Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Giant multilocular prostatic cystadenoma is a large tumor composed of acini and cysts lined by prostatic-type epithelium set in a hypocellular fibrous stroma. This rare tumor arises in men between 28 and 80 years old as a large midline prostatic or extraprostatic mass causing urinary obstruction. The epithelial lining displays prostate-specific antigen (PSA) immunoreactivity. One case was associated with high-grade prostatic intraepithelial neoplasia (PIN). Surgical excision is usually curative, although it may recur if incompletely excised. The differential diagnosis includes phyllodes tumor, multilocular peritoneal inclusion cyst, multicystic mesothelioma, müllerian duct cyst, seminal vesicle cyst, lymphangioma, and hemangiopericytoma. The light microscopic appearance of the cyst lining is useful in separating these lesions ( Table 9.1 ). One reported case was successfully treated laparoscopically.
| Type of Cyst | Location | Size | Contain Sperm |
|---|---|---|---|
| Prostatic cyst | Lateral | Variable | No |
| Seminal vesicle cyst | Lateral | Large | Yes |
| Diverticulum of ejaculatory duct or ampulla | Lateral | Variable | Yes |
| Müllerian duct cyst | Midline | Large | No |
Other benign unilocular cysts that may be sampled by biopsy include seminal vesicle cyst, ejaculatory duct cyst, and müllerian duct cyst. Location is often useful, recognizing that seminal vesicle cyst is typically lateral, whereas müllerian duct cyst is midline. Seminal vesicle cyst may contain ectopic prostatic acini and urothelium. Echinococcal cyst is usually associated with prominent inflammation, and organisms are often demonstrable.
A case of benign adenofibroma of the ejaculatory duct has been reported. This incidental autopsy finding consisted of a small polypoid mass of epithelium and stroma that projected into a cystically dilated duct. Adenomatoid tumor of the ejaculatory duct has also been described.
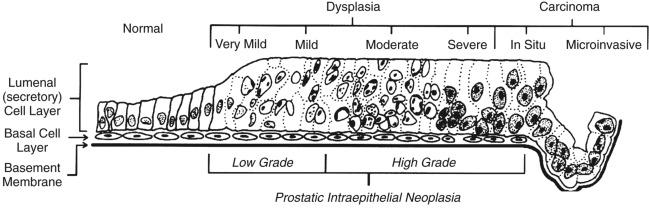
PIN refers to the preinvasive end of the continuum of cellular proliferations within the lining of prostatic ducts, ductules, and acini. High-grade PIN is the earliest identifiable stage in carcinogenesis, possessing most of the phenotypic, biochemical, and genetic changes of cancer without invasion into the fibromuscular stroma. The World Health Organization (WHO) contends that PIN is the only preinvasive lesion for prostate cancer. Other potential but unproven candidates for premalignancy in the prostate include atypical adenomatous hyperplasia (see Chapter 8 ), malignancy-associated changes arising in normal-appearing epithelium, and atrophy (see Chapter 8 ).
Initial references to the lesion we now know as PIN were apparently made by early authors such as Kastendieck and Helpap, but they did not provide reproducible criteria and distinguish their findings from mimics of PIN. McNeal emphasized the possible premalignant nature of proliferative changes in the prostatic epithelium, but his description included a variety of findings. More than 21 years later, McNeal and Bostwick described reproducible diagnostic criteria for “intraductal dysplasia” and introduced a three-grade classification system. The following year, Bostwick and Brawer proposed using PIN as a replacement for intraductal dysplasia, and this new term was promulgated in 1989 at a consensus workshop on prostate preneoplastic lesions sponsored by the American Cancer Society and National Cancer Institute.
The diagnostic term prostatic intraepithelial neoplasia was subsequently endorsed at numerous multidisciplinary and pathology-only consensus meetings. Terms such as intraductal dysplasia , severe dysplasia , large acinar atypical hyperplasia , and duct-acinar dysplasia were discouraged and have ceased to be used in routine practice. The term intraductal carcinoma (IDC) was discouraged many years ago but has seen a resurgence recently as a separate entity that may be mistaken for PIN (see later).
The 1989 conference also recommended compression of the PIN classification into two grades: low-grade (formerly PIN grade 1) or high-grade PIN (formerly PIN grades 2 and 3) ( Table 9.2 ). The clinical significance of high-grade PIN was considered substantial at that time, whereas low-grade PIN was considered largely inconsequential, a belief that has been reinforced in subsequent decades and persists today.
| Low-Grade PIN (Formerly PIN 1) |
High-Grade PIN (Formerly PIN 2 and 3) |
|
|---|---|---|
| Acinar size | Normal; not enlarged | Normal; not enlarged |
| Architecture | Epithelial cells crowding and stratification, with irregular spacing | Similar to low-grade PIN; more crowding and stratification; four main patterns: tufting, micropapillary, cribriform, and flat |
| Cytology | ||
| Nuclei | Enlarged, with marked size variation | Enlarged; some size and shape variation |
| Chromatin | Normal | Increased density and clumping |
| Nucleoli | Rarely prominent a | Prominent |
| Basal cell layer | Intact | May show some disruption |
| Basement membrane | Intact | Intact |
Interobserver agreement between pathologists for high-grade PIN is “good to excellent,” with 67% overall agreement between pairs of reviewers. However, this is not true for low-grade PIN; also, it has a much lower predictive value for cancer that limits its clinical utility, and most do not routinely report this finding today except in research studies. Thus, the term prostatic intraepithelial neoplasia is now used interchangeably with high-grade PIN by most investigators. High-grade PIN is considered a standard diagnosis that must be included as part of the reported pathologic evaluation of biopsies, transurethral resections (TURPs), and prostatectomy specimens. The diagnostic utility when cancer is already present is uncertain, but 69% of urologic pathologists report PIN in this setting.
The mean incidence rate of isolated high-grade PIN is 9% (range, 4% to 25%) of prostate biopsies. Given that there are an estimated 1,300,000 prostate biopsies performed annually, a reasonable estimate is that there are about 115,000 new cases annually of high-grade PIN without cancer and a prevalence of more than 16 million ( Table 9.3 ). The prevalence of PIN varies according to the population of men under study, with the lowest likelihood in those participating in PSA screening and early detection studies, with an incidence rate of PIN ranging from 0.7% to 20%.
| Age (y) | High-Grade % PIN |
U.S. Population (Thousands) |
PIN | ASAP a,b |
|---|---|---|---|---|
| 40-49 | 15.2 | 20,550 | 3,123,600 | 822,000 |
| 50-59 | 24.0 | 14,187 | 3,404,880 | 567,480 |
| 60-69 | 47.3 | 9,312 | 4,404,576 | 372,480 |
| 70-79 | 58.4 | 6,926 | 4,044,784 | 177,040 |
| 80-89 | 70.0 | 2,664 | 1,864,800 | 106,560 |
| Total | 53,639,000 | 16,842,640 | 2,145,560 |
a The estimated value of 4% is used throughout as the prevalence of ASAP has not been determined as a function of age.
b It should be noted that ASAP is not a diagnostic entity but rather a diagnostic category; thus the “prevalence” shown in this table represents the theoretical detection level for this histologic finding given current methods of detection if the entire population underwent biopsy.
The relationship between the number of cores sampled and the incidence of PIN on needle biopsy is controversial, although most agree that greater sampling increases the yield of both PIN and cancer. The incidence rate of PIN on 24-core saturation biopsy was 22% to 45%.
Those undergoing TURP have the highest likelihood of PIN, varying from 2.8% to 33%. In such cases, all tissue should be examined, but serial sections of suspicious foci are usually not necessary. Unfortunately, needle biopsies fail to show the suspicious focus on deeper levels in about one-half of cases, often precluding assessment by immunohistochemistry and compounding the diagnostic dilemma.
The prevalence and extent of PIN increase with patient age ( Table 9.3 ). An autopsy study of whole-mount prostates from older men showed that the prevalence of PIN in prostates with cancer increased with age, predating the onset of carcinoma by more than 5 years. A similar study revealed that PIN is first seen in men in their twenties and thirties (9% and 22% frequency, respectively), and preceded the onset of carcinoma by more than 10 years. Most foci of PIN in young men were low grade, with increasing frequency and volume of high-grade PIN with advancing age.
Race and geographic location also appear to influence the incidence of PIN after controlling for patient age. African American men have a greater prevalence of PIN than Caucasians in the 50- to 60-year-old age group, the decade preceding detection of most prostate cancers. African American men also had the highest incidence of cancer (about 50% more than Caucasians). In contrast, Japanese men living in Japan had a lower incidence of PIN than those residing in the United States, and Asians had the lowest clinically detected rate of prostate cancer. Interestingly, Japanese men diagnosed with PIN also had an increased likelihood of development of prostate cancer, indicating that PIN is a precursor of clinical prostate cancer in Asian men. Differences in the frequency of PIN in the 50- to 60-year-old age group across races essentially mirror the rates of clinical prostate cancer observed in the 60- to 70-year-old age group.
The likely causal association of PIN with adenocarcinoma is supported by the observation that the prevalence of both increase with patient age, and that PIN precedes the onset of prostate cancer by less than one decade ( Table 9.4 ). The severity and frequency of PIN in prostates with cancer are greatly increased when compared with prostates without cancer (73% versus 32% frequency, respectively). High-grade PIN in sextant biopsy carries a 50% risk for carcinoma on subsequent biopsy within 3 years, although this risk was lower when more than six cores are obtained; this decline in predictive value is expected given the increased sampling for cancer with a greater number of core biopsies (see later).
They have similar architectural and cytologic features.
Both are located chiefly in the peripheral zone and are multicentric.
There is a close spatial association of PIN and cancer.
Growth fraction of PIN is similar to cancer.
Apoptosis-suppressing oncoprotein Bcl-2 expression is increased in PIN and cancer.
The highest grade of PIN has loss of basal cell layer, similar to cancer.
Increased frequency of PIN in the presence of cancer
Increased extent of PIN in the presence of cancer
Increased severity of PIN in the presence of cancer
PIN is more closely related to cancer than benign epithelium.
For some biomarkers there is progressive loss of expression with increasing grades of PIN and cancer, including prostate-specific antigen, neuroendocrine cells, cytoskeletal proteins, and secretory proteins.
For some biomarkers there is progressive increase in expression with increasing grades of PIN and cancer including type IV collagenase, transforming growth factor-α, epidermal growth factor, epidermal growth factor receptor, Lewis Y antigen, and c-erB-2 oncogene.
High-grade PIN and cancer have similar nuclear area, chromatin content and distribution, nuclear perimeter, nuclear diameter, and nuclear roundness.
High-grade PIN and cancer have similar nucleolar number, size, and location.
High-grade PIN and cancer have similar frequency of aneuploidy.
High-grade PIN and cancer have similar frequency of allelic loss.
High-grade PIN and cancer have similar foci of allelic loss.
There is progressive increase in microvessel density from PIN to cancer.
Cancer is found to arise in the foci of PIN.
Age incidence peak of PIN precedes cancer.
PIN on biopsy has high predictive value for cancer on subsequent biopsy.
PIN , Prostatic intraepithelial neoplasia.
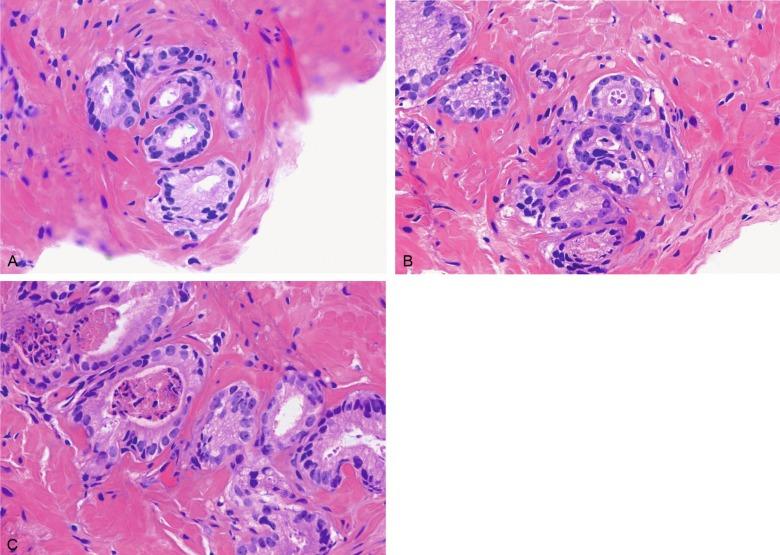
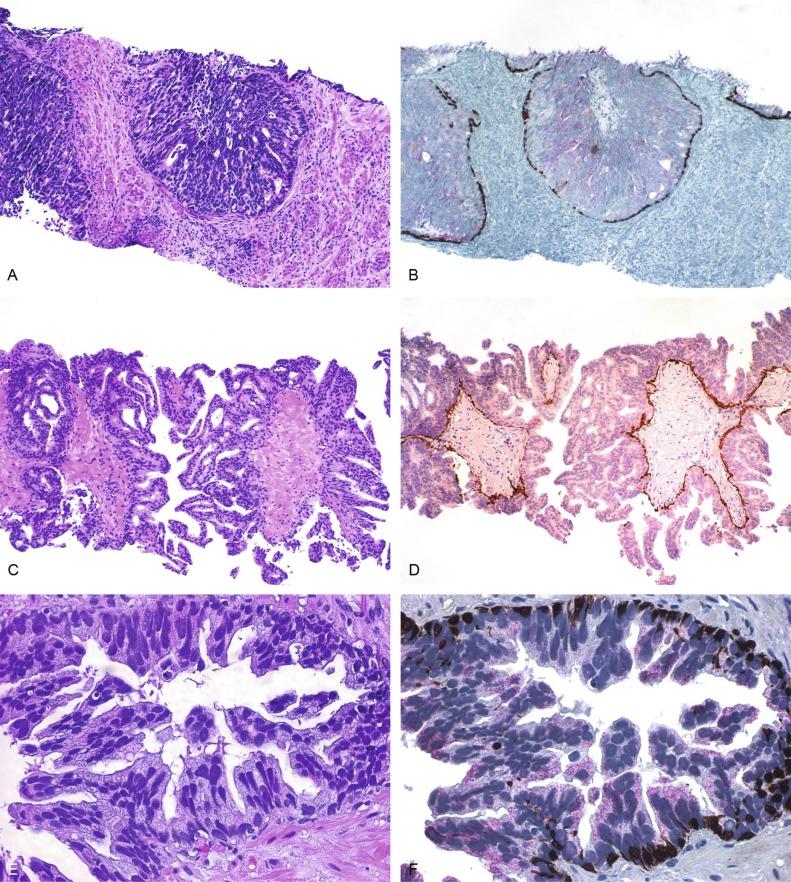
PIN is characterized by cellular proliferation within preexisting ducts and acini, with cytologic changes mimicking cancer, including nuclear and nucleolar enlargement ( Figs. 9.1 to 9.3 ). There is inversion of the normal orientation of epithelial proliferation from the basal cell compartment to the luminal surface, similar to adenoma in the colon. The requisite nucleolar enlargement must be present in at least 10% of cells within the focus to be considered diagnostic; however, overstaining, hyperchromasia, and nuclear overlap may obscure the nucleolar features. Overexpression of the MYC oncogene is responsible for increased nucleolar number and size in PIN and cancer.
