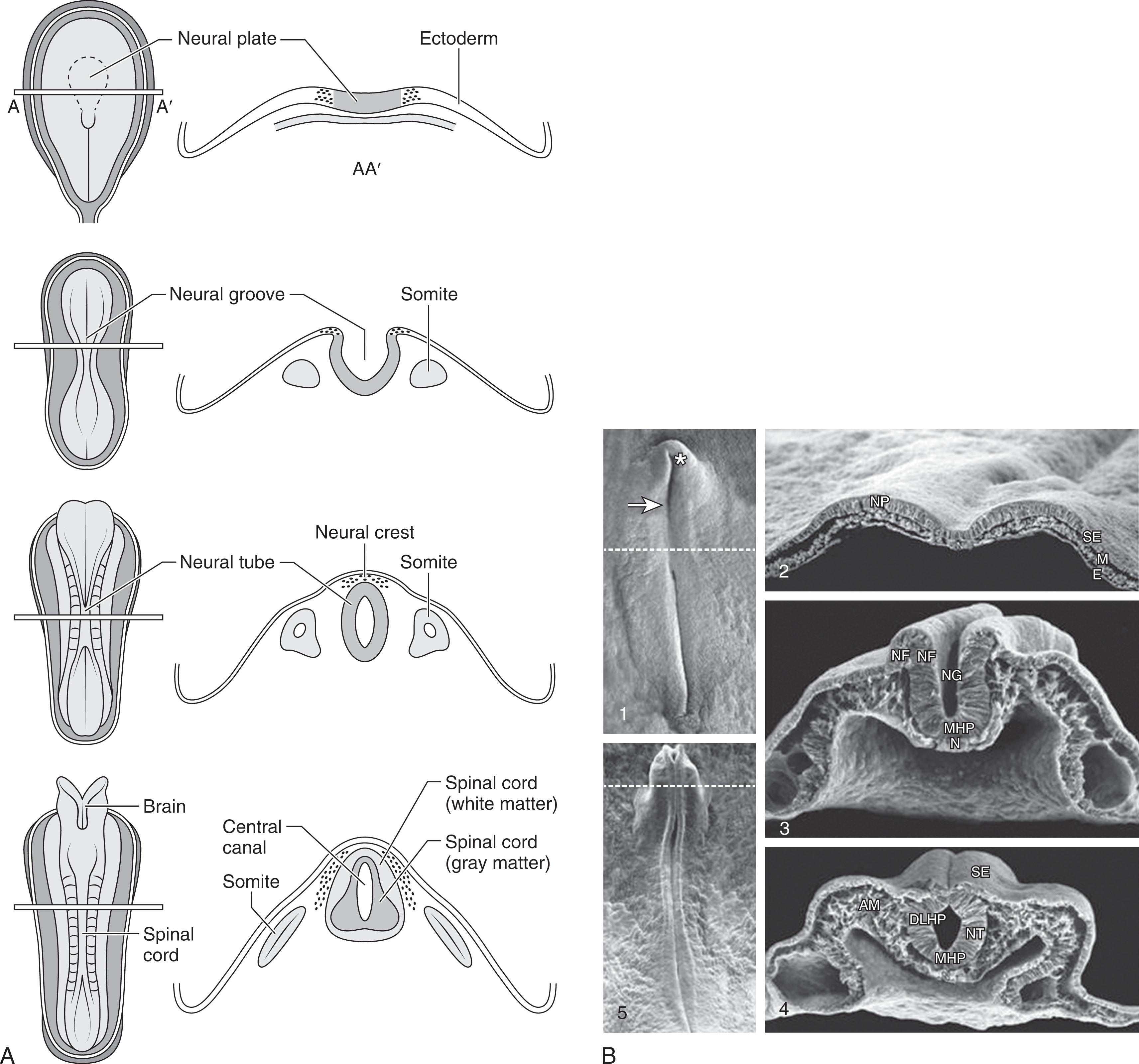
Primary neurulation occurs under the induction of the underlying notochord and chordal mesoderm during the third and fourth weeks p/c (see Box 1.1 and Figs. 1.1A and B ). The neural plate deformations required for development of the neural folds, and subsequently the neural tube, are mediated by a variety of cellular and molecular mechanisms, the most important of which involve the cytoskeletal network of microtubules and microfilaments. Under the influence of vertically oriented microtubules, cells of the developing neural plate elongate, and contraction of actin microfilaments arranged circumferentially around the apical portions of the cells results in cells with a broad base and narrow apex. These forces on the neural plate result in invagination of its midline, dorsomedial folding of its edges, and closure to form the neural tube (see Figs. 1.1A and B ). The process of neural fold bending in a dorsomedial direction also appears to involve differential proliferation and translocation of the neuroepithelial cells. Surface glycoproteins, especially cell adhesion molecules important for cell-cell recognition, as well as adhesive interactions with extracellular matrix, mediate fusion of the opposing neural folds. Other critical molecular events include action of the products of certain regional patterning genes (especially bone morphogenetic proteins and sonic hedgehog), homeobox genes, surface receptors, and transcription factors.
