Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Parenchymal brain metastases (BMs) are the most common direct neurological complication of systemic cancer. BMs are the most common intracranial tumor in adults, with 200,000–300,000 diagnosed annually in the United States ( ). In comparison, there are 35,000 new patients with primary brain tumors diagnosed each year in the United States. The precise incidence of BM is unknown and likely much higher, likely owing to improved tolerability and durability of systemic therapy and advances in imaging. Additionally, it is believed that up to 40% of adult patients with cancer are found to have undiagnosed BM at autopsy ( ).
Any solid tumor may metastasize to the brain; however, the incidence of BM varies with the tumor type as well as molecular subtype. The most common primary tumors to metastasize to the brain include lung, breast, and melanoma ( ). A population-based analysis of patients diagnosed with a primary lung, melanoma, renal, or colorectal cancer between 1973 and 2001 suggests that the true incidence percentages are lower than previously estimated ( ). Overall, lung cancer accounts for up to 50% of all patients with BM, and breast cancer accounts for up to 20%. Melanoma, renal cell carcinoma, and gastrointestinal tumors each account for an additional 5%–10% of cases ( ).
Given the advent of genomic characterization of solid tumors, the molecular subtype of the primary tumor may also influence the risk of development of BM. In breast cancer, the presence of human epidermal growth factor receptor 2 (HER2) amplification or hormone receptor (HR) negative and HER2-negative status have increased risk of development of BM ( ). In non–small cell lung cancer (NSCLC), anaplastic lymphoma kinase (ALK)–rearranged tumors, the frequency of BM in this population may be up to 40%. In ALK-rearranged NSCLC, among patients who have received prior treatment with ALK tyrosine kinase inhibitors (TKIs), the incidence of BM is approximately 45%–70%.
Additionally, the blood–brain barrier prevents systemic therapeutic agents from entering the central nervous system (CNS), thereby creating a sanctuary for tumor cells. BM can arise anywhere in the brain, and the frequency of such tumors in various locations reflects the relative proportion of cerebral blood flow. Thus 80% of metastases arise in the supratentorial compartment. For unclear reasons, pelvic and gastrointestinal primary tumors are more likely to metastasize to the posterior fossa than to the supratentorial region ( ).
Although most patients develop BM in the setting of known cancer, BM may be an initial manifestation of an underlying primary tumor in 10%–30% of cases ( ). Less than one-fourth of such patients have clinical features suggesting location of the primary tumor. Nonetheless, 80% will eventually have the primary site of tumor identified during their lifetime. Lung cancer is the most common cause of BM presenting without a known primary, accounting for two-thirds of cases. Among lung tumor metastases, in patients with advanced NSCLC, approximately 20% have BM at the time of initial diagnosis ( ). A retrospective analysis of 176 patients with newly diagnosed brain masses concluded that chest computed tomography (CT) and brain magnetic resonance imaging (MRI), if used in concert as initial diagnostic studies, would have identified a biopsy site in 97% of patients with a newly detected intracranial mass ( ). The high likelihood of a primary lung tumor and the fact that many patients with other primary tumors have lung metastases by the time they develop BM makes restricting initial radiological studies to the chest the more cost-effective approach. Because most BM are multiple, and most patients with BM have a known cancer, only 15% of solitary intracranial masses in patients not known to have cancer turn out to be metastatic tumors ( ).
Parenchymal BM generally arise from hematogenous spread, typically through the arterial circulation directly or through the lymphatic system. Tumor emboli, like all emboli, tend to lodge at the gray/white junction because the caliber of blood vessels narrows at this site. These small emboli enlarge in a spherical fashion, eventually developing central necrosis as they outgrow their blood supply. They are usually associated with substantial surrounding vasogenic edema and well demarcated from the adjacent brain. The surrounding normally functioning brain tissue displaces, and herniation occurs if the displacement is not successfully treated. Metastases from certain primary tumors (squamous cell subtype of NSCLC, melanoma, choriocarcinoma, thyroid, and renal cell carcinoma) have a tendency for intratumoral hemorrhage. This may be attributable to a tendency for neovascularization or because they invade blood vessels.
The histopathology of BM usually closely resembles that of the underlying systemic tumor. BM may have a higher labeling index (percentage of cells going through the cell cycle) than the corresponding systemic tumor, suggesting that their growth rate is faster. Additionally, there is growing understanding that there may be genetic divergence between the primary tumor and BM, which provides an opportunity to investigate further the pathogenesis of BM and therapy.
BM may occur as long as 20 years after discovery of the primary tumor. Alternatively, BM may even antedate discovery of the underlying systemic cancer, as commonly occurs with lung cancer. Patients with specific subtypes of breast cancer and melanoma may enjoy years of apparent freedom from systemic cancer prior to discovery of cerebral metastasis ( ). Conversely, the triple-negative breast cancers (TNBCs) often have a shorter period between initial diagnosis of primary disease and development of BM ( ). The presenting features are usually progressive over days to weeks, although occasional patients present acutely with seizures or stroke-like syndrome in the setting of intratumoral hemorrhage. Half of patients complain of headache, and a third have mental status changes. Most headaches are indistinguishable from tension headache ( ). The “classic” brain tumor headache, which is worse in the mornings or awakens the patient from sleep, is uncommon, and its absence does not preclude the diagnosis of a brain tumor. Headache in the absence of other symptoms is more likely to be due to multiple metastases than a single metastasis. Over time, headache from brain metastasis becomes progressively more severe and may be accompanied by nausea, vomiting, and drowsiness. Unilateral weakness and gait disturbances are other common presenting complaints. Seizures are present at diagnosis in 18% of patients with BM ( ). Mental status changes and hemiparesis are the most common findings on neurological examination; each is present in approximately 60% of patients ( ). Despite the frequent occurrence of increased intracranial pressure (ICP), papilledema is detectable in only 10% of patients.
