Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
One of the first observations of a potential neural control of kidney function was made by Claude Bernard in the middle of the 19th century, who reported a unilateral diuresis following section of the greater splanchnic nerve of the anesthetized dog. However, the critical role of the sympathetic nervous system in the control of renal function was long questioned due to the views of Homer Smith, who regarded the renal innervation to be of little importance in determining the function of the kidney. It was not until the 1960s that the innervation of the kidney and its regulation of renal function came under detailed scrutiny. Over the following three-to-four decades a wealth of information has been generated and concepts developed which have provided the foundation for how the renal sympathetic nerves regulate all aspects of vascular, tubular, and secretory functions of the kidney, both normally and in pathophysiological states of cardiovascular diseases. A number of major reviews have brought together the most significant pieces of information about the renal sympathetic nerves over this period. This knowledge is now being translated into therapeutic approaches in man, particularly in relation to hypertension, heart failure, and renal disease. The recent reports by Esler and co-workers that bilateral renal denervation in patients with resistant hypertension resulted in a profound and sustained (≥2 years) reduction in blood pressure reinforces the important contribution of the autonomic control of the kidney in determining cardiovascular homeostasis and how it may be involved in pathophysiological states. These findings are set to reinforce the drive to gain knowledge of both the efferent and afferent innervations of the kidney, and to stimulate the development of further therapeutic avenues to modulate the neural control of the kidney.
One of the first observations of a potential neural control of kidney function was made by Claude Bernard in the middle of the 19th century, who reported a unilateral diuresis following section of the greater splanchnic nerve of the anesthetized dog. However, the critical role of the sympathetic nervous system in the control of renal function was long questioned due to the views of Homer Smith, who regarded the renal innervation to be of little importance in determining the function of the kidney. It was not until the 1960s that the innervation of the kidney and its regulation of renal function came under detailed scrutiny. Over the following three-to-four decades a wealth of information has been generated and concepts developed which have provided the foundation for how the renal sympathetic nerves regulate all aspects of vascular, tubular, and secretory functions of the kidney, both normally and in pathophysiological states of cardiovascular diseases. A number of major reviews have brought together the most significant pieces of information about the renal sympathetic nerves over this period. This knowledge is now being translated into therapeutic approaches in man, particularly in relation to hypertension, heart failure, and renal disease. The recent reports by Esler and co-workers that bilateral renal denervation in patients with resistant hypertension resulted in a profound and sustained (≥2 years) reduction in blood pressure reinforces the important contribution of the autonomic control of the kidney in determining cardiovascular homeostasis and how it may be involved in pathophysiological states. These findings are set to reinforce the drive to gain knowledge of both the efferent and afferent innervations of the kidney, and to stimulate the development of further therapeutic avenues to modulate the neural control of the kidney.
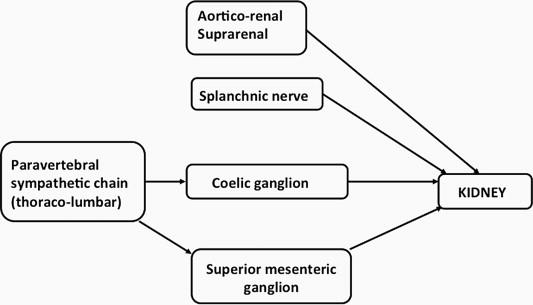
The efferent innervation of the kidney comprises pre-ganglionic fibers which arise from spinal segments T 11 –L 3 , and traverse to both pre-vertebral (thoraco-lumbar sympathetic chain) and para-vertebral ganglia (aortico-renal, splanchnic, coeliac, superior mesenteric ganglia) which give rise to the post-ganglionic fibers. There is great species variation in the overall contribution from the various ganglia, for example, with 80% arising from the ipsilateral para-vertebral ganglia (T 11 –L 2 ) in the rat and hamster, but only 50% for the cat and monkey (T 11 –L 3 ). Figure 16.1 gives a brief outline of these different neural pathways. Tracer studies with horseradish peroxidase and herpes virus have been used to demonstrate the neural pathways for the sympathetic innervation. The pre-ganglionic sympathetic nerve fibers in the rat are primarily located in the intermediolateral column of the spinal cord, from T 9 –T 13 , suggesting that they descend three to four segments before exiting the spinal column at T 11 –L 3 . A number of nuclei within the central nervous system project to these intermediolateral areas of the spinal cord, including the raphe nuclei, rostral venterolateral medulla, an A5 group, and the paraventricular hypothalamic nucleus. Together, there is a view that descending projections from the supra-spinal systems are the primary regulators of sympathetic outflow to the kidney.