Secondary neurulation is the process of caudal neural tube formation, which commences at completion of primary neurulation; that is, upon closure of the posterior neuropore around the S2 spinal level, on day 26 p/c ( Table 1.2 ). Secondary neurulation occurs in the caudal cell mass and, by forming the remaining sacrococcygeal neural tube, completes neural tube formation. Secondary neurulation gives rise to the conus medullaris and cauda equina, as well as components of the genitourinary tract and hindgut. Starting between 28 to 32 days p/c the caudal cell mass undergoes vacuolation, coalescence, and canalization, processes that culminate by 7 weeks p/c ( Box 1.2 ). At this point the vacuoles connect to the central canal of the neural tube previously formed by primary neurulation. Not infrequently, accessory lumens remain and may be important in the genesis of certain anomalies of neural tube formation (see later). After canalization the caudal cell mass undergoes retrogressive differentiation between 7 weeks p/c and into postnatal life (see Table 1.2 ). At 8 weeks p/c the spinal cord tissue extends the entire length of the spinal column. Subsequent disproportionate growth of the spinal column results in relative ascent of the conus medullaris (which contains the ventriculus terminalis), leaving the filum terminale in its wake. As a result the conus ascends to the level of L3 by 40 weeks, reaching its final level of L1–L2 by 3 months postnatal.
Canalization: 4–7 weeks of gestation
Retrogressive differentiation: 7 weeks of gestation to after birth
Canalization: undifferentiated cells (caudal cell mass) → vacuoles → coalescence → contact central canal of rostral neural tube
Retrogressive differentiation: regression of caudal cell mass → ventriculus terminalis, filum terminale
The terminology used to describe embryonic anomalies of craniospinal development is inconsistent and often imprecise, which in turn has compromised diagnosis and counseling. To remedy this situation, we first review the definitions and categorization to be used in this text. The term dysraphism is best understood by considering its root (i.e., raphe ), which is defined as a line of union between two contiguous bilaterally symmetrical structures. Dysraphism is therefore a failure of this process and in its broadest sense includes any incomplete midline closure of the developing head and spine and may involve the mesenchymal and ectodermal structures individually or in combination. Embryologically, dysraphic states of the central neuroaxis can be divided into those that occur (i) preneurulation (during gastrulation) and involve the neurenteric canal; (ii) during primary neurulation, forming the vast majority of open neural tube defects; (iii) during secondary neurulation, with disturbed development of the caudal cell mass, which is responsible for most closed neural tube defects; or (iv) during midline closure of the mesoderm and cutaneous ectoderm. Spina bifida refers only to defects of vertebral arch formation (see below); subtyping of spina bifida is based on the presence and nature of associated neuroectodermal malformations. Isolated vertebral arch dysraphism without underlying neural defects or cystic evagination of the meninges or cord results in true spina bifida occulta .
Neural tube defects refers to a disturbance in neuroectodermal development, defined embryologically as defects of primary or secondary neurulation. Anatomically, neural tube defects can be further categorized by their location relative to the first fusion point of the neural tube, at the level of the future foramen magnum. Lesions of the anterior neural tube (rostral to the foramen magnum) lead to cranial dysraphism, whereas those of the posterior neural tube (caudal to the foramen magnum) lead to spinal dysraphism . Recent research has shown that secondary neurulation is more complex than previously thought and that there is overlap between primary and secondary neural tube closure (see later).