Several neurological conditions may mimic BM both clinically and radiographically. A primary brain tumor must be a consideration, especially in patients with a single brain mass. This is a particularly important consideration in patients with breast cancer and a dural-based tumor ( ). Abscess, demyelination, progressive multifocal leukoencephalopathy, cerebrovascular disease, and the effects of radiation or chemotherapy also simulate BM, both clinically and radiographically. Although the clinical syndrome and the neuroimaging studies usually provide a diagnosis, tissue sampling is often necessary for diagnosis or for symptomatic control of disease and to facilitate adjuvant therapy.
Neuroimaging advances since the early 1970s have made the diagnosis of BM relatively straightforward in almost all cases. Noncontrast MRI is as sensitive as contrast-enhanced CT for detection of BM. Use of gadolinium-containing contrast agents dramatically improves the sensitivity of MRI, making it markedly superior to contrast-enhanced CT scanning ( Fig. 76.1 ) ( ).
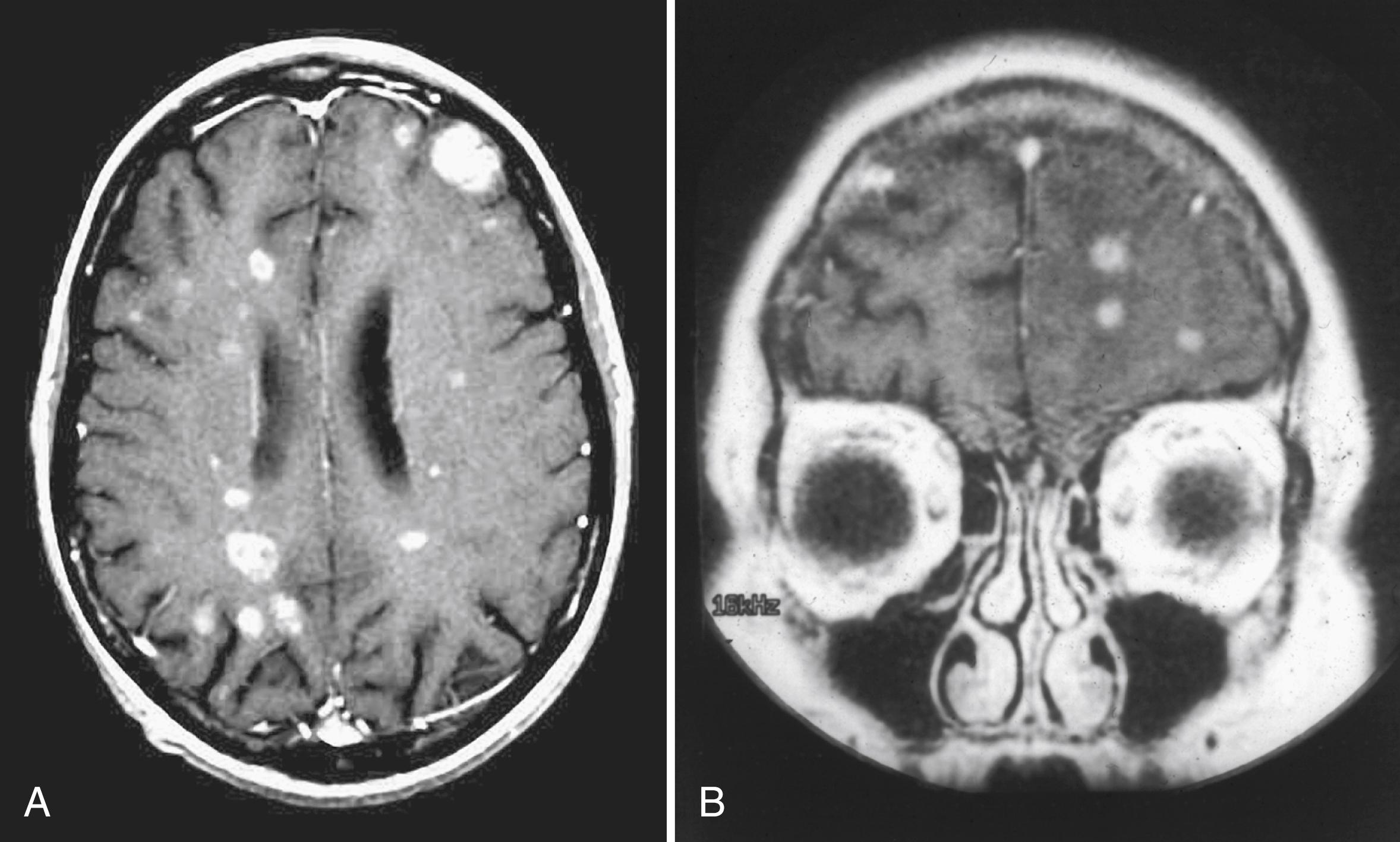
In the pre-MRI era, approximately half of all patients were found to have a single brain metastasis, likely owing to the limits of detection of CT. It is now believed that solitary brain metastasis is relatively infrequent, and it is more common to have multiple BM. Approximately, 75% of patients with BM have multiple metastases when studied with MRI ( ). Lung cancer and melanoma are somewhat more likely to produce multiple cerebral metastases, whereas renal cell, breast, and colon cancer tend to produce single metastases ( ). In addition to the number of BM (solitary vs. multiple), the size of the lesion also carries implications with regards to therapy, which will be discussed in subsequent sections on management.