There has been a continuing evaluation of the size and type of nerves forming the efferent innervation of the kidney. Analyses performed in the rabbit utilizing electron microscopy have demonstrated two types of nerve fibers with different diameters in the renal cortex, suggesting that different functionalities may exist within these different nerve populations. Detailed counts of fiber number and diameter in the rat have found that the majority of the fibers are unmylelinated (96%) with the remainder being myelinated. The average diameter of the unmyelinated fibers was approximately 1.3 μm, and while DiBona and colleagues reported a bimodal distribution, this was not observed in the report of Sato and co-workers. More recently, Fazan and colleagues investigated the situation in the mouse and found that, although the nerve fibers had a similar mean diameter, 0.76±0.02 μm, compared to the rat, the distribution was clearly uni-modal. Together, these observations provide no consistent support for the argument that different nerve fibers are directed towards the innervation of specific cell types (vascular, epithelial or granular) in order to control selected functions.
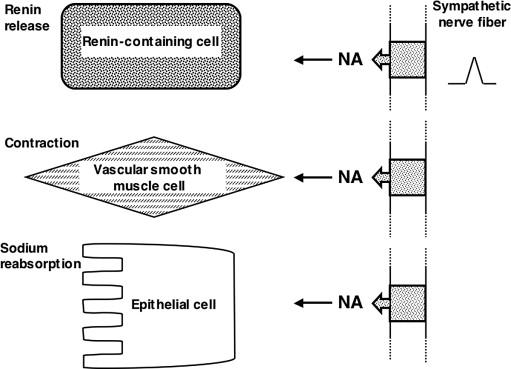
The sympathetic nerves generally traverse from the ganglia, running alongside the renal artery, and enter the hilus of the kidney where they begin to divide, with smaller nerve bundles approximately following the major divisions of the blood vessels. The sympathetic nerves begin to divide into smaller bundles which penetrate and form a network throughout the cortical and juxta-medullary areas. Early studies by Barajas and co-workers demonstrated discrete neuro-effector junctions present at the afferent and efferent arterioles, the granular cells of the juxtaglomerular apparatus, the proximal and distal tubules, and the thick limb of the ascending limb of the loop of Henle. This group of investigators, utilizing electron microscopy, went on to show that the sympathetic nerves were typical autonomic fibers having varacosities associated with the neuro-effector junctions that contained dense cored vesicles. Using a tritiated noradrenaline radiographic approach they clearly showed the presence of noradrenaline, and although there was a suggestion that acetylcholine esterase was present, there was no indication that the fibers were cholinergic. Later, more detailed studies indicated that there was a variation in the number of neuro-effector junctions along the nephron, with the greatest number being at the proximal tubule, fewer at the thick ascending limb of the loop of Henle, and the smallest number at the distal tubules and collecting duct. Interestingly, when calculated as the density of neuro-effector junctions per unit length, the density was found to be greatest in the thick ascending limb of the loop of Henle and progressively less in the distal and proximal tubules. There is also a regional variation, with the innervation being greatest along the cortico-medullary border and becoming less in the outer cortex and deeper regions of the medulla. This regional variation in innervation density is paralleled in both the vascular and tubular structures. Figure 16.2 illustrates the cell types where neuro-effector junctions have been described and the functions they may regulate.
A large body of evidence from biochemical and pharmacological studies indicates that the primary neurotransmitter arising from the sympathetic innervation is noradrenaline. Functional studies have shown that renal denervation, in the initial stages, is associated with a marked decrease in noradrenaline content of some 95%, while activation of the renal sympathetic nerves results in an elevation in noradrenaline production or spillover into the renal venous blood. The role of dopamine and potential dopaminergic nerve-mediated influences remains unclear, as all adrenergic nerve varicosities contain dopamine as an intermediary in noradrenaline biosynthesis, but there is little evidence that dopamine acts as a neurotransmitter in the neural regulation of either renal hemodynamic function, renin release or fluid reabsorption.