The distinction between open or closed dysraphic lesions is important for understanding the primary lesion and its secondary complications. Open neural tube defects have at least some continuity between the external surface of the fetus and the underlying neural tissue and at least intermittent CSF leakage. In addition, open neural tube defects are usually associated with other CNS anomalies, including hindbrain, callosal, and cerebral cortical malformations. Closed neural tube defects are skin covered, with no exposed neural tissue and no CSF leak; the defect is confined to the spine and other associated CNS anomalies are rare. As a general rule , most open neural tube defects result from disturbed primary neurulation, whereas most closed neural tube defects result from disturbed secondary neurulation, although there are notable exceptions to this rule . For example, higher (thoracic and cervical) myelomeningoceles may be skin-covered (such as cervical myelocystoceles) and sacral lesions are occasionally open.
Disorders of primary neurulation are discussed in order of decreasing severity, starting with complete failure of neural tube formation (craniorachischisis totalis), followed by disorders of anterior neural tube formation (cranial dysraphism) and disorders of posterior neural tube formation (spinal dysraphism).
Craniorachischisis totalis ( Box 1.3 ) results from essentially total failure of neurulation at a very early stage, leaving an exposed neural plate–like structure (with no overlying axial skeleton or dermal covering) involving the entire dorsal extent of the central neuroaxis ( Fig. 1.2 ). Because the neural plate is formed by 18 days p/c and first point of closure of the neural tube occurs at 22 days p/c, the onset of craniorachischisis totalis is estimated to be no later than 20 to 22 days of gestation. The precise incidence of this lesion is unknown because most cases are aborted spontaneously in early pregnancy and only a few have survived to early fetal stages.

Anencephaly (see Box 1.3 ; Figs. 1.3 and 1.4 ) has traditionally been classified as an anterior neural tube defect. However, based on human and animal observations, some consider this lesion to be a primary defect in formation of the cranial vault and its coverings, with secondary degeneration of the cranial neural contents. Specifically, it is proposed that anencephaly results primarily from (partial) absence of the cranial vault (acrania), with initial protrusion of the early fetal brain above the remaining skull bones (exencephaly) and subsequent degeneration of the underlying telencephalic mantle due to direct exposure to the amniotic fluid. According to this viewpoint, anencephaly is not a true neural tube defect because the primary lesion results from a skeletal (mesodermal) defect. Instead, the underlying pathogenetic mechanism of anencephaly invokes a “two-hit” hypothesis similar to that discussed for myelomeningoceles later. This notion that anencephaly is not a true neural tube defect is not universally held. The cranial defect usually involves the frontal bones above the supraciliary ridge, the parietal bones, and the squamous part of the occipital bone, and in the most severe cases, the cranial vault abnormality extends back to, or through (holoacrania or holoanencephaly), the foramen magnum. Defects stopping short of the foramen magnum are known as meroacrania or meroanencephaly. The underlying neural tissue defect in anencephaly most commonly involves the forebrain and variable amounts of upper brainstem (see Figs. 1.3 and 1.4 ), leaving a residual degenerated mass of hemorrhagic, fibrotic, and neuroglial tissue with little definable structure. Onset of anencephaly is estimated to be no later than 24 days of gestation. Polyhydramnios is a frequent feature.