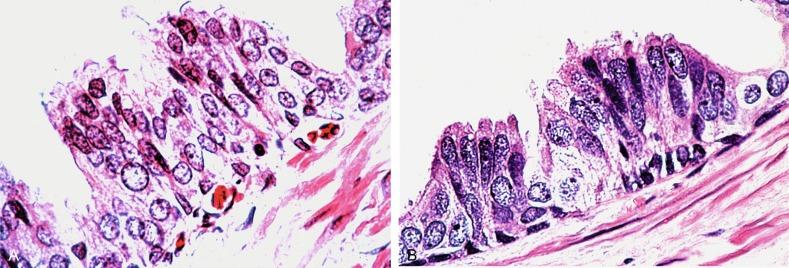
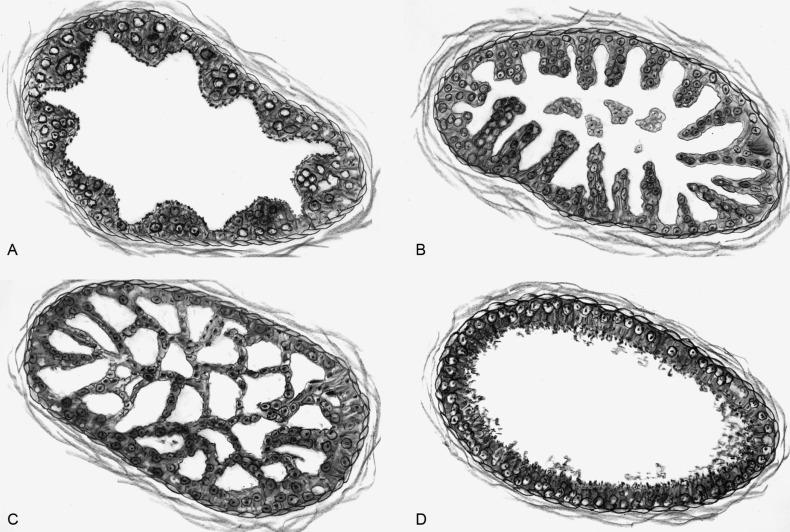
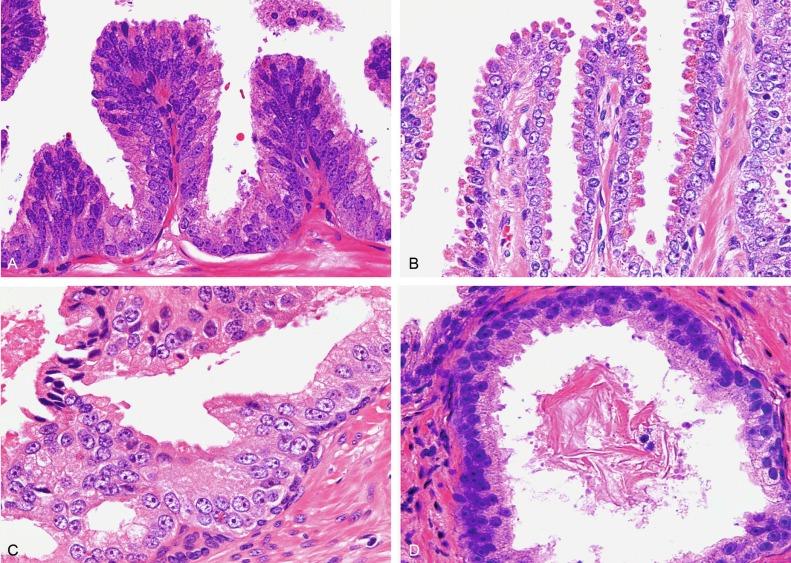
There are four main patterns of high-grade PIN: tufting, micropapillary, cribriform, and flat ( Figs. 9.1 to 9.3 ). The tufting pattern is the most common, present in 97% of cases, although most cases have multiple patterns. There are no known clinically important differences between the architectural patterns, and their recognition appears to be only of diagnostic utility. Sporadic retrospective reports have suggested that the cribriform or micropapillary patterns may indicate higher risk for coexistent cancer, but this has been refuted. Other unusual patterns of PIN include the signet ring cell pattern, small cell pattern, mucinous pattern, microvacuolated (foamy-gland) pattern, inverted (hobnail) pattern, and PIN with squamous differentiation ( Fig. 9.4 ; Table 9.5 ). The small cell pattern is usually negative for neuroendocrine markers and does not appear to be a precursor of small cell carcinoma. The hobnail pattern usually coexists with Gleason score 7 cancer.
Usual patterns
Tufting
Micropapillary
Cribriform
Flat
Unusual patterns and variants
Signet ring cell
Small cell (neuroendocrine)
Foamy gland (microvacuolated)
Hobnail (inverted)
Squamoid
The presence of extensive PIN appears to be more predictive of cancer than the more common isolated single acinus with PIN (see later). The presence of intraluminal crystalloids in combination with PIN is a more compelling indication for repeat biopsy than PIN alone.
PIN spreads through prostatic ducts in multiple different patterns, similar to carcinoma. In the first pattern, neoplastic cells replace the normal luminal secretory epithelium, with preservation of the basal cell layer and basement membrane. This pattern often has a cribriform or near-solid appearance. Foci of high-grade PIN may be difficult to distinguish from intraductal/intraacinar spread of carcinoma by routine light microscopy (see later). In the second pattern, there is direct invasion through the ductal or acinar wall, with disruption of the basal cell layer. In the third pattern, neoplastic cells invaginate between the basal cell layer and columnar secretory cell layer (“pagetoid spread”), a rare finding. Proliferative activity according to Ki67 labeling is lower in PIN (mean, 6%; range, 2% to 15%) than in ductal adenocarcinoma; the combination of histologic features and measurements of cellular proliferation help to distinguish these findings in limited tissue samples.
Ultrastructurally, high-grade PIN displays features between those of benign epithelium and adenocarcinoma. These include the presence of cells with a variable number of cytoplasmic secretory vacuoles, luminal apocrine blebs, large nuclei with coarsely clumped chromatin, enlarged nucleoli, prominent apical microvilli, intact or discontinuous basal cell layer, and intact basement membrane. Occasional acini have luminal cells abutting the basement membrane without interposition of basal cells, and other acini with extremely attenuated basal cell cytoplasmic processes contain bundles of intermediate filaments.
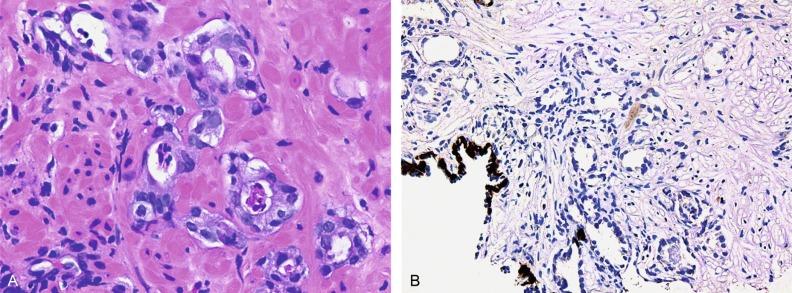
Early stromal invasion, the earliest evidence of carcinoma, occurs at sites of acinar outpouching and basal cell disruption in acini with high-grade PIN ( Figs. 9.5 and 9.6 ). Such microinvasion is present in about 2% of high-power microscopic fields of PIN and is seen with equal frequency in all architectural patterns. At the transition from histologically normal epithelium to PIN, there is a surge in phosphorylated Akt (prosurvival protein) and concomitant suppression of downstream apoptosis pathways (antisurvival proteins) that precedes the transition to invasive cancer.
The mean volume of PIN in prostates with cancer is 1.2 to 1.3 mL, and the volume increases with increasing pathologic stage, Gleason grade, positive surgical margins, and perineural invasion (PNI). These findings underscore the close spatial and biologic relationship of PIN and cancer, and may result from an increase in PIN with increasing cancer volume.
PIN and cancer are usually multicentric. PIN is multicentric in 72% of radical prostatectomies with cancer, including 63% of those involving the nontransition zone and 7% of those involving the transition zone; 2% of cases have concomitant single foci in all zones. The peripheral zone of the prostate, the area in which the majority of cases of prostate cancer occur (≥ 70%), is also the most common location for PIN. Cancer and PIN are frequently multicentric in the peripheral zone, indicating a “field” effect similar to urothelial carcinoma of the bladder. Central zone cancer is more likely to be associated with PIN in the central zone than the peripheral zone. Despite common multifocality, PIN may be a monoclonal process according to similar high-resolution genomewide signatures.
High-grade PIN and prostate cancer are morphometrically and phenotypically similar ( Fig. 9.7 ). PIN occurs primarily in the peripheral zone and is seen in areas that are in continuity with prostate cancer. PIN and prostate cancer are multifocal and heterogeneous. Increasing rates of aneuploidy and angiogenesis as the grade of PIN progresses are further evidence that high-grade PIN is precancerous. Prostate cancer and high-grade PIN have similar proliferative and apoptotic indices.
Swedish investigators claim that PIN cannot be diagnosed by fine needle aspiration (FNA) alone, although this has been refuted. PIN in combination with atypical small acinar proliferation (ASAP) suspicious for but not diagnostic of malignancy is discussed later.
Biopsy remains the definitive method for detecting PIN and early invasive cancer. Serum PSA is not significantly elevated, if at all, and PIN cannot be detected by imaging methods such as ultrasound, magnetic resonance imaging (MRI), or positron emission tomography/computerized tomography (PET/CT) scans. There is a poor correlation of PIN and PSA density. Mean PSA increased from 8 to 12 ng/mL in patients with PIN who experienced development of cancer within 2 years; those with PIN who did not have cancer during this interval had an increase in PSA from 5 to 6 ng/mL. Median PSA velocity is greater in men with PIN who were subsequently diagnosed with cancer. A velocity threshold of 0.75 ng/mL/year predicts which men with PIN experience development of cancer, and velocity was the only significant predictor of subsequent cancer detection on multivariate analysis. The ratio of free to total PSA is the same for patients with high-grade PIN and cancer.
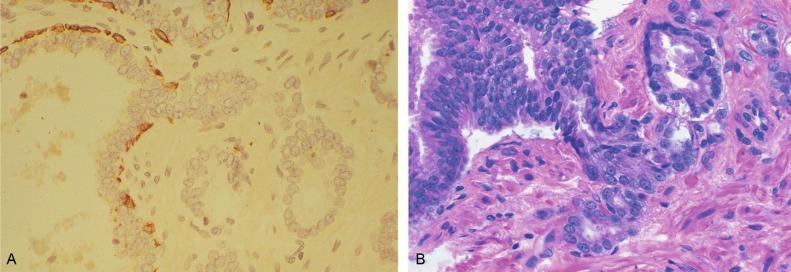
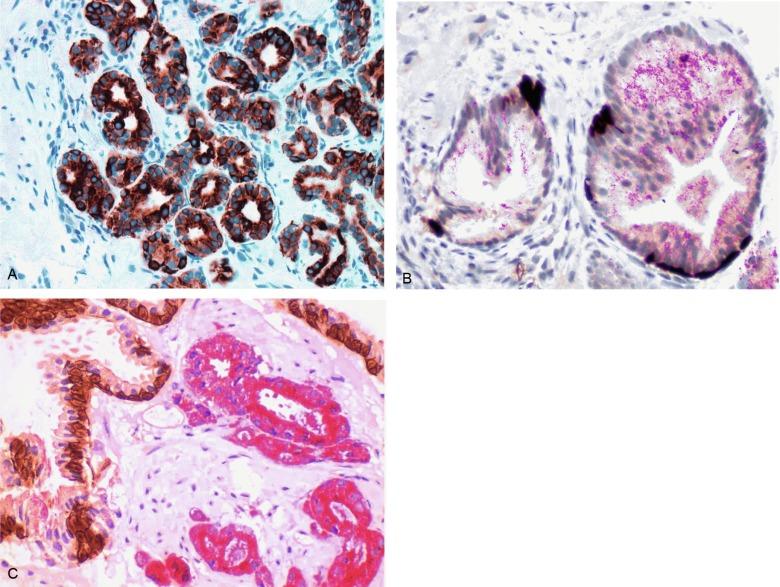
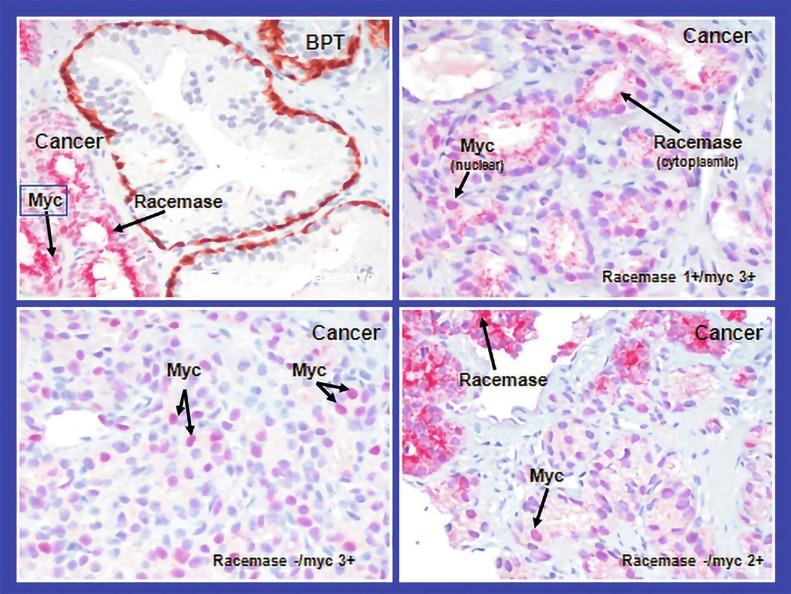
We routinely use a series of four immunostains that, in combination, are invaluable in separating benign and malignant conditions of the prostate ( Table 9.6 ). Select antibodies, such as antikeratin 34βE12 (high-molecular-weight keratin) and p63 (see later), are used to stain tissue sections for the presence of basal cells, recognizing that PIN retains an intact or fragmented basal cell layer, whereas cancer does not ( Fig. 9.8 ). In addition, racemase and c-Myc are useful for staining of the dysplastic secretory cells of PIN (see later).
Basal cells (benign: brown with diaminobenzidine stain)
Cytoplasm: keratin 34 B-E12
Nuclei: p63
Prostatic intraepithelial neoplasia and cancer cells (malignant: red with Texas red stain)
Cytoplasm: racemase (p504S)
Nuclei: c-Myc
We routinely generate unstained intervening sections of all prostate biopsies for possible future immunohistochemical staining, recognizing that small foci of concern are often lost when the tissue block is recut. One study reported loss of the suspicious focus in 31 of 52 cases.
Monoclonal basal cell–specific antikeratin 34βE12 stains the cytoplasm of most normal basal cells of the prostate, with continuous intact circumferential staining in many instances. There is no staining in secretory and stromal cells. This marker is the most commonly used immunostain for prostatic basal cells, and methods of use with paraffin-embedded sections have been optimized. Antikeratin 34βE12 is formalin sensitive and requires pretreatment by enzymes or heat if formalin-based fixatives are used. After pepsin predigestion or microwaving, there is progressive loss of immunoreactivity from 1 week or longer of formalin fixation. Heat-induced epitope retrieval with a hot plate yielded consistent strong positive results with up to 1 month of formalin fixation. Staining intensity was consistently stronger for all intervals of formalin fixation when the hot plate method was used, compared with pepsin predigestion or microwaving. Weak immunoreactivity was rarely observed in cancer cells after hot plate treatment, but not with pepsin predigestion or microwave antigen retrieval. Steam-EDTA in combination with protease enhanced basal cell immunoreactivity compared favorably with protease treatment alone in benign prostatic epithelium. Nonreactive benign acini were always the most peripheral acini in a lobule, a small cluster of outpouched acini farthest from a large duct, or the terminal end of a large duct. More proximal acini had a discontinuous pattern of immunoreactivity.
Increasing grades of PIN are associated with progressive disruption of the basal cell layer, according to studies using antikeratin 34βE12. Basal cell layer disruption is present in 56% of cases of high-grade PIN and is more frequent in acini adjacent to invasive carcinoma than in distant acini. Early invasive carcinoma occurs at sites of glandular outpouching and basal cell discontinuity in association with PIN. The cribriform pattern of PIN may be mistaken for the cribriform pattern of intraductal or ductal carcinoma, and the use of antikeratin staining is often useful in making this distinction, although exceptions occur (see later). Cancer cells consistently fail to react with this antibody, although admixed benign acini may be misinterpreted as cancerous staining. Thus, immunohistochemical stains for antikeratin 34βE12 may show the presence or absence of basal cells in a small focus of atypical glands, helping establish a benign or malignant diagnosis, respectively. We believe that this antibody can be used successfully if one judiciously interprets the results in combination with the light microscopic findings. Relying solely on the absence of immunoreactivity (absence of basal cell staining) to render the diagnosis of cancer is discouraged. Nonetheless, studies have noted that the rate of equivocal cases can be reduced considerably, by 68% or from 5.1% to 1.0%, with the addition of this immunohistochemical marker. Evaluation of prostate biopsies after therapy, such as radiation therapy, may be one of the most useful roles for antikeratin 34βE12 (see later).