Although other chapters address corticosteroids and antiepileptic drug (AED) usage, a few comments pertinent to their rational use in BM are appropriate. Corticosteroids improve symptoms associated with BM and should be considered for temporary symptomatic relief. One randomized controlled trial examined different doses in patients with BM. Patients with cerebral metastases and a Karnofsky Performance Score (KPS) ( Table 76.1 ) less than or equal to 80 were randomized in two consecutive studies to 4 mg versus 16 mg and 8 mg versus 16 mg dexamethasone. All patients received standardized whole-brain radiotherapy (WBRT) after receiving dexamethasone for 1 week. At 1 week, patients treated with lower doses had similar outcomes to those treated with higher doses of dexamethasone. Patients treated with higher doses had increased toxicity at 1 month. The authors concluded that unless patients were in danger of herniation, 2 mg twice daily was an appropriate starting dose ( ). Although corticosteroids are part of the mainstay for management of peritumoral edema associated with BM, higher doses of corticosteroids are associated with increased toxicity ( Table 76.2 ) ( ).
| KPS 100 | Normal; no complaints, no evidence of disease |
| KPS 90 | Able to carry on normal activity; minor signs or symptoms of disease |
| KPS 80 | Normal activity with effort; some signs or symptoms of disease |
| KPS 70 | Cares for self; unable to carry on normal activity or do active work |
| KPS 60 | Requires occasional assistance but is able to care for most personal needs |
| KPS 50 | Requires considerable assistance and frequent medical care |
| KPS 40 | Disabled; requires special care and assistance |
| KPS 30 | Severely disabled; hospitalization is indicated, although death not imminent |
| KPS 20 | Very sick; hospitalization necessary, active support treatment necessary |
| KPS 10 | Moribund; fatal processes progressing rapidly |
| KPS 0 | Dead |
| Involved Organ System | Side Effect |
|---|---|
| Neurological/psychiatric | Insomnia Mood lability Anxiety/depression Psychosis Increased appetite Hiccups Tremor |
| Musculoskeletal | Proximal myopathy Osteoporosis Arthralgias Avascular necrosis Decreased growth/height (pediatric patients) |
| Gastrointestinal | Dyspepsia/gastritis |
| Hematological/immunlogical | Immunosuppression-related infections (oropharyngeal candidiasis, Pneumocytis jiroveci pneumonia) |
| Endocrine | Hyperglycemia Weight gain Cushingoid habitus Adrenal insufficiency (after discontinuation) |
| Cutaneous or vascular | Acne Striae Delayed wound healing Peripheral edema |
| Ocular | Visual blurring Cataract formation |
Approximately 20% of patients with BM present with seizures and require treatment with standard anticonvulsants. The use of prophylactic anticonvulsants in patients who have not had seizures is controversial. Fewer than 20% of these patients experience seizures later in the course of their illness, and this risk does not appear to be reduced with prophylactic anticonvulsants. A systematic review of the topic, specifically among patients with BM, concluded that the risk of seizure within 3 months was low (10%) and prophylaxis was not warranted ( ). This agrees with other studies that evaluated prophylaxis in patients with all types of brain tumors (not limited to metastases) ( ). Consequently, the American Academy of Neurology (AAN) has issued a practice parameter recommending against the prophylactic use of anticonvulsants in patients with brain tumors who have not had a seizure ( ). A more recent randomized study evaluated the role of phenytoin for 1 week in seizure-free patients following surgery to reduce the risk of seizure in the immediate postoperative period. The risk of seizure was in general low (8%) during this time and the use of prophylaxis did not alter the risk. Furthermore, toxicity was higher in the group that received phenytoin ( ). A subsequent study compared levetiracetam, a newer antiseizure agent, to phenytoin in patients undergoing craniotomy for resection of supratentorial tumor ( ): 146 patients were randomized to either levetiracetam or phenytoin with primary endpoint of seizure and secondary endpoint of frequency of side effects. The incidence of seizure in the levetiracetam cohort was 1.4% compared to 15.1% with phenytoin. There were no treatment-related side effects with levetiracetam, where phenytoin was discontinued in five patients ( ), indicating that levetiracetam is preferred to phenytoin. Potential exceptions to the AAN guideline include patients with metastases from melanoma (which may be more epileptogenic because of multiplicity or hemorrhage), tumors in motor cortex, or concomitant parenchymal and leptomeningeal disease
In contrast to seizure-free patients, those with seizures do require anticonvulsant therapy. Although efficacy is similar among the available anticonvulsants, differences in pharmacokinetic profile may influence which agent is used. Anticonvulsants metabolized by the P450 system interact with corticosteroids and many common antineoplastic therapies such as irinotecan and erlotinib. Consequently, the effectiveness of a given dosage of dexamethasone may be decreased, and tumor exposure to an antineoplastic agent may be reduced. Examples of enzyme-inducing anticonvulsants include phenytoin and carbamazepine. Alternatively, newer, nonenzyme-inducing agents such as levetiracetam and lacosamide do not interact with other medications administered concurrently. In addition, the side-effect profile of the more modern agents is more favorable, further supporting their use as first-line therapies.
While the goals of radiation therapy (RT) are to alleviate neurological deficits and provide disease control, whether or not RT prolongs survival or improves quality of life has yet to be demonstrated in a randomized clinical trial. The Quality of Life After Treatment for Brain Metastases (QUARTZ) study was a phase III, randomized non-inferiority trial in which patients with NSCLC BM were randomized 1:1 to either optimal supportive care (OSC) with dexamethasone and WBRT or OSC alone ( ). The primary outcome was quality-adjusted life years (QALYs), which was generated from OS and patient-reported questionnaires. In this study, there was no difference in OS between the WBRT-treated and OSC-alone groups, with a difference in mean QALYs was 4.7 days, thus suggesting that WBRT did not provide an additional clinical benefit for patients in this group ( ).