Multiple types of adrenoceptors are present within the kidney mediating the actions of noradrenaline released from the nerve varicosities, and a range of α 1 - and α 2 -adrenoceptors subtypes exist along the renal vasculature and nephrons. At a functional level, stimulation of the α-adrenoceptors causes a vasoconstriction of vascular smooth muscle and reabsorption of fluid at tubular epithelial cells. Stimulation of β-adrenoceptors increases renin release at the juxtaglomerular granular cells. A number of molecular biological and cloning investigations have defined α 1 -adrenoceptors into α 1A -, α 1B -, and α 1D -subtypes, α 2 -adrenoceptors into α 2A -, α 2B -, and α 2C -subtypes, and β-adrenoceptors as β 1 -, β 2 -, and β 3 -subtypes, all of which are G-protein coupled receptors comprising a superfamily of membrane proteins that signal the actions of adrenaline and noradrenaline. The α 1 -adrenoceptors utilize a range of signaling pathways including activation of phospholipases A and C, mobilization of intracellular calcium stores, as well as opening of voltage-dependent and independent calcium channels which results in a rapid vascular smooth muscle contraction. Interestingly, noradrenaline binding to α 1 -adrenoceptors also activates the MAP kinase pathways which, in the longer term, are responsible for regulating growth and hypertrophy of the vascular smooth muscle. There are reports that α 1A - and α 1B -adrenoceptors are present to a similar degree in the cortex and outer stripe of the medulla, while in the inner stripe of the medulla the α 1B -adrenoceptor subtype appears to predominate. However, in terms of hemodynamic functionality, α 1A -adrenoceptors are more effective than the α 1B -adrenoceptor subtypes in causing renal vasoconstriction.
α-Adrenoceptors are also present on the epithelial cells of the nephron, where they modulate fluid reabsorption. There is evidence, at least in the proximal tubule, that α 1 -adrenoceptor stimulation engages both the phospholipase C and MAP kinase pathways, but these signal to different end-points, that is to the sodium/hydrogen exchanger, isoforms-1 (NHE1) and isoform-3 (NHE3), respectively. At the more distal sections of the nephron, the distal tubule and collecting duct, α 2 -adrenoceptors are the primary subtype, and in this segment their activation results in a decrease in cAMP which blunts the action of other factors signaling through this pathway. A key example is AVP, which stimulates both water reabsorption and ENaC insertion in this region where these actions are blunted by α 2 -adrenoceptor agonists. β 1 -adrenoceptors are found primarily on the granular renin-containing cells of the afferent arteriole, and their activation stimulates adenylyl cyclase which increases intracellular levels of cAMP. The β 2 -adrenoceptors found on the tubules, primarily collecting duct, also utilize cAMP production as the signaling molecule, but their function at this site has not been resolved.
Activation of the various signaling pathways via ligand binding to the adrenoceptors increases fluid reabsorption at the level of the proximal tubule. In the proximal tubule, the catecholamines stimulate Na/K-ATPase at the basolateral membrane, resulting in increased sodium reabsorption from the tubular lumen across the apical membrane. There is evidence from micropuncture studies, as well as in vitro studies, that the sodium–hydrogen exchanger is activated, particularly isoform 3 (NHE3), the isoform primarily responsible for the regulation of sodium entry into the epithelial cells at the proximal tubule. The NHE3 protein in the proximal tubule appears to be present in the microvillae, either incorporated into the plasma membrane where it is likely to be active or internally in the subapical vesicles, where it is considered to be inactive in terms of a transporting protein. The mechanisms involved in the translocation of the NHE3 from the plasma membrane into the subapical vesicles are complex and require the interaction of a number of proteins (NHERF1, myosin VI, ezrin, raft, non-raft, and myosin). McDonough has recently reviewed the evidence showing that when blood pressure is elevated acutely or when the sympathetic nervous system is reflexly activated there is increased movement of NHE3 into the subapical compartment where the transporter is inactive. McDonough and co-workers have put forward the concept that translocation of the NHE3 into the subapical regions is a key element whereby sodium reabsorption may be regulated by catecholamines, both in the short-term as well as over a longer timeframe, thereby contributing to cardiovascular homeostasis.
The interstitium of the kidney contains a complex milieu of hormones and factors which may vary across the cortex and medulla, but can determine the level of functions of all cell types, vascular smooth muscle, renin containing, and epithelial cells. There are modulator influences at the neuro-effector junction which can come into play and influence the amount of noradrenaline released in response to depolarization caused by the passage of an action potential. An outline of potential interactions with various agents is illustrated in Figure 16.3 .