In addition to PIN and cancer, basal cell layer disruption or loss also occurs in inflamed acini, atypical adenomatous hyperplasia, and atrophy with postatrophic hyperplasia. There may be misinterpreted as cancer if one relies exclusively on the immunohistochemical profile of a suspicious focus. Furthermore, basal cells of Cowper glands may not express keratin 34βE12, although this has been disputed. Rare (0.2%) cases of adenocarcinoma have been reported that focally or weakly express keratin 34βE12, including foci of metastatic high-grade adenocarcinoma. Basal cell hyperplasia is a histologic mimic of cancer, and use of antikeratin 34βE12 is recommended in any equivocal cases that include this lesion in the differential considerations because it is invariably strongly immunoreactive.
Cytokeratin (CK) 5 and CK14 messenger RNA (mRNA) and protein are expressed in the basal cells of benign acini and PIN, and CK14 mRNA is present in low levels in the luminal cells of some foci of PIN. Thus, if PIN is derived from basal cells, as is currently believed, CK14 translation is depressed and a low level of CK14 mRNA may persist. CK8 mRNA and protein were constitutively expressed in all epithelia of the normal and neoplastic prostate. CK19 mRNA and protein were expressed in both basal and luminal cells of benign acini. CK16 mRNA was expressed in a similar pattern as CK19, but CK16 protein was not detected. Basal cells display immunoreactivity at least focally for keratins 10, 11, 13, 16, and 19; of these, only keratin 19 is also found in secretory cells. Other keratins found exclusively in the secretory cells include 7, 8, and 18.
Prostatic basal cells do not usually display myoepithelial differentiation, in contrast with basal cells in the breast, salivary glands, pancreas, and other sites.
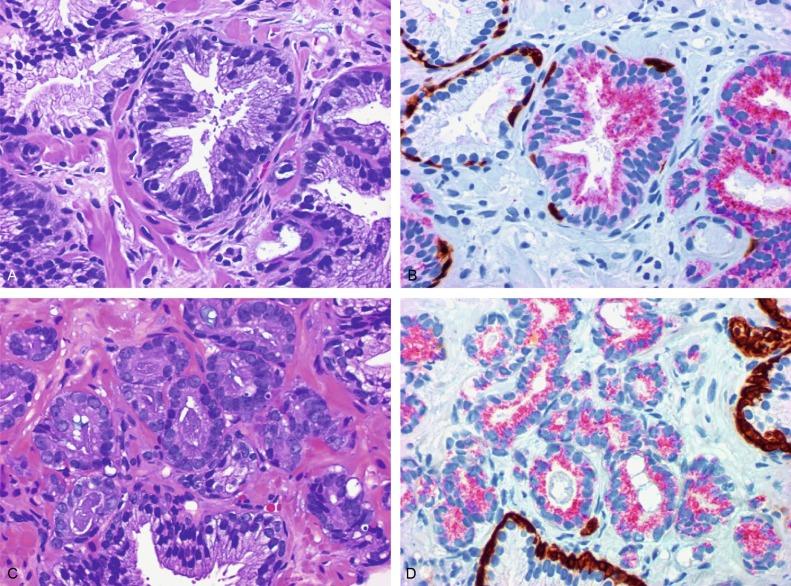
p63 is a nuclear protein that is at least as sensitive and specific for the identification of basal cells in diagnostic prostate specimens as high-molecular-weight CK staining. p63 may be more sensitive than keratin 34βE12 in staining benign basal cells, particularly in TURP specimens, offering slight advantage in diagnostically challenging cases. Basal cell cocktail (the combination of 34βE12 and p63) increases the sensitivity of basal cell detection and reduces staining variability. Triple staining with racemase, high-molecular-weight CK, and p63 is commonly used for the diagnosis of prostate cancer ( Figs. 9.8 through 9.10 ), although we believe that the addition of c-Myc (quadruple stain) optimizes diagnostic yield (discussed later in this chapter).
Aberrant (positive) p63 staining is occasionally observed in primary and metastatic prostate cancer, so diagnosis should not rely exclusively on this marker. Normal stem cells (reserve cells) are maintained by p63, and alteration of p63 expression has an oncogenic role in prostate cancer. In contrast with usual prostatic adenocarcinomas, prostate tumors with p63 expression show a mixed luminal/basal immunophenotype, uniformly lack ERG gene rearrangement, and frequently express GSTP1. Expression of cytoplasmic aberrance of p63 is associated with high ALDH1A1 expression.
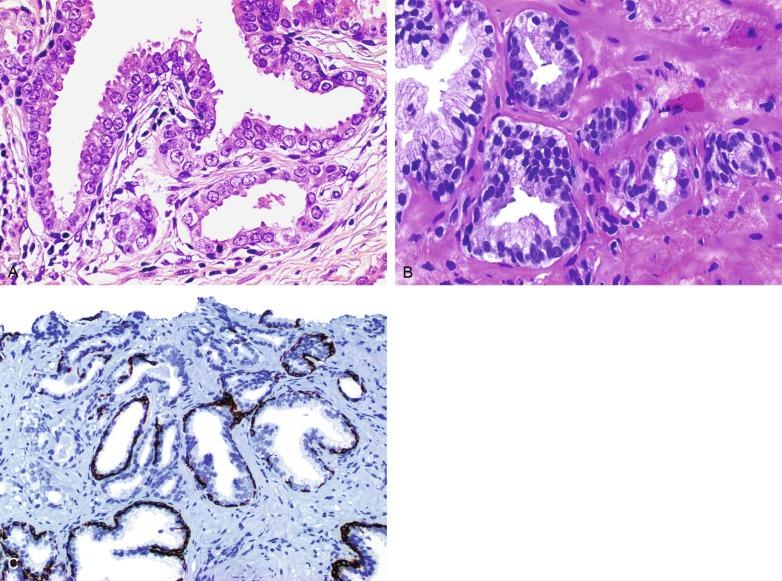
Racemase (α-methylacyl-coenzyme A [CoA] racemase [AMACR] or P504S) is invaluable for separating benign and neoplastic acini, including evaluation of PIN, ASAP, and separation of cancer from hormonally treated benign acini. This well-characterized enzyme catalyzes the conversion of several (2R)-methyl-branched-chain fatty acyl-CoAs to (S)-stereoisomers. mRNA levels of racemase are upregulated ninefold in prostate cancer. The gene for AMACR is greatly overexpressed in prostate cancer cells. Its advantage over antikeratin 34βE12 is its positive granular cytoplasmic staining in cancer cells, with little or no staining in benign acini. In PIN, monoclonal and polyclonal antibodies to racemase are positive in 77% and 91% of foci, respectively ( Fig. 9.10 ). Because racemase is not specific for prostate cancer and is present in high-grade PIN (> 90% of cases), as well as nephrogenic adenoma, this staining must be interpreted with care. The diagnosis of PIN or prostate cancer should be rendered only in combination with convincing histologic evidence. Moderate to strong racemase expression in PIN is indicative of associated adenocarcinoma. Racemase is negative in up to 27% of cancer cases.
Overexpression of racemase in PIN may be predictive of increased likelihood of cancer on repeat biopsy, but this has not been confirmed.
There is a compelling need for an immunohistochemical stain for cancer nuclei that would aid in cases in which cytoplasmic racemase staining is marginal or absent. c-Myc , a transcription factor that regulates cell proliferation, metabolism, protein synthesis, mitochondrial function, and stem cell renewal, is highly overexpressed in PIN and cancer cell nuclei, especially in those with negative racemase staining; positive c-Myc is complementary to racemase for prostate transformation. c-Myc overexpression is associated with Gleason score, unlike racemase, and may be useful for both diagnosis and prognosis of prostate cancer. There is intense nuclear staining of c-Myc in epithelial cells in 15%, 100%, and 97% of cases of benign tissue, PIN, and cancer, respectively. Mean percentage of c-Myc + cells is 0.2% (range, 0% to 5%), 34.4% (range, 10% to 50%), and 32.3% (range, 5% to 70%), respectively. In cancer acini with negative racemase, c-Myc is usually positive.
TMPRSS2:ERG gene rearrangements are highly specific for prostatic malignancy, present in about 50% of cancers and in 10% to 29% of foci of PIN, according to both immunohistochemical staining with anti-ERG antibody and TMPRSS2:ERG gene rearrangement studies. ERG rearrangement is associated with loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog), and this cooperation promotes progression of PIN to invasive cancer. ERG staining of biopsies with PIN is predictive of cancer on repeat biopsy (60% to 95% in positive cases versus 5% to 21% in negative cases). However, He et al. found a 38% risk for cancer on repeat biopsy that was independent of ERG staining status with PIN. Immunohistochemical expression of ERG in PIN is strongly predictive of ERG status in coexistent prostate cancer, with more than 95% concordance.
PIN is associated with progressive abnormalities of phenotype and genotype, which are between normal prostatic epithelium and cancer, indicating impairment of cell differentiation and regulatory control with advancing stages of prostatic carcinogenesis. There is progressive loss of some markers of secretory differentiation, cytoskeletal proteins, and multiple gene products ( Table 9.7 ). Other markers show progressive increase in expression from benign epithelium through PIN to cancer ( Table 9.7 ). A model of prostatic carcinogenesis has been proposed based on the morphologic continuum of PIN and the multistep theory of carcinogenesis.
| Increased Expression | Decreased Expression |
|---|---|
| Amphiregulin | Activated caspase-3 |
| Aneuploidy | Androgen receptor expression |
| Apoptotic bodies | Annexin I |
| Aurora-A (Aurora 2 kinase, STK-15), a protein found in centrosomes | Annexin II |
| Bcl-2 oncoprotein | Blood group antigens |
| Calcium-activated nucleotidase 1 (CANT1) | BRCA2 |
| Cell growth regulatory protein LIM domain only 2 (LMO2) | CD10 |
| Cell proliferation-associated protein Cdc46 | Fibroblast growth factor-2 Hepatocyte growth factor activator inhibitor-1 |
| c-erbB-2 (HER2) and c-erbB-3 oncoproteins | Inhibin |
| c-Fes protooncogene | Insulin-like growth factor binding protein-3 |
| Claudin-3 | Interstitial collagenase (MMP-1) |
| c-met protooncogene | Neuroendocrine cells |
| c-Myc | NKX3.1 homeobox gene-encoded protein |
| Cyclooxygenase-2 (COX-2) | Ornithine decarboxylase |
| Cysteine-rich secretory protein 3 (CRISP3) | Prostate-specific antigen |
| Dentin sialophosphoprotein | Prostatic acid phosphatase |
| Ep-Cam transmembrane glycoprotein | p-Cadherin Prostate-specific transglutaminase |
| Estrogen receptor α and β | Telomerase |
| FAS-related apoptosis signaling pathway markers FADD-FAS associating protein with death domain, pro-caspase-8, and caspase-8 | p27KIP1 |
| Gelatinase B (MMP-9), matrilysin-1 (MMP-7) and the membrane-type 1-MMP (MT1-MMP) | 5α-Reductase type 2 |
| G-protein–coupled receptor PSGR2 | 15-Lipoxygenase 1 and 2 |
| G 1 cell-cycle arrest regulator p16(INK4a) | |
| Glutathione S-transferase P1 | |
| Heat shock protein 90 | |
| Human glandular kallikrein 2 (hK2) | |
| Hypoxia-inducible factor 1α (HIF-1α) | |
| IL-6 and IL-10 | |
| Insulin-like growth factor binding protein IGFBP-rP1 | |
| Ki67 (MIB-1) expression | |
| Lewis Y antigen | |
| Macrophage inhibitory cytokine-1 (MIC-1) | |
| Matriptase, a type II transmembrane serine protease | |
| Matrix metalloproteinase-26 (MMP-26) | |
| Metallothionein isoform II | |
| Microvessel density | |
| Minichromosome maintenance protein-2 (Mcm-2) | |
| Mitochondrial protein MAGMAS | |
| Mitotic figures | |
| Mammalian target of rapamycin signaling pathway markers 4E-BP1 and p-4E-BP1 | |
| Mutator (RER + ) phenotype | |
| Neuropeptide Y (NPY) | |
| NOS-1 and NOS-2 | |
| Osteopontin | |
| Polo-like kinase-1 (PLK-1) | |
| Prolactin receptor | |
| Promoter methylation of GSTP1 gene | |
| Prostate-specific membrane antigen | |
| Prothymosin α | |
| Rac-specific guanine nucleotide exchange factor Tiam1 | |
| RNase III endonuclease Dicer | |
| Serine/threonine kinase Pim-1 | |
| Skp2 (E3 ubiquitin ligase) | |
| Slit2 | |
| Tenascin-C | |
| Transforming growth factor-α (TGF-α) | |
| Tissue inhibitor of metalloproteinases (TIMP-1 and TIMP-4) | |
| TXA 2 synthase and TXA 2 receptors | |
| Type IV collagenase | |
| Vascular endothelial growth factor (VEGF) | |
| p21 | |
| p53 | |
| p62 sequestosome 1 (SQSTM1) gene product | |
| 5α-reductase type 1 |
a The preponderance of reported results supports this conclusion.
High-grade PIN and prostate cancer share similar genetic alterations. For example, 8p12-21 allelic loss is commonly found in cancer and microdissected PIN. Other genetic changes found in carcinoma that already exist in PIN include loss of heterozygosity (LOH) at 8p22, 12pter-p12, 10q11.2, and gain of chromosomes 7, 8, 10, and 12, and the 8p24 and PTEN genes. LOH frequencies at 13q (one of the most common chromosomal alterations in high-stage cancer) is 0% in PIN versus 49% in clinical prostate cancer. Alterations in oncogene Bcl-2 expression and RER + phenotype are similar for PIN and cancer. Up to 64% of patients with PIN have LOH for the mannose 6-phosphate/insulin-like growth factor 2 receptor ( M6P/IGF2R ) gene, a marker for glycolytic metabolism.
Short telomere length in PIN and the surrounding stroma is associated with an increased risk for cancer. Telomere length is also predictive of time from the original biopsy to diagnosis of cancer. Overexpression of p4EBP1 predicted cancer with sensitivity and specificity of 63% and 100%, respectively.
PIN is epigenetically similar to carcinoma according to the percentage of methylated alleles for the APC , GSTP1 , and RARbeta2 genes. Methylation of the apoptosis-associated ASC promoter region is increased in PIN and cancer. TMPRSS2 exon 1 is fused in-frame with ERG exon 4 in 50% of prostate carcinomas and in 19% to 21% of cases of PIN but not in benign controls. PIN and cancer have higher levels of oxidative stress measured by urinary F2-isoprostane than benign prostatic tissue. Another mechanism associated with early prostatic carcinogenesis is deSUMOylation (SUMO refers to small ubiquitin-like modifier proteases), probably through enhanced cell proliferation resulting in PIN. Overexpression of p16INK4A in PIN is reportedly an independent predictor of relapse.
Microvessel density is higher in high-grade PIN than in adjacent benign prostatic tissue, and capillaries are shorter, more widely spaced, have more open lumina and curvaceous external contours. PIN vessels are lined by greater number of endothelial cells. PIN is virtually always accompanied by proliferation of small capillaries in the stroma. It is likely that PIN initially co-opts adjacent vessels, similar to other tumors, and that these vessels soon regress, only to be followed by marked angiogenesis at the cancer’s edge. Microvessel density is higher in cases with PIN associated with prostate cancer than in foci of isolated PIN or benign prostatic hyperplasia (BPH).