For those patients treated with RT, the Radiation Therapy Oncology Group (RTOG) database has permitted the application of statistical techniques such as recursive partitioning analysis (RPA) to separate patients with BM treated with WBRT into different prognostic classes based on clinical features at presentation. Patients with BM can be divided into three classes. Class 1 consists of patients with a KPS greater than or equal to 70, age younger than 65 years, primary site of tumor resected or controlled with treatment, and no extracranial sites of metastatic tumor. Such patients have a median survival of 7.1 months. Class 3 is composed of all patients whose KPS is less than 70; the median survival in this group is only 2.3 months. Class 2 contains all patients who do not fall into classes 1 and 3; class 2 patients have a median survival of 4.2 months ( ). Subsequent studies have validated these results ( ). The limited number of patients with KPS less than 70 in the RTOG dataset precluded further analysis of this population.
Survival following diagnosis of BM is dependent upon several factors, including primary tumor of origin. Given the limitations of existing prognostic indices at the time, the need for a reliable prognostic assessment for guidance of treatment led to the development of the graded prognostic assessment (GPA), which was found to be as prognostic as earlier prognostic measures and easier to use ( ). The GPA is the sum of score (0, 0.5, and 1) for four factors, comprising age, KPS, extracranial metastases (none or present), and number of BM (1, 2–3, or >3) ( Table 76.3 ). Subsequently, the diagnosis-specific GPA (DS-GPA) was proposed, incorporating both the primary diagnosis and criteria from RPA. Using data obtained from a retrospective analysis of 4259 patients with newly diagnosed BM, treated between 1985 and 2007, separate criteria were developed for patients with melanoma, lung, renal cell, breast, and gastrointestinal (GI) cancers. In this analysis, prognostic factors varied by diagnosis: in lung cancer, functional status, age, presence of extracranial disease, and number of BMs were significant, whereas only functional status was significant for breast and GI cancers ( Table 76.4 ). Separate DS-GPAs are currently under investigation for specific cancers, including NSCLC with incorporation of molecular markers and melanoma ( ).
| GPA of Newly Diagnosed BMs | Significant Prognostic Factors | GPA Scoring Criteria | ||||
|---|---|---|---|---|---|---|
| NSCLC/SCLC | 0 | 0.5 | 1 | — | — | |
| Age | >60 | 50–60 | <50 | — | — | |
| KPS | <70 | 70–80 | 90–100 | — | — | |
| ECM | Present | — | Absent | — | — | |
| No. of BMs | >3 | 2–3 | 1 | — | — | |
| Melanoma/renal cell cancer | 0 | 1 | 2 | — | — | |
| KPS | <70 | 70–80 | 90–100 | — | — | |
| No. of BMs | >3 | 2–3 | 1 | — | — | |
| Breast/GI cancer | 0 | 1 | 2 | 3 | 4 | |
| KPS | <70 | 70 | 80 | 90 | 100 | |
| Score | |||
|---|---|---|---|
| 0 | 0.5 | 1.0 | |
| Age | >60 | 50–59 | <50 |
| KPS | <70 | 70–80 | 90–100 |
| No. of CNS metastases | >3 | 2–3 | 1 |
| Extracranial metastases | Present | — | None |
With standard fractionation schemes, WBRT is tolerated well. Toxicity associated with radiation varies according to the duration of time following treatment: acute toxicity occurs during or within 6 weeks of completion of therapy, early-delayed appears up to 6 months from treatment completion, and late effects present at least 6 months after treatment. Acutely, patients may experience fatigue, alopecia, anorexia, nausea, and vomiting. Early-delayed effects may include fatigue, transient neurological worsening, or symptoms related to pseudoprogression. Headache and nausea are generally alleviated with corticosteroids and antiemetics. In poor-prognosis patients, the acute side effects of WBRT must be considered, however, as they may have a significant impact on the quality of the remaining short predicted life span ( ).
Long-term survivors of BM are at risk of suffering late complications of WBRT, of which neurocognitive effects are the most feared. Of WBRT recipients for BM, as many as 10%–30% develop cognitive impairment by 1 year if radiation doses per fraction exceed 300 cGy ( ). Symptoms commonly include poor short-term memory, abulia, gait unsteadiness, and urinary urgency. MRI frequently reveals extensive symmetrical periventricular white matter changes termed radiation leukoencephalopathy, ventriculomegaly, and sometimes cortical atrophy. The clinical picture may resemble normal-pressure hydrocephalus, but a positive durable response to ventriculoperitoneal shunt is rare ( ). Because the risk of this complication is greater with larger fraction sizes, radiation oncologists treat patients with good prognosis with 20 fractions of 200 cGy or similar regimens.
Neurocognitive decline in the setting of cancer is multifactorial, and establishing attribution is difficult. Progressive CNS disease, concurrent use of chemotherapy and other pharmacological agents (such as antiseizure medications), depression/anxiety, and neurological impairment, among other factors, may contribute. It is also recognized that patients with cancer may have cognitive impairment prior to any treatment or intervention. The true incidence of neurocognitive impairment from WBRT is unknown due to limitations of the available data to date. Many studies have relied on the Mini-Mental Status Examination (MMSE) assessment, which is not optimal given that these brief scales lack sensitivity and may not capture longitudinal decline in function when compared to formal neurocognitive testing ( ). Comprehensive neurocognitive assessments that evaluate multiple cognitive domains provide more meaningful data but require expertise and are more expensive and labor intensive. Neurocognitive endpoints are increasingly being recognized as critical determinants of outcomes and are being used as primary measures of effectiveness in modern cerebral metastases studies. In contrast, overall survival is often used as a primary endpoint despite the fact that the majority of patients die of concurrent systemic disease. As neurocognitive toxicity related to cranial irradiation may present similarly to other neurodegenerative processes, an investigation of alternate or reversible etiologies should be undertaken.