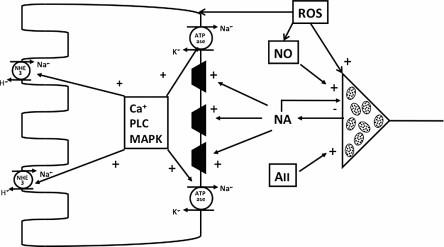
At an early stage it was recognized that pre-synaptic α 2 -adrenoceptors were able to act in an auto-inhibitory fashion, whereby when activated by noradenaline released into the neuro-effector junction, they decreased neurotransmitter release caused by subsequent depolarizations. There is evidence that this situation pertains at the kidney. α 2 -adrenoceptors are present in the kidney, and their blockade enhances noradrenaline release and renal nerve induced vasoconstriction in the dog. In the rabbit blockade of α 2 -adrenoceptors peripherally has little effect on renal nerve-induced vasoconstrictions, but potentiates the renal nerve-mediated antidiuresis and antinatriuresis.
A second important neuro-modulator is angiotensin II, which is present in the renal interstitium at high levels in conditions of increased endogenous angiotensin II production. Activation of pre-synaptic AT-1 receptors facilitates the release of noradrenaline. This was first reported by Boke and Malik using the isolated perfused rat kidney. They found that renal nerve-induced catacholamine release into the renal vein was facilitated when angiotensin II was added to the perfusate. At a functional level, studies in the anaesthetized rat using both direct and reflex activation of the renal nerves, at levels which had little or no effect on renal hemodynamics, caused decreases in fluid excretion which were blocked in the presence of an angiotensin-converting enzyme inhibitor, and restored following administration of exogenous angiotensin II. Similar observations were reported by Veelken and colleagues in the conscious rat, where administration of the AT1-receptor antagonist ZD 7155 blunted the renal nerve-dependent antidiuresis and antinatriuresis in response to an air-jet stress test. Together, these findings support the view that at both renal vascular and epithelial cell neuro-effector junctions occupation of pre-synaptic AT1 receptors enhances neurotransmission. It would seem that these receptors are fully occupied, even at low endogenous levels of angiotensin II and under normal conditions the facilitation is maximal but when production is prevented, then neurotransmission will be blunted. There may be other interactions at the post-synaptic membranes of the epithelial cells. Quan and colleagues demonstrated that the ability of angiotensin II to stimulate proximal tubule fluid reabsorption was blunted following acute renal denervation, but augmented if the renal sympathetic nerves were stimulated at low levels not affecting filtration rate. A comparable interaction was reported by Abdulla and co-workers, who found that the renal vasoconstriction caused by close renal arterial infusion of angiotensin II was blunted following acute renal denervation. Thus, these observations demonstrate that angiotensin II has modulatory activities at the neuro-effector junction, both pre-synaptically and post-synaptically, which determine the effectiveness of the neural control of renin release, fluid reabsorption, and vascular tone in the kidney.
A third potentially important factor determining noradrenaline release, and hence its impact on functional end-points, is nitric oxide (NO). All isoforms of nitric oxide synthase (NOS), endothelial (eNOS), neuronal (nNOS), and inducible (iNOS) are present in the kidney. eNOS has been found along the vasculature and glomerular capillaries, nNOS is present in the renal nerves, at low concentrations along the tubules and high concentrations in the macula densa, while iNOS is expressed constitutively in the medulla. It is possible that in the environment of the neuro-effector junction, NO may have both pre-synaptic and post-synaptic actions, and this probably contributes to the conflicting findings which have been reported. Thus, in the anaesthetized dog, the low level renal nerve stimulated noradrenaline output was enhanced following NOS inhibition and suppressed in the presence of an NO donor, consistent with an inhibitory pre-synaptic action. In contrast in the rat, NOS blockade suppressed renal nerve mediated noradrenaline output and neurally induced renal vasoconstriction. In terms of the influence of NO on renal nerve stimulated increases in sodium reabsorption, there is a lack of consistency. It is evident that NOS inhibition increases, and exogenous NO decreases, proximal tubule fluid reabsorption, suggesting an inhibitory action of NO on reabsorptive processes. Importantly, this action of NO is only evident if the renal nerves are intact. In an apparent conflict with these studies are the findings that renal nerve-induced increases in fluid reabsorption are prevented by NOS blockade, consistent with a facilitating action of NO. In attempting to clarify these differing reports, it is possible that NO could act in two ways, directly within the post-synaptic cell, either vasculature or epithelial cells, where it may blunt the neurally controlled cell function, or indirectly by facilitating neurotransmitter release. The differing balances between these two sites of action may simply reflect how the NO generating systems may be activated by the various experimental conditions of the investigations.