A critical balance exists between the proangiogenic vascular endothelial growth factor (VEGF) and the angiogenic antagonist angiopoietin-2. Angiogenin is a polypeptide involved in the formation and establishment of new blood vessels necessary for growth and metastasis of cancer. The percentage of cells staining positively for angiogenin in benign epithelium, PIN, and cancer is 17%, 58%, and 60%, respectively, confirming the potential role that angiogenin plays in neoplastic progression.
The prevalence and extent of PIN is decreased after radiation therapy, although PIN persists in 9% of biopsies after a course of three-dimensional external beam conformal radiation therapy.
After radiation therapy, PIN retains the features characteristic of untreated PIN and is readily recognized in tissue specimens. The key pathologic features include nuclear crowding, nuclear overlapping and stratification, nuclear hyperchromasia, and prominent nucleoli. The basal cell layer is present but often fragmented; racemase shows strong apical to diffuse cytoplasmic staining. The most common patterns of PIN after radiation are tufting and micropapillary, similar to those reported in untreated PIN.
The long-term efficacy of radiation treatment may depend on eradication of both cancer and precancerous lesions that could induce secondary metachronous invasive cancer. Does recurrent cancer after irradiation result from regrowth of incompletely eradicated tumor or from progression of incompletely eradicated PIN?
There is a marked decrease in the prevalence and extent of high-grade PIN after androgen deprivation therapy. This decrease is accompanied by epithelial hyperplasia, cytoplasmic clearing, and prominent glandular atrophy, with a lower ratio of glands to stroma.
In the normal epithelium, luminal secretory cells are more sensitive to the absence of androgen than basal cells, indicating that the cells of PIN share this androgen insensitivity with basal cells. The loss of some normal, hyperplastic, and dysplastic epithelial cells with androgen deprivation is probably due to acceleration of programmed cell death (apoptosis).
There is heterogeneity in the response of PIN to therapy that probably varies with type of therapy and duration. Three months of LHRH-agonist leuprolide and antiandrogen flutamide results in a 50% reduction in the incidence and extent of PIN; 6 months induces even greater reduction. Flutamide decreases the prevalence and extent of high-grade PIN and induced epithelial atrophy, and cessation of therapy results in return of PIN. A randomized prospective trial of 60 men with PIN revealed similar rates of adenocarcinoma at 1 year for those treated with flutamide or placebo (14% and 10%, respectively).
Another antiandrogen, bicalutamide, lowers mean cancer volume, mean PIN volume, and incidence of PIN after a few months. At prostatectomy, Gleason score is similar to the untreated control group. There is no evidence of higher-grade cancer emergence after treatment.
The results of 5α-reductase (finasteride) treatment of PIN are controversial, and the cumulative number of cases studied is probably too small to draw conclusions. Two reports found no apparent effect on the histologic appearance or extent of high-grade PIN, whereas a third study of three cases noted atrophy, involution, and decreased prevalence. In the Prostate Cancer Prevention Trial (PCPT), finasteride decreased the overall risk for PIN (alone and with cancer) from 12% in the placebo group to 9% in the finasteride group.
Toremifene is a selective estrogen receptor modulator that eliminates high-grade PIN and reduces the incidence of prostate cancer. After 4 months of toremifene, 72% of men treated (versus 18% of controls) had no high-grade PIN on subsequent prostate biopsies. In another study, cumulative risk for prostate cancer was reduced in patients taking toremifene compared with placebo (24% versus 31%) with an annualized rate of prevention equivalent to 7 cancers per 100 men treated. Among patients with no biopsy evidence of cancer at baseline and at 6 months, the 12-month incidence of prostate cancer was reduced by 48% with toremifene compared with placebo (9% versus 17%). The final results, however, at 3 years failed to yield a significant difference in cancer yield between untreated and treated patients (35% versus 32%, respectively).
A prospective randomized 3-year trial of selenium versus placebo in men with PIN revealed no difference in cancer yield (37% versus 36%, respectively).
The histologic differential diagnosis of PIN includes a variety of benign conditions including atrophy, postatrophic hyperplasia, atypical basal cell hyperplasia, cribriform hyperplasia, and metaplastic changes associated with radiation, infarction, and prostatitis ( Tables 9.8 and 9.9 ; see Chapter 8 ). Many of these display mild architectural and cytologic atypia, including enlarged nucleoli. The diagnostic difficulty is compounded when these findings appear in small, cauterized, or distorted specimens.
| Histologic Mimic of PIN | Differentiating Features |
|---|---|
| Inflammatory reactive changes |
|
| Urothelial metaplasia |
|
| Seminal vesicles and ejaculatory ducts |
|
| Cribriform hyperplasia |
|
| Postatrophic hyperplasia |
|
| Atypical basal cell hyperplasia |
|
| Low-grade PIN |
|
| Intraductal carcinoma |
|
| Large gland variant of Gleason pattern 3 |
|
| Mimic of Prostatic Intraepithelial Neoplasia | No. of Cases (%) |
|---|---|
| Basal cell hyperplasia | 12 (20%) |
| Benign proliferative epithelium (noncentral zone) | 10 (17%) |
| Low-grade prostatic intraepithelial neoplasia | 10 (17%) |
| Reactive changes | 10 (17%) |
| Atypical basal cell hyperplasia | 7 (12%) |
| Central zone epithelium | 5 (8%) |
| Urothelium | 2 (3%) |
| Seminal vesicle | 1 (2%) |
| Cribriform hyperplasia | 1 (2%) |
| Postatrophic hyperplasia | 1 (2%) |
| Atrophy | 1 (2%) |
Neoplastic mimics of PIN include IDC (see later), cribriform adenocarcinoma, ductal carcinoma, and urothelial carcinoma. Proliferative activity is higher in ductal carcinoma than in PIN (Ki67 labeling, 33% versus 6%). Stratified epithelium in noncribriform glands of cancer can also resemble high-grade PIN.
Inflammation may cause prostatic carcinogenesis, but the evidence to date is inconclusive or contradictory, probably because of the ubiquitous presence of inflammation in the prostate (see Chapter 8 ). Chronic inflammation is present in about 8% of biopsies and appears to confer a protective effect from PIN and cancer even after adjusting for age, digital rectal examination findings, serum PSA, and prostate volume. Inflammation is inversely related to cancer risk, and atrophy is marginally related; whereas, PIN is a significant risk factor.
Proliferative changes of the epithelium are linked to carcinogenesis in almost all epithelial malignancies. Proliferative regenerative change, referred to in the prostate as proliferative inflammatory atrophy, may be premalignant. The hypothesis is that cellular injury and regeneration characteristic of inflammatory atrophy is induced by inflammation and release of reactive oxygen species (oxidative stress) resulting from insult caused by chemicals (e.g., dietary carcinogens), physical factors (e.g., arteriosclerosis-induced ischemia), or bacteria. The regenerating cells are at increased risk for mutation, which predisposes them to cancerous initiation, promotion, and progression. The clinical implication of this hypothesis is that antiinflammatory drugs could potentially block procarcinogenic inflammatory processes. Evidence to date is inconclusive as to whether atrophy is a precursor of PIN, direct precursor of cancer that bypasses PIN, or simply an epiphenomenon (see Chapter 8 ).
What is clear is that any association with cancer requires proliferative changes, and these changes in the setting of atrophy are most often associated with inflammation. Substantial inflammation is often linked with infectious and noninfectious prostatitis, and emerging data indicate a causative role for inflammation in prostate cancer development over time, although there are conflicting data (see Chapter 8 ). The presence of CD68-immunoreactive inflammatory cells in the periprostatic white adipose tissue is associated with high-grade cancer. Despite the controversy regarding the role of atrophy (or lack thereof), virtually all authors agree that inflammation-induced oxidative stress is the most plausible explanation for initiation of prostatic carcinogenesis.
The clinical importance of recognizing PIN is based on its strong association with prostatic carcinoma. PIN in biopsies has a high predictive value for adenocarcinoma (36% in repeat biopsies within 36 months), so its identification warrants further search for concurrent or subsequent invasive carcinoma, especially when multifocal ( Table 9.10 ). This strong prediction is especially true for African American men regardless of PSA level (83% versus 7% incidence rate of cancer on rebiopsy in men with and without PIN, respectively). PIN is a stronger predictor of cancer than patient age and PSA (risk ratios: 15, 4, and 4, respectively).
| Biopsy Findings | Suggested Response b |
|---|---|
| Isolated PIN (three or fewer sites) | Repeat biopsy after 1 year if PSA rises |
| Extensive or multifocal PIN (> 3 foci) | Repeat biopsy |
| ASAP | Repeat biopsy |
| ASAP + PIN | Repeat biopsy |
| Intraductal carcinoma | Immediate repeat biopsy or definitive therapy c |
a This assumes contemporary biopsies with ≥ 10 cores. Other clinical and laboratory factors such as serum PSA must always be considered.
b Monitoring serum PSA is recommended in all cases.
c Intraductal carcinoma almost always coexists with Gleason pattern 4 and 5 cancer.
High-grade PIN in TURP specimens is also an important predictive factor for prostate cancer. Among 14 patients with PIN and BPH followed for up to 7 years (mean, 5.9 years), 3 (21.4%) experienced development of prostatic cancer. Mean serum PSA concentration was higher than in those who did not experience cancer (8.1 versus 4.6 ng/mL, respectively). All subsequent cancers apparently arose in the peripheral zone and were detected by needle biopsy. Thus, all tissue should be submitted by the pathologist for examination when PIN is found in TURP specimens. The high predictive value of PIN for the development of subsequent cancer warrants reporting the presence of PIN in TURP specimens, according to the Cancer Committee of the College of American Pathologists. On the other hand, PIN in the transition zone and central zone from Norwegian men was not predictive of subsequent cancer development.
PIN is not always predictive of adverse pathologic findings including higher Gleason grade, seminal vesicle invasion, PNI, and lymphovascular invasion (LVI). However, Auskalnis et al. reported that PIN before prostatectomy predicted higher Gleason score and TNM stage. PIN on biopsy predicted contralateral lobe involvement. PIN was not predictive of biochemical failure at 32 months in patients undergoing prostatectomy and androgen deprivation therapy. Conversely, in a multivariate case-control study of 615 Swedes followed for more than 10 years, those with PIN were 89% more likely to die of prostate cancer.
The extent of PIN in needle biopsies is another strong predictor of cancer. The positive predictive value of PIN for existing cancer is 64%, with a sensitivity of 28% and a specificity of 81%. For those with minute foci of PIN (isolated PIN that involves only one core) identified on an extended core biopsy, repeat biopsy may not be necessary within the first year in the absence of other clinical indicators of cancer such as elevated PSA. Cancer detection is greater in patients with multifocal high-grade PIN than in those with unifocal PIN (70% versus 10%, respectively). When four or more cores contain PIN, the predictive value for cancer on subsequent biopsy is 39%. Kronz et al. found that the number of core samples with PIN was the only independent histologic predictor of a cancer diagnosis; risk for cancer was 30.2% with one or two cores with PIN, 40% with three cores, and 75% with more than three cores. Recutting of blocks with PIN reveals that 12% of patients have prostatic adenocarcinoma that was not previously detected.
PIN coexists with cancer in more than 85% of prostatectomies, and the likelihood of finding cancer increases with time between procedures: 32% incidence rate of cancer on repeat biopsy performed within 1 year, compared with a 38% incidence rate after 1 year.
The predictive value of PIN declines when a greater number of cores are obtained, probably contributing to the decline in predictive accuracy for cancer in recent years. The main factor is use of extended biopsy techniques that result in more thorough prostate sampling and in higher cancer detection rates; thus, there is a smaller pool of patients with an isolated diagnosis of PIN. Another factor is the lower detection rate and difficulty in the detection of the remaining small cancers; larger significant tumors may also escape detection. These factors lead to a higher frequency of negative repeat biopsies and may reflect a new steady state and a new low plateau in the predictive accuracy of this marker. In one report, the investigators demonstrated that with six core biopsies for both the initial and rebiopsy, the risk for cancer after PIN was 14.1% compared with 31.9% in the group that had eight cores or more on follow-up with an initial six-core biopsy. The risk for cancer on biopsy within 1 year after a diagnosis of PIN (13.3%) was relatively low if good sampling (eight or more cores) was initially performed.
Widespread PIN requires repeat biopsy, probably within 3 to 6 months. Isolated PIN probably does not require immediate repeat biopsy unless the serum PSA climbs. However, a recent survey of German urologists found that the majority undertake repeat biopsy regardless of extent of PIN.
Follow-up biopsy is suggested for patients with PIN within 1 year if the serum PSA increases or there is clinical suspicion for cancer ( Table 9.10 ). Some urologists perform saturation biopsies consisting of more than 16 biopsies in one session in an effort to definitively exclude cancer. Most authors agree that identification of PIN in the prostate should not influence or dictate therapeutic decisions. We are aware of 21 radical prostatectomies that were purposely (3 cases) or inadvertently performed (18 cases) in patients whose biopsies contained only high-grade PIN; all but two of the cases contained adenocarcinoma in the surgical specimen (D.G. Bostwick, personal communication, 2007).
Multiple chemoprevention trials using PIN as a risk factor for prostate cancer have met with variable success ( Table 9.11 ).
| Chemopreventive Agent | Trial Phase a | Trial Features | Results | Reference |
|---|---|---|---|---|
| Green tea catechins | 1 | 12 months of treatment; 60 men with PIN randomized to treatment or placebo | Lower incidence of cancer (3% versus 30%) | Bettuzzi et al. |
| Flutamide (antiandrogen) | 1 | 12 months of treatment; 60 men with isolated PIN randomized to treatment or placebo | No reduction in cancer (14% versus 10%) | Alberts et al. |
| Selenium, vitamin E, and soy isoflavonoids | 1 | 6 months of treatment; 71 men with PIN; no randomization or controls | Decline in prostate-specific antigen predicted lower risk for subsequent cancer | Joniau et al. |
| Bicalutamide (antiandrogen) | 1 | 6 months of treatment; 20 men with isolated PIN; control group of 22 untreated men with PIN | Lower incidence of cancer (10% versus 27%) and lower extent | Bono et al. |
| Herbal extract Zyflamend b | 1 | 18 months of treatment; 15 men; no randomization or controls | No side effects | Capodice et al. |
| Lycopene | 1 | 12 months of treatment; 40 men randomized to treatment or placebo | Lower incidence of cancer | Mohanty et al. |
| Toremifene (antiestrogen) | 2 | 12 months of treatment; 176 men randomized to treatment or placebo | Lower incidence of cancer (9.1% versus 17.4%) | Price et al. |
| Flutamide (antiandrogen) | 2 | 12 months of treatment; 172 men randomized to treatment or placebo | Lower incidence of cancer (11.2% versus 30.2%) | Zhigang et al. |
| Toremifene (antiestrogen) | 3 | 36 months of treatment; 1467 men randomized to treatment or placebo | Slightly lower cancer incidence (32% versus 35%) | Taneja et al. |
| Finasteride (5α-reductase inhibitor) | 3 | 84 months of treatment; 9454 men randomized to treatment or placebo (secondary end point) | Lower incidence of cancer (6.0% versus 7.1%) | Thompson et al. |
| Dutasteride (5α-reductase inhibitor) | 3 | 48 months of treatment; 8122 men randomized to treatment or placebo (secondary end point) | Lower incidence of cancer (3.7% versus 6.0%) | Andriole et al. |
| Selenium, vitamin E, and soy isoflavonoids | 3 | 36 months of treatment; 303 men with PIN randomized to treatment or placebo | No difference in risk for cancer (26%) | Fleshner et al. |
a Trial phase is estimated because most of these trials were not U.S. Food and Drug Administration registration trials.
b Extract consists of holy basil, green tea, ginger, Baikal skullcap, rosemary, Chinese goldthread and barberry, oregano, hu zhang, and turmeric.