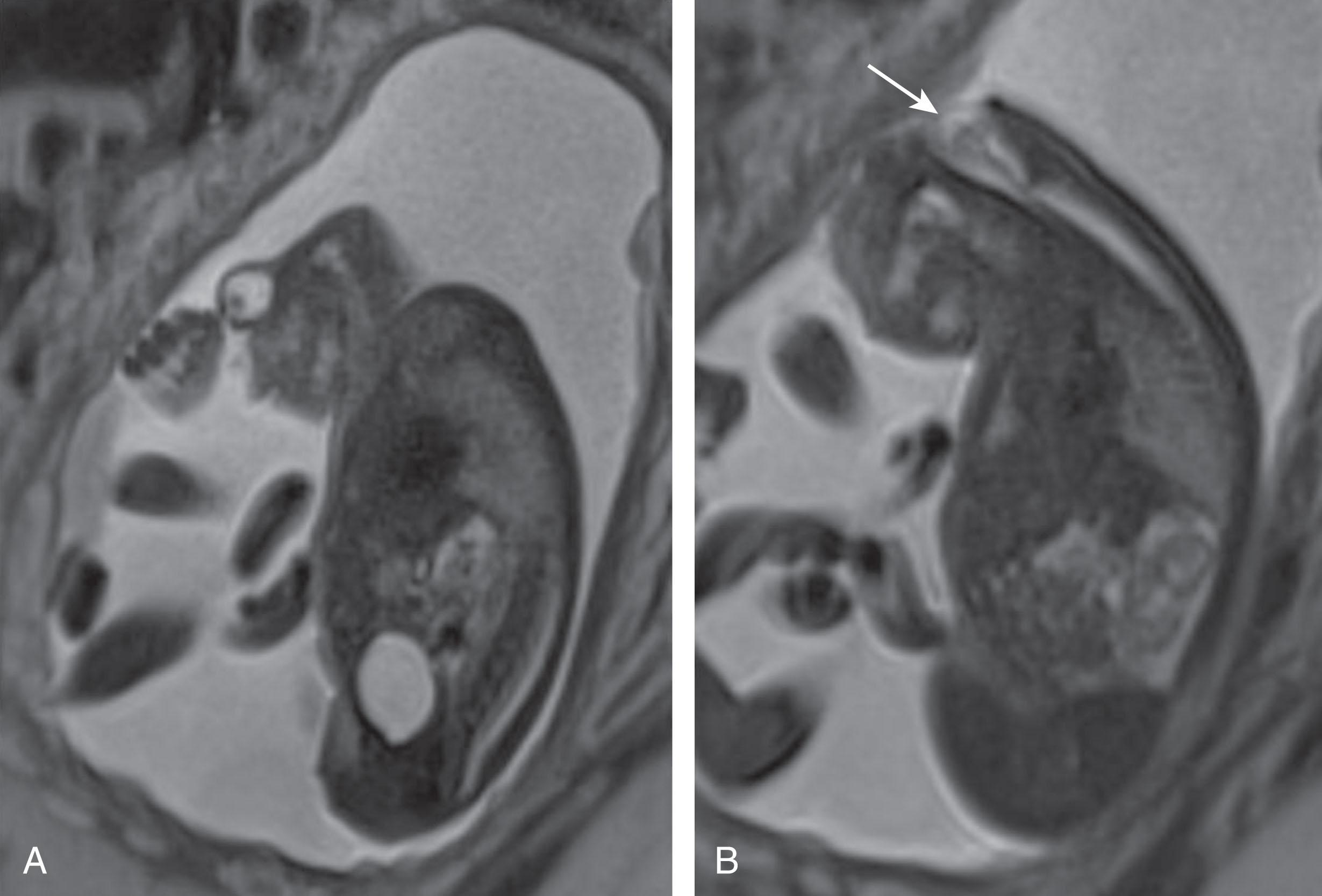
Approximately 75% of anencephalic infants are stillborn, and the remainder die in the neonatal period. The disorder is not rare, and epidemiological studies reveal striking variations in prevalence as a function of geographical location, sex, ethnic group, race, season of the year, maternal age, social class, and history of affected siblings. The risk increases with decreasing social class and with a history of affected siblings in the family. Since the late 1970s, the incidence of anencephaly in the United States, like that of myelomeningocele (see later), declined to approximately 0.2 per 1000 live births in 1989, remaining relatively stable since then, with the most recent estimate (2009–2011) of 0.28 per 1000 live births. Both genetic and environmental influences appear to operate in the genesis of anencephaly (see later discussion of myelomeningocele). Recently, maternal use during pregnancy of the selective serotonin reuptake inhibitor paroxetine has been implicated in the development of anencephaly.
Because the skull bones begin to ossify around 11 weeks p/c, the cranial defect is readily identifiable by second trimester fetal ultrasound. In fact, the proportion of anencephaly detected prenatally has been reported to be as high as 96% to 100%. Systematic prenatal detection and elective termination of pregnancy complicated by anencephaly has resulted in a sharp decline in the number of live-born cases.