The generation of cellular energy results in the production of reactive oxygen species which comprise radicals that can damage both nuclear and cytoplasmic proteins and phospholipids. A raised output of reactive oxygen species is recognized as a state of oxidative stress that is associated with a range of metabolic and cardiovascular diseases. Reactive oxygen species comprise superoxide anions, H 2 O 2 , and other reactive radicals which are able to influence both neurotransmission and the responsiveness of the target cells to the neurotransmitter. The actions of the reactive oxygen species may be either direct or indirect, as a consequence of their ability to reduce the bioavailability of NO. There are reports that systemic infusion of tempol, a synthetic diffusible superoxide dismutase mimetic which scavenges superoxide anions, reduced blood pressure and renal sympathetic nerve activity to the same extent before and following NOS blockade. These findings would be compatible with the argument that in states of oxidative stress, for example hypertension, increased production of superoxide anions could enhance the activity of the sympathetic nervous system, and hence the level at which it influences kidney function. The enzymes NAD(P)H and superoxide dismutase are present in both the cortex and medulla of the kidney, with the level of the latter being somewhat higher in the medulla. This group demonstrated that oxidative stress increased the activity of NAD(P)H, but not superoxide dismutase, in the cortex but not the medulla. This would suggest that in renal oxidative stress whereas superoxide anion generation would increase, the level of scavenging would remain unaltered, implying greater activity of the reactive oxygen species. This is important in terms of renal sympathetic nerve activity, as Shokoji et al. demonstrated that direct application of tempol or DETC, a blocker of superoxide dismutase, directly onto renal sympathetic nerve fibers decreased and increased nerve traffic, respectively. These findings imply that oxidative stress in the kidney can itself alter the level of reactive oxygen species in the local environment, which may directly affect the level of renal sympathetic nerve activity and renal nerve-dependent function.
The earliest studies by Cohnheim and Roy using a renal plethysmograph, demonstrated that renal volume was decreased following asphyxia or when the cut ends of the renal nerves were stimulated. Indeed, these early plethysmographic studies also identified the spinal origin of the vasoconstrictor fibers. The advent of the modern flowmeters to measure renal blood flow dynamically allowed studies in anaesthetized dogs, cats, rabbits, and rats which convincingly demonstrated that direct electrical stimulation of the renal nerves caused frequency related reductions in renal blood flow. Furthermore, reflex activation of the renal sympathetic nerves, either as a consequence of activation of the baroreceptor reflex by reduction in carotid sinus pressure or activation of the somatosensory system resulted in a renal nerve-mediated reduction in renal blood flow. Together, these reports indicate that renal nerves cause contraction of the vascular smooth muscle of the resistance vessels, thereby reducing blood flow. While the innervation of the afferent arteriole would cause a reduction in renal blood flow because it is the major resistance bed within the kidney, the impact and relative importance of a neurally induced vasoconstriction at the efferent arteriole is less clear cut in terms of its overall contribution to the reduction in renal blood flow. Luff and co-workers were able to identify at the ultrastructural level two types of fibers in the rabbit that were differentially distributed, one type solely innervating the afferent arteriole and a second type evenly distributed to both afferent and efferent arterioles, which they argued enabled an independent regulation of the two resistance vasculatures. However, an alternative view is that because of the differing wall thicknesses and lengths of the afferent and efferent arterioles, even if both vessels constricted to a similar degree, the efferent arteriolar constriction would have a greater impact on glomerular filtration pressure, and hence filtration rate.