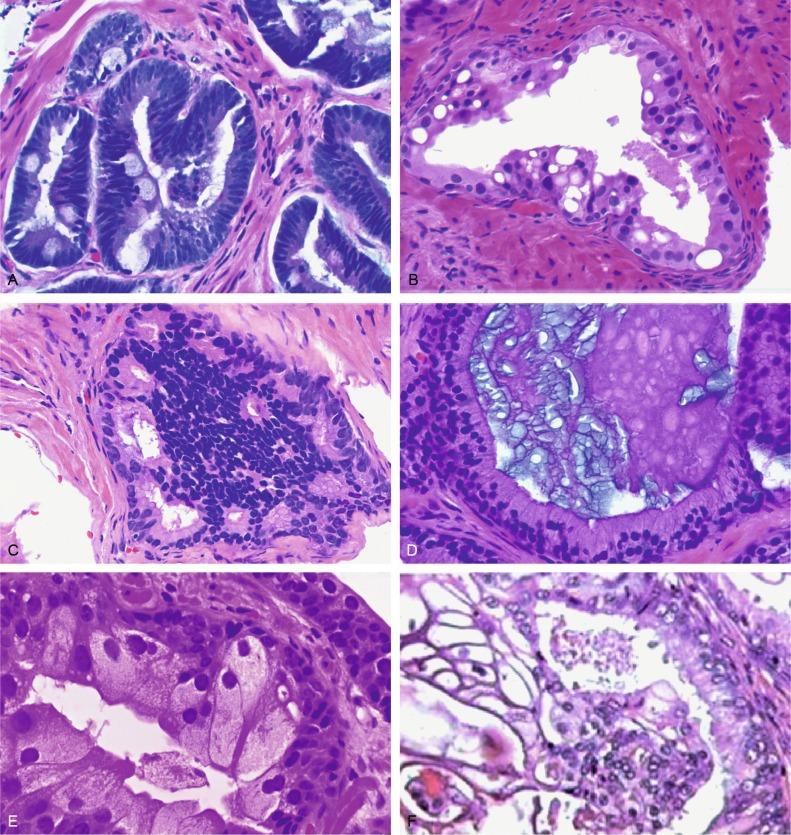
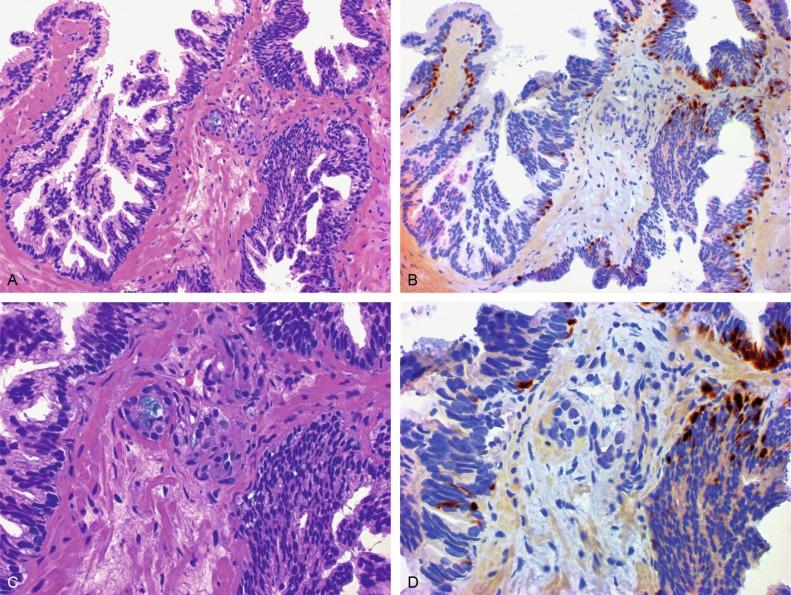
Up to 6% of contemporary needle biopsies and 1% of TURP contain collections of small acini that are suspicious for cancer but fall below the diagnostic threshold. These are reported as ASAP suspicious for but not diagnostic of malignancy ( Figs. 9.11 to 9.13 ). Cancer has been identified in subsequent biopsies in a significant number of cases, indicating that this finding is a useful predictor (see later). Identification of ASAP warrants repeat biopsy for concurrent or subsequent invasive carcinoma.
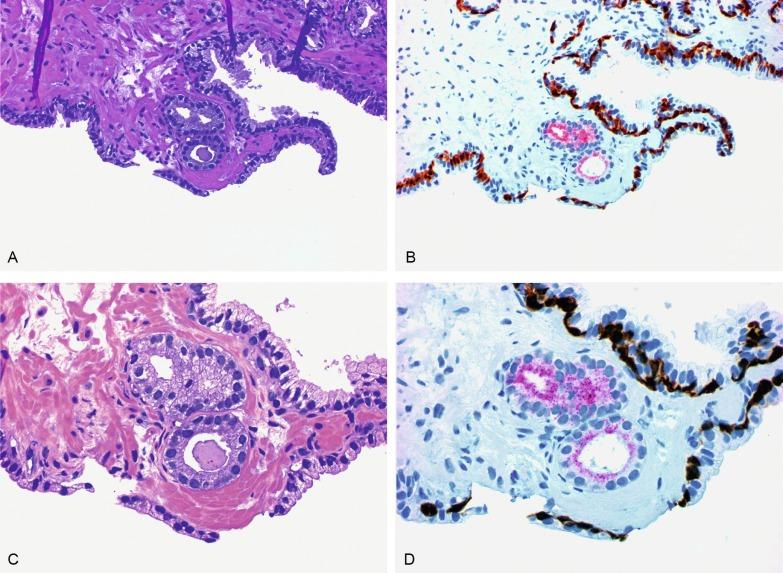
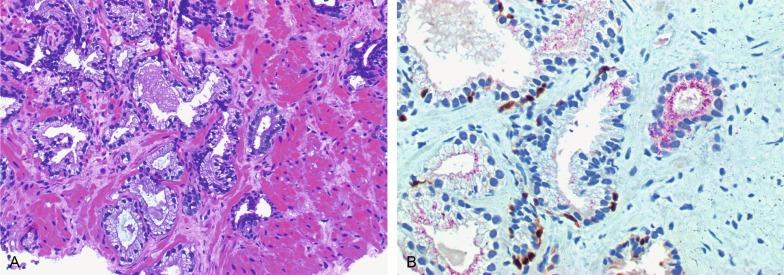
The prevalence of ASAP in men in the United States is estimated to be 2,145,560 ( Table 9.3 ). It should be noted that ASAP is a diagnostic category, but not a true histopathologic entity (most cases probably represent undersampled adenocarcinoma), so the prevalence data are simply a gauge of the magnitude of the diagnostic problem of ASAP that would be encountered if all men underwent biopsy (biopsy is the only method for detection of ASAP).
ASAP represents our inability to render an incontrovertible diagnosis of cancer in a needle biopsy. The focus of concern is invariably no larger than two dozen acini, less than the size of the head of a pin, so the major concern is overdiagnosis of cancer based on insufficient evidence. The diagnostic difficulty with ASAP usually results from one or a combination of the reasons listed in Table 9.12 . All of these may hinder the diagnosis of carcinoma, but in such cases the possibility cannot be definitively excluded. The need for this category is based on our “absolute uncertainty.” That this need exists is manifested by the variety of terms or synonyms currently in use that include the word atypical to describe this diagnosis, although atypical small acinar proliferation is now the preferred term and is most widely used clinically around the world. The diagnosis of ASAP indicates to the clinician that the biopsy in question exhibits histologic features that are neither clearly malignant nor benign, and that follow-up of the patient is warranted.
Small number of acini in the focus of concern (invariably less than two dozen acini)
Small focus size, average 0.4 mm in diameter
Focus present at core tip or biopsy edge, indicating that the focus is incompletely sampled
Loss of focus of concern in deeper levels
Distortion of acini raising concern for atrophy
Lack of convincing features of cancer (insufficient nucleomegaly or nucleolomegaly)
Clustered growth pattern mimicking a benign process such as atypical adenomatous hyperplasia
Foamy cytoplasm raising concern for foamy gland carcinoma
Focally positive high-molecular-weight cytokeratin
Positive p63 staining
Negative racemase immunostain
Histologic artifacts such as thick sections or overstained nuclei
Tangential cutting of adjacent high-grade prostatic intraepithelial neoplasia
Architectural or cytologic changes (nucleomegaly and nucleolomegaly) caused by inflammation or other lesions
For pathologists, three questions need to be answered before the diagnosis of ASAP or cancer in a small lesion: (1) Would you be absolutely confident of this biopsy diagnosis if it were followed by a negative prostatectomy? (2) Would another pathologist agree with the diagnosis of cancer? and (3) Can you confidently support the diagnosis of adenocarcinoma based solely on this biopsy? If the answer to any of these questions is no, then we recommend use of the more conservative ASAP diagnosis. In this setting, we believe that “atypical small acinar proliferation” is a valid diagnostic category if it is used judiciously, and that maximum information has been obtained from the available tissue. Other evidence useful in supporting a cancer diagnosis, including patient age, serum PSA concentration, and results of high-molecular-weight CK, p63, racemase, and c-Myc studies, cannot substitute for convincing hematoxylin and eosin (H&E) microscopic findings. To avoid bias, the above information should be considered only in combination with microscopic examination.
The histologic features that most often preclude a definitive diagnosis of malignancy are the small size of the focus (70% of cases), disappearance on step levels (61%), lack of significant cytologic atypia such as nucleolomegaly (55%), and associated inflammation (9%), raising the possibility of one of many mimics of adenocarcinoma ( Fig. 9.14 ). Other causes include negative staining for high-molecular-weight CK or p63, atrophic changes or inflammation accompanying glands lacking a basal cell layer, and the presence of associated PIN.
Immunohistochemical stain for racemase facilitates the diagnostic support provided by antikeratin 34βE12, p63, and c-Myc, particularly in equivocal biopsies such as ASAP ( Fig. 9.15 ). These immunostains resolve 76% of ASAP diagnoses. We routinely use these important techniques in the diagnostic workup of atypical prostate lesions on needle biopsies, thereby decreasing the incidence of ASAP while reducing the risk for false-negative results and the need for additional biopsies. Strand et al. demonstrated that preparing new recut sections and performing immunostains allowed definitive diagnosis of carcinoma in 22% of cases that would otherwise have been signed out as ASAP.
Diagnostic criteria differ between ASAP and minimal cancer ( Table 9.13 ). First, the mean number of acini and length of the focus of concern in ASAP (11 acini) are about one-half of those of minimal cancer (17 acini). The small size of a focus of concern is the commonest source of difficulty in ASAP, accounting for 70% of ASAP diagnoses. Infiltrative growth is a constant feature of prostate cancer, but also occurs in 68% to 75% of ASAP cases. All cancers have at least mild nuclear enlargement; whereas, ASAP may have little or no significant enlargement. Prominent nucleoli may be obscured by nuclear hyperchromasia, and this occurs more commonly in ASAP than in minimal cancer. The greater frequency of mitotic figures in cancer (up to 10% of cases) stands in contrast with that of benign acini or ASAP. However, mitotic figures are too rare to be a reliable diagnostic finding in small foci of cancer. Blue-gray acidic luminal mucin may be a useful discriminator, occurring in 61% of cancers compared with 42% of ASAP cases. Eosinophilic proteinaceous secretions are nonspecific, occurring with similar frequency in cancer and ASAP (73% versus 66% to 74% of cases, respectively). Crystalloids are uncommon and nonspecific, occurring in 19% of cancers and 16% of cases of ASAP. Prior studies reported incidence rates of crystalloids in 6%, 13%, and 22% of ASAP foci in 5% of benign biopsies. These data confirm that crystalloids do not pose an increased risk for cancer in subsequent biopsies. Moderate to severe atrophy accompanies more cases of ASAP than cancer. Small foci of postatrophic hyperplasia (which is invariably associated with typical atrophy and has “moderately enlarged” nuclei in 39% of cases) and typical atrophy are sometimes interpreted as ASAP. Obscuring inflammation is responsible for ASAP diagnoses in 30% of cases; however, 20% of minimal cancers also have associated acute or chronic inflammation.
| Findings | ASAP | Minimal Cancer a |
|---|---|---|
| Architectural | ||
| Linear extent (mm), mean ± SD | 0.4 ± 0.3 | 0.8 ± 0.5 |
| No. of acini, mean ± SD | 11 ± 10 | 17 ± 14 |
| Infiltrative growth | 75% | 100% |
| Cytologic | ||
| Nuclear hyperchromasia | 44% | 9% |
| Nuclear enlargement (scale of 0-3), mean ± SD | 1.2 ± 0.8 | 1.8 ± 0.7 |
| Prominent nucleoli in at least 10% of cells | 55% | 100% |
| Mitotic figure(s) | 0% | 10% |
| Luminal | ||
| Blue mucin | 6% | 33% |
| Stroma and adjacent acini | ||
| High-grade prostatic intraepithelial neoplasia in same slide | 23% | 57% |
| Moderate to severe atrophy | 59% | 35% |
Interobserver agreement for ASAP among urologic pathologists was higher than that of other pathologists (κ, 0.39 versus 0.21, respectively), but complete (100%) agreement was reached by the experts in only 7 of 20 biopsies. The experts diagnosed adenocarcinoma (49%) more often than the nonexperts (32%), and agreement was particularly poor for foci comprising fewer than six acini. The authors concluded that consultation would be of value with a specialized pathologist for ASAP before rendering the diagnosis of carcinoma.
Stratification of ASAP does not increase the predictive accuracy for cancer on repeat biopsy despite multiple attempts. We stratified suspicion in each ASAP case into three levels: ASAPB (ASAP suspicious for but not diagnostic of malignancy, favor benign), ASAPS (ASAP suspicious for but not diagnostic of malignancy), and ASAPH (ASAP highly suspicious for but not diagnostic of malignancy). ASAPB was used for cases in which we deemed the focus of concern unlikely to be cancer but could not with absolute certainty exclude the possibility. Conversely, ASAPH was used for cases in which the focus was almost certainly carcinoma, but a confident diagnosis of cancer could not be rendered. ASAPS was used for cases with intermediate suspicion.
In stratifying ASAP into these levels of suspicion, three criteria emerged as significant. Infiltrative growth was present in just over one-half of ASAPB cases but almost all ASAPH cases. ASAPS and ASAPH had greater degrees of nuclear enlargement than in ASAPB but were confounded more frequently by nuclear hyperchromasia. Hyperchromasia involved more than one-half of cases of ASAPS and ASAPH, often obscuring nuclear detail and nucleoli; nevertheless, we noted a nonsignificant trend toward prominent nucleoli in a higher percent of the cases as suspicion increased. Two other nonsignificant trends were noted with increasing suspicion for malignancy: more frequent coexistent high-grade PIN and less frequent moderate to severe atrophy.
Multiple studies revealed nonsignificant trends for increasing risk for subsequent cancer with increasing suspicion. Stratification of ASAP also did not predict the normalized percent of involvement by cancer on positive repeat biopsy. Thus at present the level of suspicion should not alter follow-up recommendations.
ASAP is predictive of prostate cancer in up 39% to 65% of repeat biopsies. Saturation biopsy “substantially” increases the cancer detection rate with or without the presence of ASAP.
ASAP represents undersampled cancer in at least 40% of cases. Iczkowski et al. observed that some men with ASAP in the first set of biopsies and benign findings or high-grade PIN in the second biopsy may still contain cancer that was not detected.
False-negative results in untreated men with documented adenocarcinoma occurred in 23% of repeat sextant biopsies. These results suggest that the current practice of performing 6 to 12 biopsies per prostate does not lower the frequency of ASAP. A declining volume of cancer at prostatectomy was noted more than a decade ago and is probably reflective of increased screening and multiple sampling. Thus as smaller-volume cancers are detected through increased sampling, many will be undersampled and not resolvable by immunostains, likely leading to an irreducible rate of ASAP diagnosis. Saturation biopsy increases the yield of ASAP.
What prostatic sites should be sampled at repeat biopsy? One study found that sampling only the side or site initially diagnosed as ASAP missed cancer in 39% of patients whose cancer was later detected exclusively at other sites, suggesting that the entire prostate should be rebiopsied.