Measures to mitigate the neurocognitive toxicity associated with WBRT have been under investigation. Neuroprotective strategies to date have included prophylactic use of memantine, an oral N -methyl- d -aspartate (NMDA) inhibitor, and use of hippocampal-sparing techniques during delivery of radiation. In a randomized, placebo-controlled study of 508 patients receiving WBRT, 256 patients received memantine starting 3 days prior to start of WBRT, which was continued for 6 months ( ). Due to patient attrition, only 149 total patients were eligible for analysis, among which there was no difference in rate of memory decline between the two treatment groups, which was the primary endpoint. There was a trend toward delaying time to development of cognitive decline which favored the memantine-treated cohort; however, this was not statistically significant ( ). In addition to memantine, donepezil, an anticholinergic typically used for mild to moderate Alzheimer’s disease, has also been studied in this context, with modest benefit ( ).
Hippocampal-avoidance radiation has been studied as well, given the role of the hippocampus in memory formation. A single-arm, phase II study of hippocampal-sparing was done in 113 patients with BM without involvement of the hippocampus. In comparison to historical controls, use of hippocampal avoidance was associated with lower rates of decline in recall in the 42 evaluable patients. In the subsequent phase III trial, the addition of memantine to hippocampal-avoidance WBRT led to reduction of risk of cognitive failure by 26%, in comparison to hippocampal-sparing WBRT alone. There were no differences noted in toxicity, progression-free survival (PFS), OS, or intracranial progression ( ).
BMs are extremely common in small cell lung cancer (SCLC), being present in 10% of patients at diagnosis, increasing to 20% during therapy, and by some reports, up to 80% of patients with SCLC will have BM at autopsy ( ). At 2 years post-diagnosis, the cumulative risk of brain metastasis is 47% for patients with limited disease and 69% for those with extensive disease. Presumably the brain is a pharmacological sanctuary for microscopic tumor against systemic chemotherapy, which does not penetrate the intact blood–brain barrier. This has led to numerous trials designed to test whether prophylactic cranial irradiation (PCI) would decrease the incidence of brain relapse and improve survival in patients who achieved systemic CR in limited-staged SCLC (LS-SCLC, defined as disease confined to one hemithorax). A consistent finding was that PCI in LS-SCLC significantly decreased the risk of cerebral metastases. An often-cited meta-analysis of these studies indicated that PCI reduced the risk of subsequent brain metastasis (59% vs. 33% at 3 years) and modestly increased 3-year survival from 15.3% to 20.7% ( P = .01) ( ). There was also suggestion of a dose response, although this was not confirmed in a recent randomized study in which patients were randomized to 25 or 36 Gy ( ).
The role of PCI in patients with incomplete response to treatment or with extensive small-cell lung cancer remains unclear. The poor prognosis of these patients (median survival of 9 months) brings into question the utility of PCI. Slotman et al. randomized patients with extensive small-cell lung cancer that responded to treatment to observation or PCI. Patients treated with PCI had a cumulative risk of symptomatic BM of 14.6% compared to 40.4% among observation patients. Patients treated with PCI also had longer median overall survival (6.7 months vs. 5.4 months) with 1-year survival also favoring the PCI treated group (27% vs. 13%) ( ).
Although PCI appears to be well-tolerated, controversy still remains over whether the benefits of PCI outweigh its toxicities, particularly leukoencephalopathy. Two large prospective randomized trials of PCI did not document increased neuropsychological deficits among PCI recipients. Others argue that the small numbers of long-term survivors in these trials precluded accurate assessment of the risk of leukoencephalopathy, and that because PCI benefited only about a fourth of its recipients, it should not be considered standard therapy.
For several decades, neurosurgeons resected single BM in selected patients and argued that surgery produced better results than radiotherapy alone, particularly noting improvement in the percentage of long-term survivors. In 1990, a seminal randomized controlled trial verified the neurosurgeons’ contention. In this study, eligible patients had a single surgically accessible metastasis identified by contrast CT or MRI scan. Patients with highly radiosensitive primary tumors were excluded. Enrolled patients were randomized to biopsy followed by WBRT (36 Gy in 12 fractions) versus resection and WBRT. Patients who underwent surgical resection of the metastasis followed by RT developed fewer local recurrences (20% vs. 52%) and significantly improved survival (40 weeks vs. 15 weeks) compared to those patients who received only a biopsy and RT. Patients who underwent surgical resection also had improved performance status and a reduced risk of dying as a result of neurological causes. Multivariate analysis showed that surgery and longer time between diagnosis of the primary tumor and the development of BM were associated with increased survival, whereas disseminated disease and increasing age were associated with decreased survival ( ). A significant prolongation in overall survival and improvement in quality of life among patients who underwent resection prior to whole-brain radiation was also noted in the randomized study by . The only patients who benefited from surgery, however, were those with stable extracranial disease. The results of these studies are in contrast to findings by in their randomized study in which there was not survival benefit among patients with BM undergoing surgery. However, a higher proportion of patients in this study had active systemic disease, possibly influencing the results, as these were likely patients with poorer performance status. Thus, in patients with surgically accessible single BM and absent or controlled systemic cancer, surgical resection became the standard of care.
Alternatively, focal radiation to the surgical bed may reduce the risk of local recurrence, as patients with a solitary brain metastasis undergoing resection have 50%–60% risk of local recurrence over the following year ( ). Several retrospective studies examining the role of postoperative radiosurgery to the surgical bed found that focused radiation is efficacious in controlling local tumor growth and maintaining long-term quality of life ( ). Stereotactic radiosurgery (SRS) to the resection cavity has been a preferred approach for patients following resection of a solitary brain metastasis, thus improving local control and avoiding potential for neurocognitive side effects. In a randomized phase III trial of 194 patients with 1 resected brain metastasis and surgical cavity less than 5 cm, patients were randomized to either SRS or WBRT. At the time of follow-up, there was no difference in overall survival; however, cognitive decline was more frequent in the WBRT cohort ( ). In a separate, randomized phase III trial comparing SRS to observation, 132 patients with BM (up to three treated with surgical resection) were randomized to either observation or SRS ( ). Primary endpoint was local recurrence in the resection cavity: 72% of patients treated with SRS remained free from disease at 12 months in comparison to 43% of patients on observation ( ).