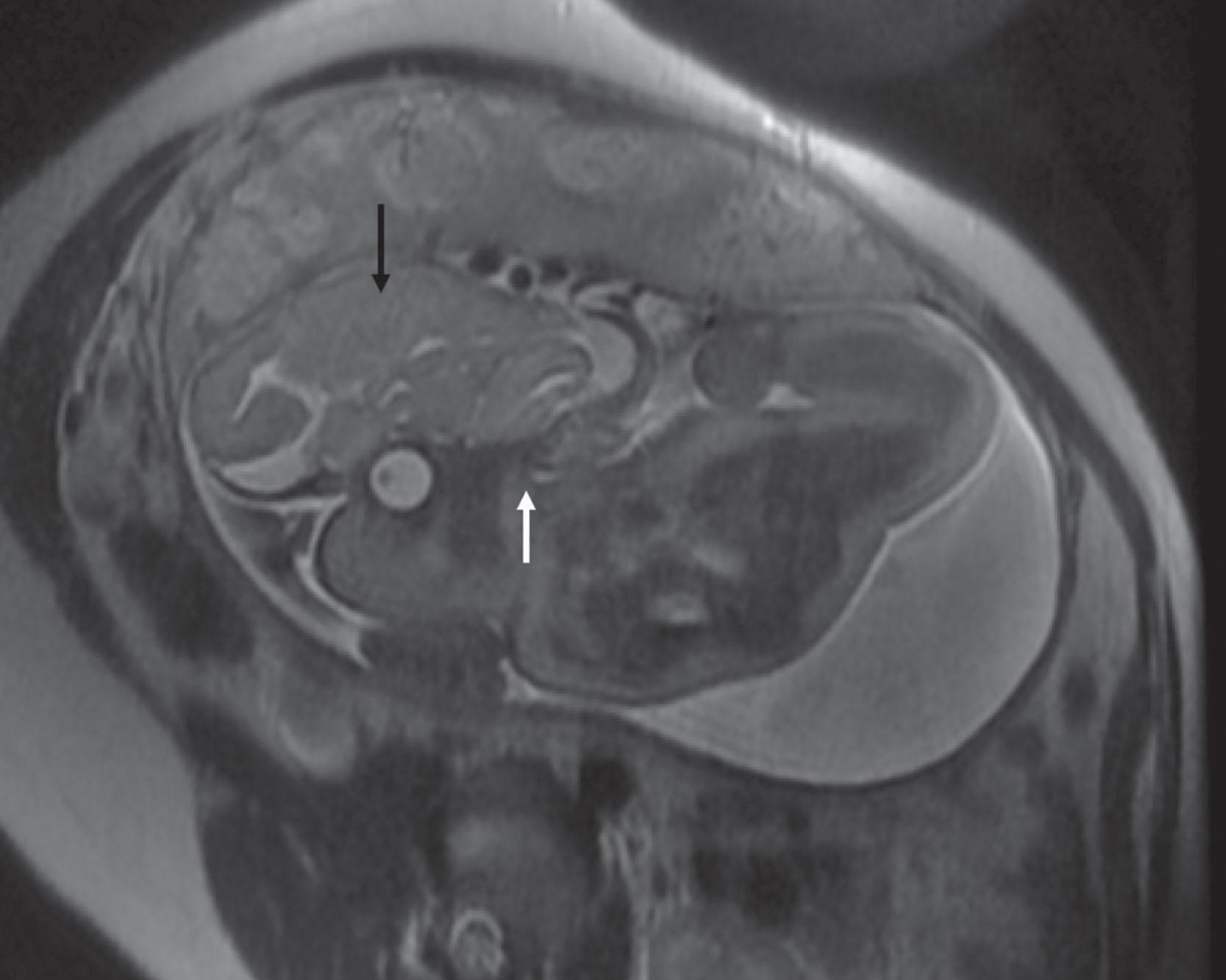
Encephaloceles (see Box 1.3 ) were previously considered a restricted disorder of neurulation involving anterior neural tube closure , although the precise pathogenesis of these lesions remains unknown. It may be better to consider these lesions as developmental disorders of cranial mesoderm in which the cranial defect is associated with a cystic extracranial extension of meninges, neural tissue, and CSF. The notion that encephaloceles are not disorders of primary neurulation is supported by the fact that the herniated brain parenchyma is relatively normal and shows no evidence of defective neurulation. Many encephaloceles are skin-covered—that is, closed—lesions. When the cystic lesion includes parts of the ventricular system, the term meningoencephalocystocele is used ( Fig. 1.5 ). Lesions that involve primarily or only the overlying meninges or skull, without obvious inclusion of neural elements, are called cranial meningoceles ( Fig. 1.6 ); these are thought to be later in onset and account for up to 20% of cystic occipital lesions. Encephaloceles occur most commonly (70% to 80%) in the occipital region ( Fig. 1.7 ), less commonly in the frontal ( Fig. 1.8 ) and frontoparietal regions ( Fig. 1.9 ), and least commonly in the temporal and parietal regions. Frontal encephaloceles may protrude into the nasal cavity (fronto-ethmoidal encephaloceles; Fig. 1.10 ). Fronto-ethmoidal encephaloceles may be due to disturbed separation (nondisjunction) of the neural ectoderm from the cutaneous ectoderm during final closure of the rostral neuropore. In the typical occipital encephalocele, the protruding brain is usually derived from the occipital lobe and may be accompanied by dysraphic disturbances involving cerebellum and superior mesencephalon. The neural tissue in an encephalocele usually connects to the underlying CNS through a narrow neck of tissue. The protruding mass is usually represented by a closed neural tube with cerebral cortex, exhibiting a normal gyral pattern, and subcortical white matter. As many as 50% of cases are complicated by hydrocephalus. Encephaloceles located in the low