In a provocative report, the investigators recommended immediate prostatectomy in patients with the biopsy diagnosis of ASAP. They suggested that the risk for subsequent cancer is 100% in prostatectomy specimens. We urge caution in recommending expansion of the indications for prostatectomy to include patients with ASAP. ASAP is best considered as a diagnostic risk category and not a true entity.
It is often difficult with small foci in biopsies to separate cancer from suspicious foci (ASAPS) when there is coexistent high-grade PIN; the difficulty is based on the inability to separate tangential cutting of the larger preexisting acini of PIN (that may appear as small separate adjacent acini) from the smaller discrete acini of cancer ( Fig. 9.16 ). In such cases, we prefer the term ASAP + PIN (referring to the coexistence of the two lesions, ASAP and high-grade PIN, in the same high-power microscopic field) to avoid overdiagnosis of tangential cutting of PIN and cancer. ASAP is placed first because of its stronger predictive value for subsequent cancer.
ASAP + PIN, found in up to 16% of all biopsies, has an intermediate predictive value of about 33% to 60% for cancer. Thus it is slightly lower than isolated ASAP (37%) but higher than isolated PIN. In four studies, PIN occurred with ASAP in 17%, 23%, 31%, or 41% of cases with ASAP, but most foci were not adjacent or contiguous. In a study of 12 patients with PIN and adjacent atypical glands (ASAP), 75% had cancer on repeat biopsy. The prostate cancer risk increased in the ASAP subgroups according to the extent of PIN in the initial sample, with ASAP + multifocal PIN carrying a 71% prostate cancer risk.
Differences in predictive values for cancer seen in multiple studies of PIN + ASAP arise from multiple causes. First, contiguous foci might have an intrinsically higher predictive value for cancer than those that include noncontiguous lesions. Second, selection bias present in cases referred for consultation also may have influenced some study results as compared with unselected primary cases in other cohorts. Third, the number of patients reported in some studies was so small that skewing of data in either direction might occur. Finally, the number or method of biopsy may influence results. For example, use of transperineal template saturation biopsies at 5-mm intervals throughout the prostate revealed that ASAP for two or more cores of high-grade PIN on a previous transrectal ultrasound-guided (TRUS) biopsy strongly indicated the presence of cancer on saturation biopsy. Patients who were diagnosed with high-grade PIN, or who had two or more cores with high-grade PIN or ASAP, cancer was detected in 53%, 89%, and 83%, respectively. High-grade cancer rates (Gleason score ≥ 7) in patients with ASAP and two or more cores of high-grade PIN were 20% and 80%, respectively.
The best available evidence today indicates that the presence of either or both lesions in needle biopsies is still a predictor for concurrent/subsequent cancer compared with patients who lack these lesions.
PIN was more than twice as frequently associated with minimal cancer (57%) than ASAP (23%). About one-half of cases of ASAP are probably undersampled cancer, and the smaller mean size of the foci in contemporary specimens decreases the likelihood of sampling accompanying high-grade PIN.
Although precancerous conditions are usually defined by histopathologic findings such as PIN, it is possible that nonmorphologic or subtle changes may also be predictive of either coexistent or subsequent prostate cancer. The hypothesis of malignancy-associated changes in the epithelium is based initially on subvisual architectural and nuclear chromatin features of otherwise benign epithelium adjacent to PIN or cancer according to a Bayesian belief network or image analysis.
Since the original descriptions, multiple other reports have suggested field-cancerization genetic or epigenetic damage in normal-appearing epithelium adjacent to PIN and prostate cancer, analogous to the situation with the urothelium of the urinary bladder and other sites. Mcm-2 expression as a measure of cell proliferation was higher in normal acini and PIN near cancer than those that were more distant. Likewise expression of the apoptosis-related marker a-casp3 was also elevated in normal acini near cancer, although Bcl-2 expression was not. Similar elevated field effect expression has been observed with telomerase, ERG, HOXC4, HOXC5, MME, PSMA (prostatic membrane antigen), SSTR1, BAX, Bcl-2, and the estrogen inducible protein pS2; lower expression was seen with glutathione S-transferase class π (GSTP).
Other molecular changes in benign epithelium of biopsies may predict subsequent prostate cancer. High levels of the cell survival molecule Akt-1, sometimes observed in normal epithelium, are an independent predictor of biochemical recurrence. High expression of other markers reportedly predicted cancer in subsequent biopsies, including EPCA-1 and P2X, but our laboratory was unable to reproduce these findings (DG Bostwick, unpublished observations, 2006). Mitochondrial DNA deletion assay was the first commercially available test of benign epithelium in biopsies that predicted prostate cancer in subsequent biopsies with a sensitivity and specificity of 84% and 54%, respectively (receiver operating characteristic [ROC] curve, 0.75).
Many genes show no difference in expression of benign epithelium that was and was not associated with malignancy (lack of field effect), including racemase, hepsin, fatty acid synthase, myosin VI, SPOCK, TPD52, and EZH2.
A recent study noted a fourfold increased risk for prostate cancer in men with increased number of epithelial CD4 + T regulatory cells in benign tissue.
IDC remains a controversial lesion because of publication of multiple definitions ( Table 9.14 ). The term was first introduced in 1972, but criteria were lacking and probably included numerous cases of what would be called ductal carcinoma today. Even now the distinction between intraductal and ductal carcinoma is uncertain.
| First Author | Definition |
|---|---|
| McNeal (1996) | Cancer extension within the branches of a single segment of the duct-acinar system. Three patterns of intraductal carcinoma include trabecular, cribriform, and solid/comedo, which represent progressive dedifferentiation with a reciprocal increase in proliferation. Basal cell layer is intact. |
| Guo (2006) | Same as McNeal (1996) with addition of either marked nuclear atypia (nuclear size six times normal or larger) or comedonecrosis; also added papillary pattern without fibrovascular cores. |
| Cohen (2007) | |
Five major criteria:
|
|
Three minor criteria:
|
McNeal and Yemoto defined IDC as cancer extension within the branches of a single segment of an intact duct-acinar system, usually with close proximity to cancer ( Table 9.14 ). The lumen-spanning cell masses followed normal duct contours and maintained a basal cell layer. This lesion was interpreted as being part of the evolution and spread of invasive carcinoma rather than a precursor. In most cases the cribriform pattern predominated. IDC usually coexists with invasive carcinoma, and previous authors had referred to this as “noninvasive ductal carcinoma.”
Subsequent authors attempted to refine these criteria to include quantitative measures such as nuclear size (six times normal or larger) and duct-acinar diameter (more than twice that of normal peripheral zone acini) ( Table 9.14 ). Similar to patterns of ductal carcinoma, IDC was noted to be either cribriform, solid, or papillary, although fibrovascular cores were absent ( Fig. 9.17 ). Intramucosal spread to the ejaculatory ducts was described by Sanchez-Salazar et al.
European urologic pathologists rely on solid intraductal growth (100% of colleagues), dense cribriform (96%), loose cribriform/micropapillary with nuclear size more than 6 × normal (83%) or comedonecrosis (74%) and dilated ducts greater than 2 × normal (39%). Nuclear size is interpreted as nuclear area by 74% of colleagues and nuclear diameter by 21%. Pure IDC in biopsies is reported by 100% and Gleason graded by 30% (48% assigned Gleason pattern 5 regardless of the presence or absence of basal cells). All perform immunohistochemistry in such cases to rule out invasive cancer. An intraductal component associated with invasive cancer is included in determination of tumor extent and number of cores involved by 74% and 83% of colleagues, respectively.
The presence of IDC on diagnostic needle biopsy is an independent predictor of cancer-specific survival after adjusting for clinical prognostic factors and treatment modality, but is not significant after adjustment for the 2014 International Society of Urological Pathology (ISUP) grade groupings.
Small cell pattern with rosettes may occur centrally within foci of IDC, similar to PIN, but has no apparent independent clinical significance. Furthermore, NE markers are usually negative.
Unlike PIN, IDC is strongly associated with aggressive prostate cancer, including high Gleason grade and large tumor volume ( Tables 9.15 and 9.16 ). Most important are the extent of the process (PIN is usually limited in its extent to a small number of acini at most), acinar size (PIN is found within preexisting acini without significant distortion or expansion of the acinar contours, whereas IDC is usually present in greatly expanded acini and ducts), and cytologic features (PIN is rarely if ever frankly anaplastic, whereas noninvasive and invasive ductal carcinoma often exhibit high-grade nuclear abnormalities such as nucleomegaly, anisonucleosis, and marked hyperchromasia); comedonecrosis is never observed in PIN but is occasionally observed in IDC. The incidence of IDC is higher in Japanese patients than in Americans (35% versus 13%, respectively), which is independent of Gleason score and cancer stage.
| Features | Intraductal Carcinoma | Ductal Carcinoma a | Cribriform/Papillary PIN |
|---|---|---|---|
| Acini | Large or greatly expanded (at least two to three times normal diameter); may represent true ducts and large ductules | Large or greatly expanded; may represent true ducts and large ductules | Small (normal acinar size), with smooth round contours |
| Lumen-spanning growth | Yes | Yes | Yes (cribriform) |
| Papillary growth | Papillae without fibrovascular cores | Papillae with fibrovascular cores | Papillae without fibrovascular cores |
| Cell shape | Pleomorphic | Variable | Relatively uniform without marked nuclear pleomorphism |
| Cell size | Enlarged six times normal | Enlarged, variable | Enlarged two to three times normal |
| Mitotic figures | Frequent | Variable | Infrequent |
| Comedonecrosis | Sometimes | Sometimes | Never |
| Solid or dense cribriform pattern | Sometimes | Sometimes | Never |
| Basal cell layer | Intact or fragmented | Usually absent | Intact or fragmented |
| Molecular genetics ( Table 9.16 ) | Much greater abnormalities than PIN | Greater abnormalities than PIN; greater concordance with intraductal carcinoma | Some abnormalities, but not substantial |
a Cribriform pattern of ductal carcinoma may be indistinguishable from cribriform pattern of acinar carcinoma.
| Cribriform/Papillary Prostatic Intraepithelial Neoplasia | Intraductal Carcinoma | Invasive Carcinoma | |
|---|---|---|---|
| Loss of heterozygosity | 9% | 60% | 0% a ; 29% b |
| TMPRSS2-ERG gene fusion | 0% | 75% | 75% c |
| PTEN loss | 0% | 100% d | 100% |
Unfortunately, uncertainty persists regarding precise criteria for separating IDC from PIN because of the identification of borderline cases with overlapping features or coexistent features, especially those with cribriform or near-solid growth. IDC mixed with invasive carcinoma may occasionally share architectural and cytologic features with cribriform PIN, and all may rarely coexist; in these cases, PIN is identified as an intraductal/intraacinar cribriform proliferation within small, smoothly rounded acini with low-grade nuclei that do not fulfill the typical criteria for IDC. Shah et al. found that the cribriform pattern of IDC was present in 18% of radical prostatectomies, was more common in cancer with Gleason score ≥ 7, ranged in size from 0.2 to 9.0 mm, and contained comedonecrosis in 33% of cases. Pleomorphic nuclei or giant nuclei at least 6 × of the adjacent nuclei were present in 28%. By comparison, isolated cribriform proliferation with malignant cells distant from cancer, referred to by some as isolated atypical cribriform proliferation (we call these IDC when the cells display anaplasia), was usually associated with Gleason score 6 cancers, was smaller (range, 0.2 to 1 mm), and never displayed comedonecrosis or pleomorphic or giant nuclei. Genetic studies may be useful for borderline cases (see later). PTEN and ERG expression may be useful in separating IDC and PIN (incidence rates of 61% versus 0% and 30% versus 0%, respectively).
IDC is an aggressive phenotype of prostate cancer that predicts high-grade, high-stage metastases and poor response to androgen deprivation therapy, external beam radiation therapy, and chemotherapy. It should be separated from PIN and, when present, reported in prostate biopsies and prostatectomy specimens, even when there is coexistent adenocarcinoma. Some authors consider IDC to be equivalent to Gleason pattern 4 or 5 adenocarcinoma, and diagnosis on needle biopsy should prompt therapeutic intervention rather than surveillance or repeat biopsy, as is the case for PIN. IDC occasionally coexists with Gleason grade 3 cancer.
IDC with cribriform architecture is present in 2% of autopsies and 1% of cystoprostatectomies with prostate cancer.
IDC displays significant genetic abnormalities. LOH is usually absent in Gleason grade 3 cancer, infrequent in PIN (9%) and Gleason grade 4 cancer (29%), but common in IDC (60%). The cribriform pattern of IDC, when present within 3 mm of invasive carcinoma, displays TMPRSS2-ERG gene fusion in 75% of cases, with 100% concordance with the cancerous component. Cytoplasmic PTEN loss is identified in 84% of cases of IDC and 100% with the cribriform pattern; concordance with invasive carcinoma is greater than 95%. IDC is associated with pathogenic germline DNA-repair gene mutations.
Cribriform IDC has a higher rate of biochemical failure and cancer-specific death than acinar carcinoma with or without cribriform growth. Gleason score 7 cancer with cribriform or IDC is independently associated with poorer biochemical recurrence-free survival after prostatectomy, but not after radiotherapy. IDC using the McNeal criteria is an independent risk factor for progression after prostatectomy. Androgen deprivation therapy induces regressive changes in PIN and prostate cancer but appears to have a minimal effect on IDC.
Prostate cancer is the most common cancer of men in the United States and is third only to lung and colorectal cancer as a cause of cancer death. In 2018, an estimated 29,430 Americans died of prostate cancer, and 164,690 new cases will be diagnosed. For all men, the overall probability is 1 in 8. Despite prevalence at autopsy of up to 80% by age 80 years, the clinical incidence is much lower, indicating that most men die with rather than of prostate carcinoma. Little is known about the causes of prostate cancer despite its high incidence and prevalence. Adenocarcinoma accounts for more than 95% of prostatic malignancies.
Adenocarcinoma does not have specific presenting symptoms and is usually clinically silent, although it may cause urinary obstructive symptoms mimicking nodular hyperplasia. Consequently, cancer is occasionally manifest initially in metastatic sites such as cervical lymph nodes and bone. The diagnosis may be made in the following clinical instances: (1) routine surveillance with digital rectal examination shows a nodular or diffusely enlarged prostate (clinical stage T2, T3, or T4); serum PSA level is greater than 2.0, 2.5, or 4 ng/mL (clinical stage T1c); or imaging and biopsies are positive for malignancy (lesion-directed, random, systematic, MRI-fusion, transperineal, transrectal, or saturation needle biopsies); (2) incidental carcinoma in TURP specimens (clinical stage T1a and T1b carcinoma); (3) metastatic adenocarcinoma of unknown primary; and (4) carcinoma of the prostate presenting as a rectal mass (prostate carcinoma rarely produces an eccentric or circumferential rectal and perirectal mass with or without mucosal involvement of the rectum).
Prostate cancer poses a greater risk for American men, especially African American men, than any other nonskin cancer. The veritable epidemic of prostate cancer has resulted in part from successful efforts at early detection with the use of the serum PSA test, thereby narrowing the still-enormous gap between the clinical incidence (8% lifetime risk) and autopsy-based prevalence (80% by age 80 years) ( Fig. 9.18 ). Physicians are unable to accurately stratify patients into those who will have progressive cancer and those who will not (cannot separate the “tigers” from the “pussycats”). An equally great problem is determining which men are at greatest risk for development of clinically apparent prostate cancer.
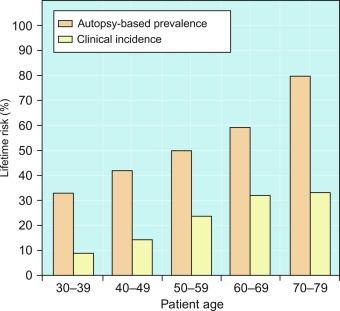
Prostatic adenocarcinoma is rare in patients younger than 40 years. Fewer than 50 cases of adenocarcinoma have been reported in each of the following groups: children younger than 12 years, adolescents, and young adults between 20 and 25 years old. In all these cases, the cancer was poorly differentiated, clinically aggressive, and unresponsive to hormonal therapy and radiation therapy.