Although the evidence presented in the previous section documents current concepts regarding treatment of solitary lesions, 50% of patients present with multiple BM. Autopsy studies indicate that 60%–85% of patients with BM have multiple lesions ( ). In the past, multiple brain lesions represented a relative contraindication to surgery due to the presumed poor survival of these patients. However, application of principles learned in studies regarding surgery for solitary lesions has yielded evidence suggesting that patients with multiple tumors may derive benefits from surgical resection similar to those of patients with solitary lesions. Currently, there is no level I evidence regarding the surgical treatment of patients with multiple BM ( ). Some retrospective reviews have suggested a role for surgery in select patients, though the overall benefit of resection of a single symptomatic brain metastasis in the setting of multiple BM is unknown.
One of the first large studies to review surgery for patients with multiple BM was , which retrospectively reviewed 56 patients with multiple BM. In group A, one or more lesions were not surgically removed, and in group B all lesions were resected. Patients in group B were compared to a cohort of patients with solitary lesions who underwent surgical resection matched by primary tumor, time from cancer diagnosis to brain metastasis, and status of systemic disease at the time of surgery (group C). Variables such as KPS, age, primary tumor, and systemic disease were similar between all three groups. All patients underwent postoperative WBRT. Median overall survival was 6 months for group A and 14 months for both group B and group C. Rates of surgical morbidity and mortality were similar between all groups, and a higher number of craniotomies per surgery was not associated with increased complication rate. Thus, surgery for resection of all lesions in patients with multiple tumors, up to 3 total, was determined to be as safe and effective as surgery for solitary tumors and provided significant survival benefit in patients amenable to surgical resection of all lesions.
retrospectively reviewed patients who underwent surgery for BM. Of 208 cases reviewed, 76 patients had multiple lesions and 17 of these underwent resection of two or more metastases. No differences in overall survival were seen when comparing patients with solitary metastases to those with two or three metastases. Evaluation of subgroups based on RPA class was performed. The authors concluded that surgical resection should be considered in patients with solitary and multiple BM, but that RPA class III solitary lesions and RPA class II multiple lesions may benefit more from SRS alone.
Based on available literature, surgical resection for patients with multiple metastases is indicated in a select group of patients. These patients have a low number of lesions (two or three) which are surgically accessible. Other favorable factors include stable systemic disease, high performance status, and age less than 65. However, patients who meet these criteria represent a relatively small subset of all patients who present with multiple metastases. Only approximately one-third of patients diagnosed with BM will be possible surgical candidates ( ).
Patients with a large number of BM are often not considered surgical candidates. In the retrospective reviews evaluating surgery in patients with multiple metastases ( ), most patients underwent one or two craniotomies to access one to three tumors. In general, better outcomes were observed in cases where all tumors were resected, and no patient had more than three craniotomies. However, certain clinical scenarios may warrant consideration for surgical intervention in these patients. For example, patients who present with poor or declining functional status in the setting of numerous BM may have a dominant lesion, or a specific lesion suspected of being the primary cause of the neurological decline. If the culprit lesion is removed, the patient’s functional status may improve rapidly. Examples include lesions causing hydrocephalus or lesions with significant edema causing mass effect with midline shift and resulting altered mental status ( ). Lesions affecting eloquent structures such as primary motor or speech areas may also cause significant neurological impairment either directly or indirectly, and surgical resection may allow significant rapid improvement in performance status. In these clinical settings, resection of a limited number of lesions thought to be directly responsible for neurological decline may allow for restoration of higher functional status and improved survival. However, as discussed in earlier section, the overall benefit of this approach has yet to be determined.
Radiation has historically played an important role in the management of cerebral metastases. Its role, however, has evolved over the years as technology advanced. Consequently, several treatment paradigms have evolved. Central to the debate on cerebral metastases is balancing the toxicity of treatment against control of cerebral disease.
WBRT has long been a staple in the management of cerebral metastases. The rationale behind its use lies in the recognition of microscopic deposits seeding the brain in addition to macroscopic, visible disease. As such, it served to not only treat the visible disease but also prevent the development of new tumors. Consequently, neurological morbidity associated with progressive and new disease could be averted. The benefits of WBRT in patients with single metastatic deposits were first documented in the seminal randomized study by in which patients who underwent a resection were randomized to radiation or observation. Although overall survival was identical between the two groups, the rate of local and distant control was better among treated patients. In addition, there was a significant reduction in neurological death.
Despite the apparent benefits of WBRT, long-term neurological toxicity remains a notable concern. This is particularly true as the number of long-term survivors increases. The exact incidence of neurocognitive impairment from WBRT is unknown. With neurocognitive testing, abnormalities can be seen in nearly half of patients after WBRT ( ). Furthermore, treatments for the sequelae are limited. WBRT can be administered only once as the risks increase further with additional courses. As such, the routine use of WBRT in patients with limited disease limits future options in the event of CNS recurrence. Consequently, the routine use of WBRT was brought into question. With the evolution of stereotactic radiotherapeutic techniques, alternative treatment paradigms were developed. Specifically, treating macroscopic visible disease in select patients with a limited number of metastatic deposits (usually <4) and deferring WBRT became a common approach. However, the negative impact of CNS progression must be weighed against the disadvantages of early WBRT.
Like conventional surgery, SRS has emerged as a means of enhancing long-term local control of BM while also reducing risk of acute toxicity. SRS is a technique of external irradiation that uses multiple convergent beams to deliver a high single dose of radiation to a radiographically well-circumscribed treatment volume. SRS is generally administered either with a Gamma Knife apparatus or a modified linear accelerator. With either technique, the use of a stereotactic head frame secured to the head with screws and the radiation delivery system allow for great precision, with a rapid drop-off in radiation dose within millimeters of the target lesion, sparing normal brain the potentially deleterious consequences of high-dose radiation.