occipital (below the inion) or high cervical regions and combined with deformities of lower brainstem and of base of skull and upper cervical vertebrae characteristic of the Chiari type II malformation (associated with myelomeningocele [see later]) comprise the Chiari type III malformation. This type of encephalocele contains cerebellum in virtually all cases and occipital lobes in approximately one half of cases ( Fig. 1.11 ). Partial or complete agenesis of the corpus callosum occurs in two-thirds of cases. Venous structures may be included in the cyst, and anomalous venous drainage (aberrant sinuses and deep veins) is present in about one-half of patients and may complicate the surgical approach to these lesions.






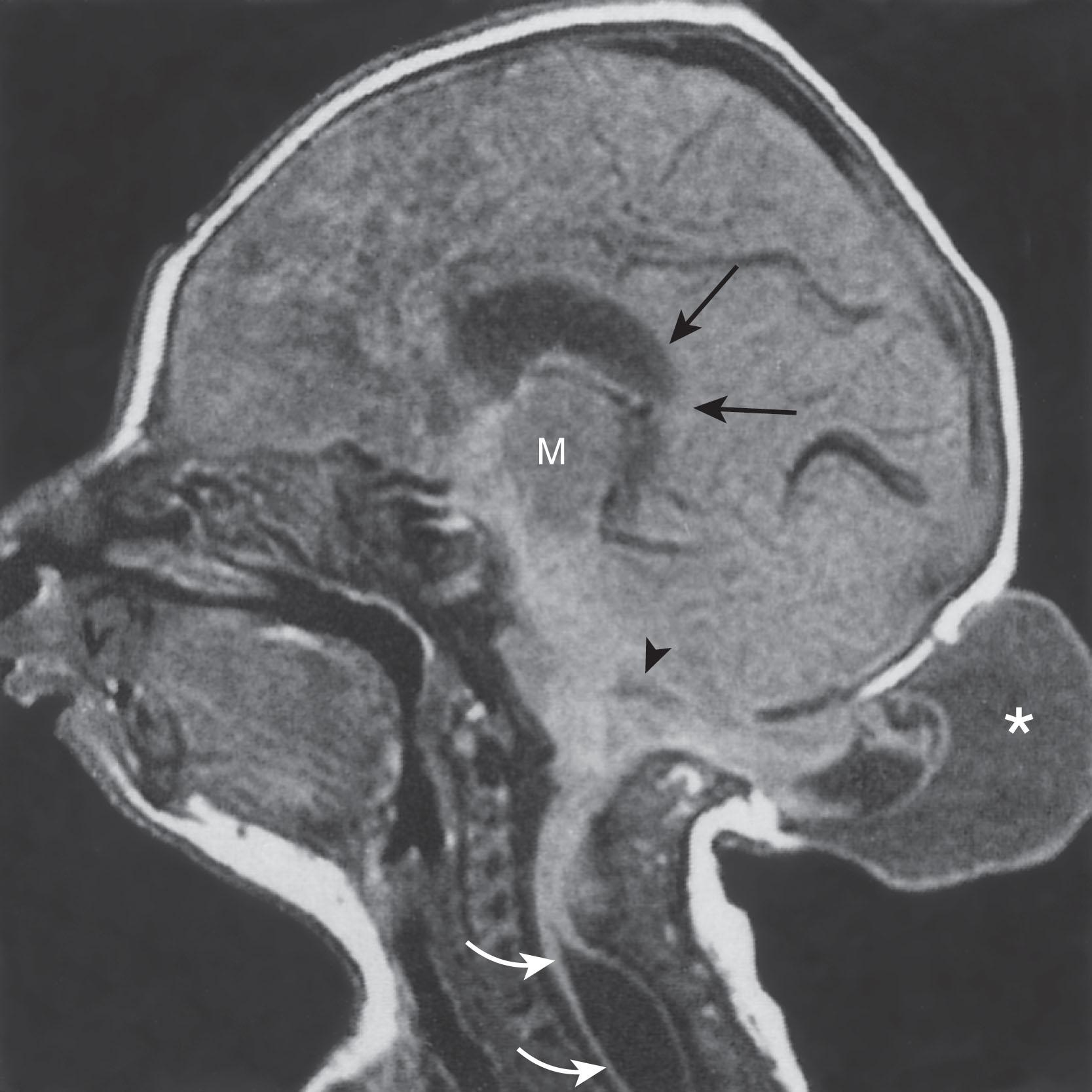
When there is a combination of the bony defects in the occipital cranium and the upper cervical vertebrae, with or without an encephalocele, plus marked hyperextension of the neck, the term iniencephaly is used ( Fig. 1.12 ). This condition is associated with multiple other somatic malformations and is almost uniformly fatal.
Onset of the most severe lesions is probably no later than the approximate time of anterior neural tube closure (24 days p/c) or shortly thereafter. Infants with encephaloceles not uncommonly exhibit associated malformations. A frequently co-occurring CNS anomaly is subependymal nodular heterotopia. The most commonly recognized syndromes associated with encephalocele are Meckel syndrome (characterized by occipital encephalocele, microcephaly, microphthalmia, cleft lip and palate, polydactyly, polycystic kidneys, ambiguous genitalia, other deformities) and Walker-Warburg syndrome (see Chapters 6 and 37 ). These disorders, as well as several other less common syndromes associated with encephalocele, are inherited in an autosomal recessive manner. Maternal hyperthermia between 20 and 28 days of gestation has been associated with an increased incidence of occipital encephalocele, as well as with other neural tube defects (see later).