After age 40, the incidence increases quickly. Autopsy studies of thoroughly evaluating prostates from men without clinical evidence of cancer have shown a very high level of latent (clinically occult) cancer. The incidence of prostatic adenocarcinoma is much higher in men of African ancestry (100 per 100,000) than in men of European ancestry (70.1 per 100,000), and men of African ancestry in the United States have the world’s highest mortality rate from prostatic adenocarcinoma. The prevalence of latent cancer is similar in different geographic and ethnic groups despite wide variation in the incidence of clinically apparent adenocarcinoma. The incidence is low in American Indians, Hispanics, and Asians, but high in American men of African and European ancestry.
Prostate cancer mortality varies considerably from country to country. High rates have been reported in the United States, particularly among African Americans, whereas low rates have been found in China and Japan. There are advantages and disadvantages in using mortality data to examine the underlying risk for prostate cancer. Incidence data are often unavailable, so mortality is a commonly used surrogate. However, mortality is a function of incidence, survival, and ascertainment and selection biases. International differences in mortality may reflect differences not only in the underlying risk for development of prostate cancer but also of differences in survival or ascertainment/reporting (death certificate) bias. Remarkably, the incidence of new cases surged in Japan in 2002 after the announcement that the Japanese Emperor, Akihito, had prostate cancer. Comparative autopsy study revealed a similar proportion of cancer in Russian Caucasian and Japanese men. More than 50% of cancers are Gleason score ≥ 7 in Japanese men and nearly 25% in Russian Caucasian men, raising questions about: (1) previous assumptions related to Asian prostate cancer, and (2) the notion of significant versus insignificant cancers.
The prevalence of carcinoma is most strongly related to age, with the prevalence doubling about every 14 years, and the age-specific prevalence of latent cancer at autopsy is remarkably constant across countries and ethnic groups. Data from the Connecticut Tumor Registry estimated the age-standardized prevalence rate to be 841.6 per 100,000 in 1994, an increase of 126% over the 1982 rate. In contrast, the prevalence in Japan remained largely unchanged from 2005 to 2014 (14% and 12% and Gleason score > 6 of 6% and 7%, respectively). However, another report from that country noted a great increase from 21% to 43% between 1983 to 1987 and 2008 to 2013, respectively. The dramatic differences in prevalence between these autopsy studies from Japan cannot be explained. Systematic literature review concluded that “the prevalence of incidental tumors was relatively low in earlier studies of Japanese men, although more recent study estimates are similar to the rest of the world.”
Incidental prostate cancer is less aggressive than clinically apparent prostate cancer according to stage, surgical margin status, and Gleason score. Totally sampled cystoprostatectomy specimens with bladder cancer also contain clinically undetected incidental prostate cancer in about 42% of cases (range, 15% to 68%) with the highest incidence in older men. Cystoprostatectomies with prostate cancer have lower stage and lower Gleason score than prostatectomy cases. Incidental prostate cancer in cystoprostatectomy cases is usually stage pT2a or pT2b (59% and 29%, respectively). Incidental prostate cancer is usually low grade (Gleason scores 5 and 6 in 25% and 46% of cases, respectively), much lower than for clinical prostate cancer (5% and 21%, respectively). Only 9% of incidental cancers are Gleason scores 8 to 10. The frequency of positive margins is lower than in clinical cancer (7% versus 52%, respectively).
The relationship between incidental, latent, and clinical prostate cancer may be explained by two hypotheses. One contends that incidental and latent carcinomas are identical histologically to lethal cancer, but have never acquired biologically aggressive features. The other hypothesis contends that clinically innocuous cancers are simply the smallest tumors, and cancer acquires the capacity to metastasize as a function of the passage of time, increasing volume, and “biological tumor progression,” a function of the mutational instability of all cancers, which becomes manifest in proportion to the number of mitotic events. We agree with Selman that the term latent carcinoma is a medical misnomer and should not be used.
Risk factors can be classified as endogenous or exogenous, although some factors are not exclusively one or the other (e.g., race, aging, and oxidative stress). There are numerous endogenous risk factors for prostate cancer, including family history, hormones, race, and aging and oxidative stress ( Table 9.17 ). Exogenous risk factors include diet, endocrine disrupting chemicals, and occupation.
Family history
Diet
Fat
Cadmium
Zinc
Obesity
Alcohol
Hormones
Smoking
Sexual activity
Early sexual activity
Multiple sexual partners
Occupational exposure
Agricultural fertilizers and pesticides
Rubber
Ionizing radiation
Venereal diseases
Herpesvirus type 2
Cytomegalovirus
Vasectomy
Benign prostatic hyperplasia
Prostatic intraepithelial neoplasia
Atypical small acinar proliferation
Prostatic hyperplasia is frequently seen in association with prostatic adenocarcinoma ( Fig. 9.19 ). There are several compelling similarities, including increasing incidence and prevalence with age, concordant natural history, hormonal requirements for growth and development, and response to androgen deprivation therapy. However, no causal relationship has been established or seriously suggested.
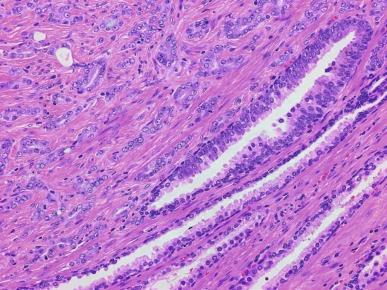
Introduction of the thin needle for transrectal biopsy of the prostate in the 1980s, together with the use of serum PSA, revolutionized early detection efforts for prostate cancer. These two advances were mutually beneficial, feeding off each other to effectively replace the large-bore transperineal needles and exclusive reliance on digital rectal examination for cancer detection. The rate of postbiopsy infection with the thin needle declined from 7% to 39% to 1%, and hemorrhage with urinary clot retention decreased from 3% to less than 1%. The false-negative rate declined from 25% to 11%, and there was an improvement in the quality of the tissue sample obtained, usually with little or no compression artifact at the lateral edges of the specimens. Also, the 18-gauge (18G) needle allows multiple biopsies of the prostate with minimal discomfort, particularly with the use of topical anesthetics such as lidocaine. Today it is hard to imagine practicing urology and urologic pathology without PSA and multiple biopsies.
A greater number of prostate biopsies are obtained currently, and more biopsy cores are submitted than ever before, creating a huge interpretive burden for the pathologist. It is estimated that more than one million biopsies are performed annually in the United States, with each biopsy consisting of on average about 10 to 12 cores, creating an estimated ≥ 10 million prostate tissue samples for the pathologist to interpret.
This burden is compounded by several factors that have increased the difficulty in prostate interpretation. First, many patients now undergo biopsy for elevated serum PSA with no other clinical evidence of cancer, resulting in an enormous number of biopsies that often contain only a small or microscopic suspicious focus. Second, numerous diagnostic pitfalls and mimics of prostate cancer have been described or refined, including postatrophic hyperplasia and IDC (see earlier in this chapter and Chapter 8 ). The great number of prostate biopsies being generated magnifies the risk for encountering rare or unusual lesions and the potential for misinterpretation of small foci. The concept of ASAPS was introduced in the late 1990s, accounting for about 2% of biopsy diagnoses. Finally, ≥ 10 biopsies (≥ 5 from each side) have largely replaced the single bilateral cores of 20 years ago and the sextant biopsies from 10 years ago, providing multiple specimens from each patient.
How can we improve the yield of cancer from prostate needle biopsies? Table 9.18 describes the known variables that influence the diagnostic yield of prostate biopsies. Fixed, uncontrolled factors included patient-related factors and prostate-related factors; however, biopsy method-related factors are controllable by the urologist and pathologist to increase the diagnostic yield of cancer and are thus deserving of additional consideration.
Patient risk factors
Patient population (e.g., screening population versus urologic practice)
Patient symptoms
Serum prostate-specific antigen
Clinical stage
Patient age
Patient race
Prior biopsy findings (e.g., prostatic intraepithelial neoplasia, atypical small acinar proliferation)
Prostate-related factors
Prostate volume
Transrectal ultrasound and other imaging findings
Urologist-controlled factors
Number of needle cores obtained
Method of biopsy (e.g., random, ultrasound-guided, magnetic resonance imaging–targeted, etc.)
Location of biopsy (e.g., laterally directed biopsies versus midline, etc.)
Amount of tissue obtained (e.g., biopsy “gun” used; operator skill)
Pathologist-controlled factors
Histotechnologist’s skill in processing and cutting prostate biopsies
Number of needle cores embedded per cassette
Number of tissue cuts obtained per specimen
Pathologist’s skill in prostate biopsy interpretation
The increase from 6 to 12 cores improved prostate cancer detection by 29%, and all were greater than 0.5 cm 3 in volume. By obtaining 10, 12, and 13 core biopsies, cancer detection rates increased by 26%, 22%, and 35%, respectively. The number of biopsy cores correlates with probability of prostate cancer detection ( Fig. 9.20 ). Saturation biopsy (often defined as > 12 or 16 cores) may be of greatest value in men with persistent suspicion of cancer after negative initial biopsy and in those with ASAP or multifocal PIN.
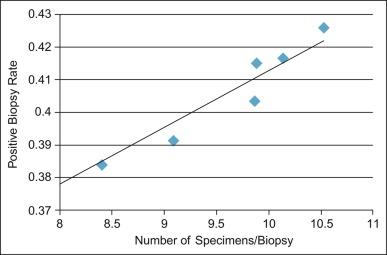
Cancer detection rate with the routine 18G biopsy needle (40%) is similar to that with the narrower 20G needle (35%), but pain is significantly less with 20G. The diagnostic yield of biopsy is improved by ultrasound-targeted or MRI-fusion–targeted biopsies, but the magnitude of the increase in accuracy remains controversial. Use of a 29-mm cutting length increases cancer yield 18% above that of a 19-mm cutting length, although the correlation of length and cancer yield has been contested. The combination of six transperineal and six transrectal biopsies resulted in cancer detection of 49%, and the detection rate increased 7% and 9% compared with the transperineal and transrectal groups alone, respectively. Increased core length per container improves cancer detection rate and Gleason score accuracy, and 12-mm cores have optimal sensitivity (42%) and specificity (62%) for cancer detection. Transperineal mapping biopsies are more accurate in determining clinical risk than transrectal biopsies and are less likely to induce sepsis, but transrectal biopsies are more popular in Europe than the United States. Template-guided transperineal saturation biopsy detects 64% of clinically significant cancers. Most of the undetected lesions are those with small volume. Approximately 20% to 30% of patients have clinically significant undetected lesions in a different lobe or different quadrant from the detected lesions in the biopsy.
Quantitation of cancer length in biopsies is discussed in the following paragraphs.
The detection rate of cancer in biopsies is higher with longer cores, particularly at the apex. For example, a 20-mm core from the right apex has 27% probability of cancer detection versus 18% for 10 mm. Only 1% of single-core biopsies are longer than 20 mm. The amount of tissue obtained by biopsy varies widely, and cumulative core sample (likely to be an inadequate sample) is less than 50 mm in 4% of biopsies. The dependence of diagnosis on tissue length is strongest at the apex. Nonglandular sampling is directly associated with shorter core lengths, with a 6-mm minimum threshold. Most urologists now routinely obtain at least 10 to 12 specimen vials per biopsy for prostate biopsies.
Lateral mid-gland and lateral base biopsy cores have the highest cancer detection rates for all prostate volumes, perhaps because of extensive sampling of the peripheral zone by lateral biopsies. Mid-gland and base biopsy cores have a relatively low yield, especially in small prostates, because of sampling of the central zone, where the prostate cancer incidence is known to be low.
Overall cancer detection rate with saturation biopsy is 44%, increasing to 52% with additional biopsies of the anterior apical region. Cancer detection rate increases 23% with a modified sextant protocol by directing the needles only into the more lateral aspect. Conversely, ultrasound-directed biopsies may be omitted when using 10-core biopsy protocols because the yield of these biopsies was less than 2%. Rebiopsy detection rate is higher with 20 rather than 12 cores.
Multiparametric magnetic resonance–transrectal ultrasound fusion–targeted biopsy may improve accuracy of: (1) detection rate of clinically significant cancer previously missed by TRUS biopsy; (2) identification of extraprostatic extension (EPE); and (3) categorization of aggressiveness based on diffusion-weighted imaging. Accuracy is influenced by interobserver variability in imaging interpretation. As a triage test, there may be a substantial number of false-negative cases with clinically significant cancer, suggesting that transperineal systematic biopsy may be superior for triage. Combining multiparametric magnetic resonance–transrectal ultrasound fusion–targeted biopsy with systematic biopsy improves the diagnostic accuracy of either single modality without increasing the rate of insignificant prostate cancer.
Prostate biopsies are particularly difficult to embed and cut because of their small size and tendency to fragment and curve. Flat embedding of the biopsy cores enhances the amount of tissue that is examined by the pathologist. Laboratories that process prostate biopsies with other tissues of differing density and consistency (e.g., breast biopsies with abundant fatty tissue) usually handle all specimens the same way, optimizing results for some tissues but often resulting in prostate biopsies that are too thick to interpret or are overstained. Excessively thick tissue specimens are two or three cells in thickness rather than the optimal one to two cells in thickness, precluding adequate assessment of nuclear and cytoplasmic details in foci of concern. Similarly, overstained sections (the most common problem in our consultation practice) contain obscured nuclear chromatin without recognizable nucleoli. These problems are compounded in biopsies with small foci that are suspicious for malignancy and in younger patients (those in their 40’s and 50’s) who have abundant proliferative epithelium that may mimic malignancy. The problems noted here apply doubly to interpretation of high-grade PIN and ASAP, accounting for both overdiagnosis (the most common mistake in current practice, in our experience) and underdiagnosis. Separate processing of the delicate prostate needle cores is recommended by the European Society of Uropathology.
Multiple needle biopsies submitted in one or two containers tend to entangle and fragment and are difficult to embed in a single plane during processing. The resulting loss of tissue surface area makes a definitive diagnosis difficult in many cases, resulting in equivocal pathology reports. If multiple cores are embedded in one cassette, it is necessary to take care that all are separated. We carefully embed up to six cores in parallel arrays per cassette after differential inking and find the cancer yield equivalent to one core per cassette with significant reduction in labor cost and effort. In a study from Finland, there was no significant difference in cancer detection rate when up to nine cores were embedded per cassette.
There is variation between laboratories in the number of serial tissue cuts obtained from each needle core for routine examination. To avoid the serious problem of undersampling, we routinely obtain six separate cuts (two adjacent sections from three separate levels) from the paraffin block for H&E staining; additional intervening sections are placed on another slide and saved for immunohistochemical stains or special studies ( Fig. 9.21 ). We consider the recommendation of the European Randomized Study of Screening for Prostate Cancer to be inadequate (they recommend only two cuts in total), probably missing up to 3% of cancers with such limited sampling. In our experience, recutting the block for additional levels with small suspicious foci is useful in about one-half of cases, with usually no more than four additional slides before the tissue specimen is exhausted. Rogatsch et al. compared biopsy cores submitted floating free in formalin with those that were stretched and oriented at biopsy and before formalin fixation, and found that the diagnostic rate of cancer increased from 24% to 31%.
Select future trends in biopsy handling and clinical significance are presented in Table 9.19 . Precise localization of cancer by site-specific labeling and three-dimensional mapping of extended saturation biopsies enables the use of targeted focal therapy such as cryosurgery. Quality-assurance measures should focus on the quantity and quality of biopsy samples. The ultimate goal of cancer detection—prediction of outcome for the individual patient—can be augmented by advanced methods of database analysis, such as artificial intelligence. Furthermore, there is continuing improvement in the definition and treatment of clinically insignificant cancer. Finally, molecular biology is beginning to revolutionize the field of diagnostic pathology.