SRS offers several advantages to conventional surgery. SRS offers the potential of treating lesions in locations generally considered surgically inaccessible. Metastases in eloquent cortex, basal ganglia, thalamus, and even the brainstem can be treated with relatively low risk. In addition, multiple lesions can be treated with SRS simultaneously with significantly less risk. SRS is also more cost-effective than surgery and can be performed in an outpatient setting. A technical limitation of SRS, compared to conventional surgery, is the inability to treat metastases greater than 3.5 cm in median diameter due to excessive radiation toxicity. SRS has been frequently used to treat up to 4 BM; a greater number of lesions typically is treated with WBRT. SRS is sometimes associated with increased symptomatic edema, often necessitating treatment with corticosteroids and sometimes requiring surgical intervention. Surgery will alleviate mass effect quickly and allow for more rapid and complete discontinuation of corticosteroids.
Numerous single-institution experiences with radiosurgery for single or oligometastatic brain lesions have been published. In a review summarizing published series comprising more than 2000 patients treated over 8 years in the 1990s, found that SRS achieved permanent local control in more than 80% of patients, with complications in fewer than 10% (mainly radiation necrosis). Outcome appeared independent of the number of metastases treated. Median survival following SRS is approximately 9–10 months, very similar to surgical series. This raised the question of whether SRS can be performed in lieu of resection. An RTOG clinical trial has affirmed this hypothesis by randomizing patients with one to three BM to WBRT with or without SRS. Patients with a single brain metastasis had a significant survival benefit as well as improved performance status from the addition of SRS, as did patients younger than 50 and those in RPA class 1 ( ). Collectively, although the available data suggest similar efficacy of SRS and surgery, a randomized study comparing the two modalities has not been performed. Nonetheless, SRS may be an alternative to resection in select patients.
One consistent and remarkable finding across numerous SRS series is that metastases from highly radio-resistant tumors like melanoma and renal cell carcinoma, which respond very poorly to fractionated radiotherapy, respond virtually as well to SRS as tumors far more sensitive to conventional radiation. However, intracranial failure rates of highly radio-resistant tumors without WBRT were 25.8% and 48.3% at 3 and 6 months, respectively, according to a recent phase II trial conducted by the Eastern Cooperative Oncology Group. Therefore, delaying adjuvant WBRT may be appropriate for some subgroups of patients with radio-resistant tumors, but routine avoidance of WBRT should be approached judiciously ( ).
Approximately 7% of metastases treated with SRS transiently increase in diameter on scan, reflecting a radiation reaction. According to an evidence-based review by the American Society for Therapeutic Radiation and Oncology (ASTRO), in selected patients with small (<4 cm) BM (up to three in number and four in one randomized trial), radiosurgery boost with WBRT improves local brain control compared to WBRT alone. Similarly, in patients with a single brain metastasis, radiosurgery boost following WBRT improves survival. In selected patients treated with radiosurgery alone for newly diagnosed BM, overall survival is not altered ( ). However, omission of upfront WBRT was associated with markedly poorer local and distant brain control ( ).
The relative effectiveness of radiosurgery versus surgery alone in patients with BM has never been ascertained. A retrospective review from the Mayo Clinic compared the efficacy of neurosurgery versus radiosurgery in local tumor control and patient survival in patients with solitary BM. There was no significant difference in patient survival ( P = .15) between the 74 neurosurgery patients and the 23 radiosurgery patients. The 1-year survival rates for the neurosurgery and radiosurgery groups were 62% and 56%, respectively. There was a significant ( P = .020) difference in local tumor control, but none of the radiosurgery group had local recurrence compared with 19 (58%) in the neurosurgery group ( ). Although surgeons occasionally remove two or even three BM, surgery is generally restricted to single lesions, whereas multiple lesions generally present no problems for radiosurgery. Radiosurgery appears more cost-effective than surgery, although surgery alleviates symptoms of mass effect much more rapidly and reliably than SRS. In a single-institution retrospective review of 213 patients with large, previously treated BM (> 4 cm), 66 were treated with SRS alone and 157 were treated with surgical resection followed by SRS. The rate of local recurrence at 1 year was lower in the surgery and SRS group (20.5% vs. 36.7%); however, the rate of radiation necrosis was higher at 1 year (22.6% vs. 12.3%) ( ). Phase III trials comparing these two modalities have been proposed, but no major multicenter study has yet been undertaken.
The role of WBRT following radiosurgery for BM is also uncertain. Two retrospective cohort studies have examined this issue. compared outcomes in 158 patients treated with radiosurgery alone versus 78 receiving radiosurgery plus fractionated WBRT. All patients had three or fewer BM. The overall median survival was 5.5 months, with no difference between treatment groups. However, median survival in patients without extracranial tumor was increased in patients getting both forms of radiation (15.4 vs. 8.3 months, P = .08). A trend existed for superior local control in patients getting combined therapy. In a phase III EORTC trial of 359 patients with one to three BM treated with either SRS or surgical resection, 100 patients were randomized to observation and 99 to WBRT following SRS, while 79 patients were randomized to observation and 81 to WBRT following surgery. Although there was decreased intracranial relapse rate in the WBRT-treated group, there was no difference in overall survival between the observation group and WBRT (10.7 and 10.9 months) ( ).