Skin-covered encephaloceles have normal maternal serum and amniotic fluid alpha-fetoprotein (AFP) levels. Diagnosis by fetal ultrasonography in the second trimester has been well documented. As with myelomeningoceles, open encephaloceles are often associated with decreased extra axial CSF spaces by fetal imaging. Diagnosis before fetal viability has been followed by elective termination; later diagnosis may require delivery by cesarean section.
Neurosurgical intervention is indicated in most patients. Exceptions include those with massive lesions and marked microcephaly. Surgery is necessary in the neonatal period for ulcerated lesions that are leaking CSF. An operation can be deferred if adequate skin covering is present. Preoperative evaluation has been facilitated by the use of computed tomography (CT) and, in particular, magnetic resonance imaging (MRI) scans. Outcome is difficult to determine precisely because of variability in selection for surgical treatment. In a combined surgical series of 40 infants, 15 infants died (38%), many whose complications can be managed more effectively now in neurosurgical facilities. Of the 25 survivors, 14 were of normal intelligence (56%), although often with motor deficits, and 11 exhibited both impaired intellect and motor deficits (44%). Not surprising, prognosis varies inversely with the extent of herniated neural tissue, with cranial meningoceles (i.e., no obvious neural tissue in the cyst) having the best prognosis. Outcome is more favorable for infants with anterior encephaloceles than those with posterior encephaloceles. Thus in one series of 34 cases, mortality was 45% for infants with posterior defects and 0% for those with anterior defects. Normal outcome occurred in 14% of the total group with posterior defects and in 42% of those with anterior defects.
In the following, we first consider disorders of primary neurulation, which are usually open neural tube defects and originate earlier in embryonic development. Disorders of primary neurulation are by far the most common and clinically relevant spinal dysraphic conditions. Thereafter we discuss disorders of secondary neurulation, which more commonly result in closed neural tube defects.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here