A number of studies have reported a disparity in the magnitudes of the reduction in renal blood flow and glomerular filtration rate produced by renal nerve stimulation. Studies in the anaesthetized rabbit, cat, and rat demonstrated that modest neurally induced reductions in renal blood flow (of 15–20%) were accompanied by either no change or a small 2–5% reduction in glomerular filtration. However, if angiotensin II activity was reduced by converting enzyme inhibitors, angiotensin II type 1 (AT-1) receptor blocking drugs or β-adrenoceptor antagonists, the neural impact on glomerular filtration rate became greater, with the magnitude of reductions in glomerular filtration rate becoming roughly proportionate with renal blood flow. These reports gave rise to the important concept that the renal nerves, indirectly via locally produced angiotensin II acting at the efferent arteriole, could ensure that over a modest range of variation in renal blood flow, the glomerular filtration rate, and hence filtered load presented to the nephrons, was maintained at a relatively constant level.
An important question is whether under basal, unstressed conditions the renal sympathetic nerves have a tonic influence on basal blood flow through the kidney. The reasons for this uncertainty reside in the manner of the experimental studies, whether anaesthetized or conscious preparations were used, the degree of surgical stress and type of anaesthesia used, and, to a degree, the species under study. Thus, in a number of reports in the anaesthetized rat, there was very little change in renal blood flow following acute renal denervation. However, in these studies there was often a relatively long period of time between basal measurements, the surgical manipulation and denervation of the kidney, and the post-surgery measurements, with the result that it was difficult to determine whether changes had taken place. In an attempt to resolve this issue, Kompanowska-Jezierska and colleagues inserted an electro-cautery wire around the bulk of the nerves, and instantaneous denervation occurred when a current was passed to destroy the neural tissue. Under these conditions, it was found that over the first 10 to 20 minutes an increase of some 20% in blood perfusing the outer cortex occurred when measured by laser Doppler flowmetry, suggesting there was sufficient activity within the renal nerves to decrease basal renal blood flow. This view was to a degree supported by the conscious rabbit reports of Malpas and co-workers, who found that seven days after renal denervation, renal blood flow was some 55% to 65% higher in the denervated compared to the innervated kidney, the large difference most likely indicative of greater basal sympathetic outflow in the rabbit.
The studies of Miki and co-workers using the conscious rat showed that while basal renal blood flow in the groups of animals with either intact or denervated kidneys could not be distinguished, an increase in renal sympathetic nerve activity during grooming and movement caused a proportionate decrease in blood flow to the intact kidneys of some 15%, whereas that to the denervated kidneys tended to increase in line with blood pressure. The relationship between renal blood flow and stress-induced activation of the sympathetic nervous system was the basis of the study undertaken by Brod and co-workers. They demonstrated in man that, in the unstressed state, administration of an adrenergic blocking drug, dibenamine, had no effect on para aminohippurate (PAH) clearance (effective renal plasma flow), but if the patients were tense, anxious, and stressed, the dibenamine administration was associated with a rise in PAH clearance compatible with the view that there was a tonic renal nerve-induced reduction in renal blood flow. Thus, the degree of tonic influence of the renal sympathetic nerves on basal hemodynamics is dependent on the level of stress impinging on the subjects, and in the normal conscious state the renal sympathetic nerves have relatively little impact.
The measurement of blood flow through the cortical and medullary regions of the kidney is difficult and fraught with technical limitations. Videomicroscopic techniques have been used to evaluate blood flow through single vasa recta vessels. The limitation of many techniques used such as the Rhubidium-86 methodology is single estimations from one kidney, the H 2 -washout method is the accuracy of curve fitting of the data, and the trapping of labeled microspheres in glomerular is only two to three measurements per kidney. More recently, there has been greater use of laser-Doppler technology, which allows continuous measurements of blood perfusion through the cortex and medulla of the same kidney. The limitations of this technique are that the values recorded are not flow, but are a flux measurement derived from the product of the velocity as well as the number of red cells moving through the volume of tissue illuminated by the laser. Consequently, the values arising from this technique represent qualitative rather than quantitative evaluations of blood flow.