Site-specific labeling
3D mapping
Focal therapy
Quality of biopsies
Number of biopsies
Quality assurance in urologic pathology
Use of neural networks and advanced measures of outcome
Improved understanding of “clinically insignificant” cancer
Molecular Diagnostics From Needle Biopsies
Interest in FNA in the United States is minimal because of the ease of acquisition and interpretation of the 18G needle core biopsy. Both techniques have similar sensitivity in the diagnosis of prostatic adenocarcinoma, and both are limited by small sample size; they are best considered as complementary techniques. Complications of FNA occur in less than 2% of cases and are like those with needle core biopsy including epididymitis, transient hematuria, hemospermia, fever, and sepsis.
FNA produces clusters and small sheets of epithelial cells without stroma. This enrichment for epithelium allows evaluation of single-cell morphology and the relationship between cells. Benign and hyperplastic prostatic epithelium consists of orderly sheets of cells with distinct margins creating a honeycomb-like pattern. Benign nuclei are uniform with finely granular chromatin and indistinct nucleoli; basal cells are often present at the edge. Prostatic carcinoma is distinguished from benign epithelium by increased cellularity, loss of cell adhesion, variation in nuclear size and shape, and nucleolar enlargement ( Fig. 9.22 ). A recent case of prostatic synovial sarcoma was diagnosed by FNA and confirmed by fluorescent in situ hybridization (FISH) analysis for SYT rearrangement on the cell block.
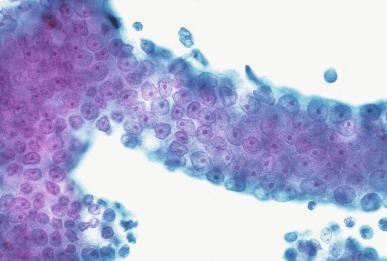
The regions of the prostate sampled by TURP and transrectal needle biopsy tend to be different. TURP specimens usually consist of tissue from the transition zone, urethra, periurethral area, bladder neck, and anterior fibromuscular stroma. Occasionally, TURP specimens may also contain small portions of seminal vesicle tissue. Radical prostatectomies performed after TURP show that the resection does not usually include tissue from the central or peripheral zones, and not all of the transition zone is removed. Most needle biopsies consist only of tissue from the peripheral zone, seldom including central or transition zones.
The role of TURP in dealing with urinary obstructive symptoms has declined in the past decade because of the introduction of ablative techniques such as the yttrium-aluminum-garnet (YAG) laser, as well as prostate-reducing medications such as 5α reductase inhibitors (e.g., finasteride, dutasteride).
The incidence rate of cancer in TURP specimens for nodular hyperplasia is about 5%. Well-differentiated adenocarcinoma found incidentally in TURP chips usually has arisen in the transition zone ( Fig. 9.23 ). These tumors are frequently small and may be completely resected by TURP. Transition zone cancer location is associated with better biochemical recurrence-free survival. Poorly differentiated adenocarcinoma in TURP chips usually represents part of a larger tumor that has invaded the transition zone after arising in the peripheral zone.
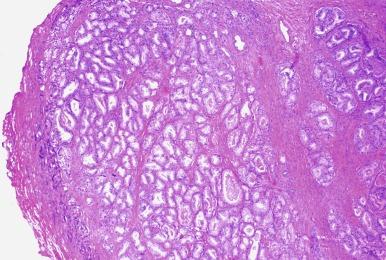
TMPRSS2:ERG and ETV1 are upregulated in acini in the peripheral zone compared with the transition zone, and these fusions account for 50% to 80% and 20% of prostate cancers, respectively. These results indicate that the benign and neoplastic glands of the two zones display distinct molecular differences and zonal-specific expression, and that ERG and ETV1 may play a role in the development and progression of cancer.
The optimal number of chips to submit for histologic evaluation is a minimum of six cassettes for the first 30 g of tissue and one cassette for every 10 g thereafter.
In patients with massive benign hyperplasia, open surgical enucleation may be preferred to TURP. The specimen usually consists exclusively of transition zone tissue and periurethral tissue with grossly visible nodules.
There are two main surgical approaches to prostatectomy. The first, retropubic prostatectomy, is the most popular approach in the United States, allowing staging of lymph node biopsies with frozen section evaluation before removal of the prostate when desired. The second surgical approach, perineal prostatectomy, does not allow lymph node biopsy during the same operation because of the anatomic approach used. Refinements in technique include nerve-sparing prostatectomy, robotic prostatectomy, and laparoscopic prostatectomy, all of which are gaining greatly in popularity.
The completeness of pathologic examination of prostatectomy specimens affects the determination of pathologic stage ( Tables 9.20 and 9.21 ). Partial sampling results in missing 29% of cases with positive margins and 20% of those with EPE. There is an increase in positive surgical margin rate (12% versus 59%, respectively) and pathologic stage with complete sectioning compared with limited sampling (sections of palpable tumor and two random sections of apex and base). Complete sectioning shows a higher detection rate of EPE (34% versus 55%, respectively) and seminal vesicle invasion (8% versus 15%, respectively) than lesser methods of sampling, even in prostates removed as part of a cystoprostatectomy. Complete sectioning also correlates with greater cancer-free survival in organ-confined and specimen-confined cases. Sehdev et al. compared 10 different methods of prostatectomy sampling using complete sectioning as the gold standard and favored two partial sampling methods ( Fig. 9.24 ) that balanced the extra time and expense of additional sections versus the risk of missing important predictive information. The first method was submission of every posterior section plus one midanterior section from right and left sides; if either of these anterior sections shows sizable tumor, all ipsilateral anterior slides are examined. This method detects 98% of tumors with Gleason score ≥ 7, 100% of positive margins, and 96% of cases with EPE (mean, 27 slides). The second method is the same as the first but with submission only of sections ipsilateral to the previous positive needle biopsy. This method detects 92% of tumors with Gleason score ≥ 7, 93% of positive margins, and 85% of cases with EPE (mean, 17 slides).
Prostate (5.5 × 3.8 × 3.5 cm) and seminal vesicles (4 × 3.2 × 1 cm) are submitted and weigh 40 and 15 g, respectively. Tumor is identified grossly involving both sides of the prostate extensively, chiefly on the right.
Pelvic lymphadenectomy tissue (right, 5.5 × 3 × 1 cm and 3 × 1 × 1 cm; left, 4.5 × 2 × 1 cm and 3 × 0.5 × 0.5 cm) submitted separately.
Radical retropubic prostatoseminovesiculectomy:
ADENOCARCINOMA (GLEASON GRADE 4 + 3 = 7). ISUP 2014 GRADE GROUPING 3. GLEASON PATTERN 4 COMPRISES 60% OF THE CANCER. TERTIARY PATTERN 5 COMPRISES 5% OF THE CANCER.
Size: about 27.72 cm3
Location: bilateral peripheral zone and transition zone
Resection margins: negative
Perineural invasion: extensive
Capsule: bilateral invasion and extensive multifocal right-sided extraprostatic extension (cumulative 1.1-cm linear length)
Premalignant change: patchy high-grade prostatic intraepithelial neoplasia
Pelvic lymph nodes: metastases to 2 of 9 right and 1 of 6 left pelvic lymph nodes
Apex: involvement of the right anterior and posterior and left posterior quadrants without extension to the margin
Bladder base: negative
Seminal vesicles: positive on the right side
Vascular/lymphatic invasion: extensive
Other: nodular hyperplasia
DNA content (flow cytometry): tetraploid (block C8; 60% cancer)
TNM (2017 revision) stage: T3bN1M: not applicable
This is optional and at the discretion of the treating surgeon. If frozen sections are requested, then all lymph nodes and perinodal adipose tissue are submitted for evaluation. Permanent sections of all frozen tissue should be obtained.
Each prostate is weighed, measured in three dimensions, and inked. Inking is performed by coating with India ink (or another preferred color) and rapid (1-2 seconds) immersion in acetone to create ink adherence to the tissue. Some use more than one ink to mark different anatomic sites (e.g., left and right sides).
Conization or shave margins are acceptable at the discretion of the pathologist; however, each pathologist is encouraged to use only one of these methods for all of their cases. Cancer involving the apex or base is not considered EPE even if the margins are positive (exception: when adipose tissue is present and cancer is in contact).
After fixation, the apex and base are amputated at a thickness of about 4 mm. The apical slice is divided into four quadrants, and each is serially sectioned at 3-mm intervals in the vertical parasagittal plane, similar to a cervical conization specimen, and submitted separately. The section from the bladder base is sectioned in a similar manner, although the amount of tissue was usually less, with divisions as hemispheres rather than quadrants. Cancer touching ink is considered a positive margin. For conization the apex usually requires quadrant sectioning, and we routinely use abbreviations for the right anterior apex (RAX), left anterior apex (LAX), right posterior apex (RPX), and left posterior apex (LPX). Similarly, the base is sampled, usually into left and right halves as left bladder base (LBB) and right bladder base (RBB), respectively. Advantages include greater localization of the cancer and determination of proximity to the margin when the margin is negative.
A thin, translucent shaving of the entire face of the apex and face of the bladder should be taken. Any evidence of cancer in these specimens indicates a positive margin. Advantages include encompassing the entire surface of the apex and base for analysis.
The seminal vesicles are amputated from the prostate at the junction of the two organs without incising the prostate itself; a slice encompassing each seminal vesicle at this junction should be submitted for routine histologic examination. If cancer is identified in the seminal vesicles or adjacent soft tissues, then the seminal vesicles should be serially sectioned in a manner similar to the prostate and submitted entirely for histologic review.
The prostate is serially sectioned as thinly as possible (about 4- to 5-mm-thick sections) by knife in a coronal plane perpendicular to the long axis of the gland from the apex of the prostate to the site of the amputated seminal vesicles. Orientation and ordering of the slices are maintained throughout to allow spatial reconstruction later of the prostate. The transverse sections are submitted in total for routine processing through neutral-buffered formalin (or equivalent alternative fixative) and sectioning as whole-mount sections (preferred) or after subdivision into two parts, four parts, or more, depending on the size of each slice. A record should be maintained so that the prostate can be reconstructed at histologic review. Routine sections should be stained with hematoxylin and eosin.
The volume of the prostate is calculated using the formula for a prolate ellipsoid, defined as length × height × width × 0.532 (correction factor for a prolate ellipsoid); no shrinkage correction factor is necessary because these measurements are made in fresh specimens.
On each slide the exact outlines of carcinoma and ablated tissue are dotted in different color inks. The area of EPE is measured by an ink line drawn along the prostatic surface, and sites of positive surgical margins are indicated with a plus (+) sign.
Cancer volume is calculated by the grid method. In brief, a transparent grid of premeasured squares is placed over the slides, and the number of squares overlying carcinoma is counted; the total number of squares per case is multiplied by the area of each square, and the sum is multiplied by the thickness of each slice of the prostatectomy. Slice thickness is calculated by dividing the fresh tissue measurement of the long axis of the prostate minus 4 mm for conization (accounts for apical section amputation) (2 mm for shave margins) by the total number of slices of the prostate. To include the volume of cancer in the apical and basal sections, the number of grids overlying cancer from these sections is multiplied by 0.09 cm 2 and then by 0.5 cm (section thickness). Final cancer volume is multiplied by 1.25, to account for tissue shrinkage caused by fixation.
The area of EPE for each case is calculated as the sum of measured line lengths on traced slides multiplied by the section thickness. The volume of tumor in the seminal vesicles is calculated by multiplying the number of grids overlying tumor in the muscular wall of the seminal vesicles by the section thickness. Positive surgical margins are defined as ink touching tumor cells (or foci of ablation); the number of separate foci with positive surgical margins is evaluated. Determinants of pathologic staging include evaluation of seminal vesicle involvement (unilateral or bilateral, as well as volume of tumor), EPE (unilateral or bilateral, as well as sites of perforation and area of EPE), and lymph node involvement (unilateral or bilateral, as well as number of nodes involved). Do not use focal versus established terminology.
The orientation of the long axis of the tumor is determined, as well as the greatest diameter of the predominant tumor nodule. Tumor location and ablated tissue location are recorded as transition zone and/or nontransition zone, with no attempt to determine precise site of tumor origin because of significant overlap of tumor and multifocality. The number of tumor foci and ablated tissue foci are counted; tumor > 2 mm from a tumor nodule is considered a separate focus by convention. Perineural invasion and vascular/lymphatic invasion are considered focal (present in less than three separate foci when evaluated by × 400 microscopic fields) or extensive (three or more foci involved).
The following variables are evaluated: Gleason primary pattern, Gleason secondary pattern, Gleason score (sum of the primary and secondary patterns), and ISUP grade grouping. The percentages of Gleason primary patterns 4 and 5, and the sum of primary patterns 4 and 5 are estimated in 10% increments. The Gleason grading scheme is noted (e.g., classic Gleason grading, ISUP 2005 modification, ISUP 2014 modification).
The presence and extent of EPE in clinical stage T2 adenocarcinoma (and hence clinical staging error) is also related to the number of blocks processed. Current guidelines for the evaluation of radical prostatectomy specimens emphasize information that should be included in the pathology report but leave the decision regarding partial or complete sampling to the pathologist. The 2009 ISUP consensus conference recommended standardization of pathology reporting of prostatectomy specimens. In response to the question relating to how much of the prostate should be blocked, participants considered both partial and complete embedding of prostates to be acceptable if the method of partial embedding is stated. Pathologists have to balance the extra costs and time involved in processing entire specimens against the risk of missing important prognostic information.
All methods begin with weighing the specimen and measuring in three dimensions: apical to basal (vertical), left to right (transverse), and anterior to posterior (sagittal), with the maximum length of each dimension recorded. Prostate weight with and without the seminal vesicles should be recorded. For ultrasonographic measurements, radiologists often describe the shape of the prostate as a prolate ellipsoid (length × height × width × 0.532), but this is only a rough estimate that shows considerable variability. Separate measurements are made of the seminal vesicles.
The gland should be fully fixed before sectioning. Several inking systems are available. It is recommended that a minimum of two different colors be used to secure correct left-side and right-side identification. The number of colors may be increased to three or four to facilitate identification of anterior and posterior. The adhesion of the ink is subsequently improved by immersing the specimen in acetone or Bouin fixative.
The apical and bladder neck margins are a common location for positive margins and EPE. The cone method is preferred for blocking the apex and the base. A thick section is amputated from the apex and the base, then cut and embedded sagittally. Sagittal slicing offers the advantage over radial slicing of producing uniform thickness tissue blocks. The shave method of the apical margin is no longer recommended because it causes either underdiagnosis or overdiagnosis of positive margins. Assessment of EPE is also more difficult with shave margin blocks. However, thin shave margins at the base are not as undesirable as apical shave margins. The bladder neck margin consists of thick muscle bundles outside of the prostate, so a positive thin shave margin at this site indicates EPE.
The remaining specimen is serially sectioned at 4 to 5 mm thickness by knife to create transverse sections perpendicular to the long axis of the prostate from the apex to the tip of the seminal vesicles. Partial and complete sampling differ by the amount of prostate tissue submitted.
The Bostwick Laboratories protocol using whole mount sections for preparing and reporting prostatectomy specimens is illustrated with multiple examples in Fig. 9.25 . Complete and careful submission of tissue for histologic evaluation allows the following: unequivocal orientation of specimen and tumor (left, right; transition zone, peripheral zone; anterior, mid, posterior; apex, base, etc.); evaluation of the extent and location of positive surgical margins; assessment and quantitation of the extent and location of EPE and seminal vesicle(s) invasion; quality-control data for the surgeon, particularly in regard to surgical margins in nerve-sparing or robotic prostatectomy. This method also facilitates postoperative measurement of tumor volume for correlation with imaging studies, as desired; evaluation of tumor grade (percentage of poorly differentiated adenocarcinoma); fulfillment of all recommendations by the Cancer Committee of the College of American Pathologists; and comparison of results with published studies.
Standard template protocols are useful because of the frequent multifocality of prostatic adenocarcinoma, the inability to fully identify the location and extent of tumor by examining randomly chosen tissue slices, and the inability to grossly identify positive surgical margins and EPE. Despite our personal preference for complete submission, most pathologists undertake partial submission, and this is practical for most cases, does not require special processing or large cassettes, and provides all necessary clinical information.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here