Fractionated stereotactic radiotherapy (FSRT) is another radiotherapeutic modality that utilizes multiple beams that converge on a well-defined target. Similar to SRS, use of multiple beams reduces exposure to normal tissue by spreading out the dose as it passes through normal tissue before converging on the target lesion. In addition, the intensity of the individual beam can be modulated, allowing greater control of dose distribution. The increased conformality of the treatments allows for the delivery of a higher dose per fraction, which may have radiobiological advantages. FSRT does not require external fixation and can be done with a mask similar to conventional radiation. Fractionation requires multiple treatments, often single doses administered over 3–5 days. Treatment planning is more complex and requires more time. Compared to SRS, FSRT is not limited by the size of the lesion, so larger lesions can be treated. However, higher doses can still be delivered to target lesions. As such, it has emerged as another stereotactic option for patients with cerebral metastases. At this juncture, FSRT is not as well studied as SRS, although the available data suggest the efficacy and risk of the two modalities are similar ( ).
Although the mainstay of management for BM has been primarily surgery and RT, as discussed in previous sections of this chapter, systemic therapies have emerged as an attractive and, at times, first-line option for treatment of BM, specifically if synchronous with the diagnosis of extracranial disease. Advances in genomic characterization have led to improved understanding of the molecular underpinnings of solid tumors, thus paving the way for identification of important driver mutations and disease-modifying pathways which are being exploited for therapeutic benefit. While standard cytotoxic chemotherapy remains a significant component of treatment for some diseases, including some lung and gastrointestinal cancers, targeted therapies and immunotherapies are increasingly used for both solid tumors and BM.
Epidermal growth factor receptor (EGFR) is a member of the erbB family and is frequently overexpressed in NSCLC ( ). Mutations in EGFR are found in 15% of NSCLC patients and have been associated with adenocarcinoma histology, younger age at diagnosis, light or never smokers, women less than 35 years, and patients of Asian descent ( ). As the presence of EGFR confers sensitivity to EGR TKI, use of these agents, in patients with metastatic NSCLC harboring activating mutations in EGFR, is the standard of care ( ).
Erlotinib is a first-generation EGFR TKI which was first shown to have activity against EGFR-mutated NSCLC BM. In a cohort of 17 patients treated with erlotinib, in the EGFR-mutated cohort, there was longer median time to progression (TTP) and higher objective response rate when compared to EGFR-wildtype patients. In a later phase II study of 48 patients with NSCLC BM, in the EGFR-mutated cohort, erlotinib was associated with longer median survival (PFS and OS) as well as a higher intracranial response rate ( ).
Osimertinib is a third-generation EGFR TKI which was initially used in EGFR-mutated NSCLC patients harboring T790M mutations, which may confer resistance to earlier generation EGFR TKIs ( ). It is also recognized for improved CNS penetration and response. In a phase III trial comparing osimertinib to chemotherapy (platinum and pemetrexed) in patients with NSCLC, among patients with BM, the osimertinib-treated cohort had longer median PFS (8.4 months vs. 4.2 months) ( ).
In patients with NSCLC with fusion of ALK and echinoderm microtubule-like protein 4 (EML4), clinical characteristics are similar to that of EGFR: younger age, adenocarcinoma histology, female, and minimal smoking history. Alterations in ALK are present in 3%–7% of NSCLC and are mutually exclusive of other NSCLC-associated driver mutations, including EGFR and KRAS ( ). Crizotinib is a first-generation ALK TKI which was prospectively investigated in patients with stable, previously treated BM ( ). In patients treated with crizotinib, median PFS was 9 months, as compared to 4 months in the chemotherapy-treated arm. There are challenges, however, to broader use of crizotinib, including development of resistance. Additionally, a subset of patients who have been treated with crizotinib without history of BM often develop CNS disease as first site of relapse ( ).
Alectinib and ceritinib are both second-generation ALK TKIs which have shown improved PFS and intracranial response rates in patients with ALK-rearranged NSCLC BM, in comparison to standard chemotherapy ( ). In three separate phase III studies, alectinib, in comparison to crizotinib, demonstrated higher CNS penetration and delayed the risk of CNS progression in patients with baseline intracranial disease. Brigatinib is a third-generation ALK TKI which has also demonstrated improved intracranial response rates, particularly in patients with acquired resistance to the earlier-generation ALK TKIs ( ). Similarly, lorlatinib is a dual ALK and ROS1 inhibitor which has gained use given its CNS penetration and ability to overcome ALK resistance. In a phase I study of lorlatinib in 54 patients with either ALK or ROS1-rearranged NSCLC, 39 patients had BM at baseline, 24 of which were evaluable ( ). Among the 19 patients with ALK-rearranged NSCLC BM, intracranial objective response was seen in 8 patients ( ). A subsequent phase II study, patients with either ALK or ROS1-rearranged NSCLC, were treated with lorlatinib one on six dose expansion cohorts based on prior exposure to earlier-generation ALK TKIs ( ). Among 30 patients in cohort 1 (ALK TKI-naïve), 8 patients had baseline CNS disease, of which 3 were measurable, and objective intracranial responses were observed in 2 patients. In cohorts 2–5, 133 of 198 patients who have been treated with at least one ALK TKI had baseline CNS disease. In the 81 evaluable patients, intracranial responses were observed in 51 patients and median duration of response was 14.5 months ( ).
HER2 is overexpressed in up to 30% of breast cancer patients and is predictive of response to HER2-directed therapies, including trastuzumab, pertuzumab, ado-trastuzumab emtansine, and lapatinib ( ). More important, these HER2-directed agents have demonstrated improved outcomes in HER2-positive CNS disease, including parenchymal and leptomeningeal disease ( ). In an observational study of factors determining outcomes in HER2-positive disease and BM, patients treated with trastuzumab had median OS of 11.6 months, versus 6.1 months in those who did not receive trastuzumab ( ). Lapatinib is a small-molecule inhibitor that has been investigated for use in HER2-positive BM. In the LANDSCAPE trial, the combination of lapatinib and capecitabine was associated with longer overall survival rate at 6 months and prolonged time to intracranial disease progression in patients with untreated BM ( ). Among 44 of 45 evaluable patients, 29 had objective CNS response (65.9%) and all patients had at least a partial response ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here