There have been a series of investigations addressing how and whether the renal sympathetic nerves may differentially regulate blood flow through the cortex and medulla. Early studies in this area applying the H 2 -washout approach in the anaesthetized dog indicated that adrenergically-mediated decreases in flow were of comparable magnitude in both cortical and medullary regions. Similar findings were reported using the Rhubidium-86 methodology, in that stimulation of the renal sympathetic nerves caused equivalent decreases in flow through both cortex and medulla. By contrast in later reports, Rudenstam et al. used laser-Doppler flowmetry in the rat and observed a relative resistance of medullary perfusion to decrease in response to renal sympathetic nerve stimulation. Evans and co-workers, using the same technique in the anaesthetized rabbit, demonstrated smaller reductions of the perfusion in the medulla than in the cortex or total renal blood flow. Further studies in rabbits showed that he magnitude of reduction was similar across the medullary region, irrespective of the depth at which measurements were made (inner or outer medulla). This, to a degree, contrasts with the findings in the anaesthetized rat, where the inner medullary perfusion was less responsive than the outer medullary area to renal nerve stimulation. The reasons underlying the differences reported in the sensitivity of the two vascular regions (cortex versus medulla) to adrenergic stimulation are unclear, but they may reside in the differing characteristics of the afferent and efferent arterioles (which may also vary between outer and inner cortical regions), possible variations in innervation density, species variation or the mix of paracrine and autocrine factors residing in the interstitium in these different regions. The potential interactions of all these factors have been considered in detail in recent reviews.
Claude Bernard first noted that section of the splanchnic nerve of the anaesthetized dog caused an increase in urine flow. Questions arising from this finding were whether the raised fluid excretion was due indirectly to an increase in glomerular filtration rate, whether it was the result of a direct action on the tubular reabsorptive processes of the epithelial cells or a combination of these mechanisms. This problem was addressed by Bonjour and co-workers using the anaesthetized dog, who convincingly demonstrated that the elevated urine flow and sodium excretion subsequent to the section of the renal sympathetic nerves was independent of any changes in glomerular filtration rate. They concluded that the raised fluid excretion reflected a direct influence of the nerves on tubular function. Thereafter, this view was supported by a series of reports using micropuncture techniques which directly examined reabsorptive rates along accessible segments of the nephron. Bello-Reuss and colleagues observed in the anaesthetized rat that section of the splanchnic nerves had a minimal effect on single nephron glomerular filtration rate, but was associated with significant decreases in both absolute and fractional sodium and water reabsorption at the proximal tubule. These conclusions were supported by other investigators at that time, using comparable techniques to directly measure tubular function.
The removal of the influence of the renal sympathetic nerves represents only one way of illustrating their action, and to fully appreciate their influence a corresponding series of studies were necessary in which the renal nerves were activated. In a ground-breaking study, LaGrange and co-workers, using the anaesthetized dog, found that direct electrical stimulation of the nerves at levels that were sub-threshold for changing either renal blood flow or glomerular filtration rate, caused a 30–40% fall in water and sodium excretion, which was interpreted as a direct action of the renal nerves on tubular fluid reabsorption. A similar situation was found to exist in the rabbit and rat, in that electrical stimulation of the renal nerves at levels sub-threshold for decreasing renal blood flow, decreased both urine flow and sodium excretion. These observations were supported by micropuncture studies which more directly evaluated the tubular actions of the nerves. These studies demonstrated that the absolute and fractional sodium and water reabsorption of the proximal tubule was increased by low frequency direct electrical stimulation of the renal nerves which was without effect on single nephron filtration rate. Moreover, DiBona and Sawin found in the anaesthetized rat that low frequency stimulation of the renal nerves, at rates which did not alter renal blood flow or glomerular filtration rate, also increased fluid reabsorption at the thick limb of the ascending loop of Henle.
Thus, there is a large body of information which substantiates the view that the renal sympathetic nerves, when activated at low rates which have minimal effects on renal hemodynamics, have a direct action on the transport processes of the epithelial cells of the proximal tubule and the thick ascending limb of the loop of Henle. The situation regarding the distal tubule and the collecting duct has not been investigated in depth, primarily because of the technical hurdles required to evaluate reabsorption in these segments in vivo . Interestingly, Bankir and colleagues have reviewed evidence for the concept that activation of vasopressin V2 receptors along the collecting duct not only increases water abstraction, but also activates epithelial sodium channels (ENaC) in the principal cells of this nephron segment. The EnaC-mediated antinatriuresis takes place in association with V2-induced increases in cAMP, and occurs at high plasma levels of vasopressin typically seen in pathophysiological states. The possibility arises that activation of α 2 -adrenoceptors along this nephron segment, which are known to suppress cAMP, could interact with vasopressin to determine the level not only of water retention, but also of the sodium due to ENaC insertion. The relationship between vasopressin and adrenoceptor activation at this nephron segment remains a source of investigation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here