Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neck masses represent a diverse group of pathologic processes and require a broad differential diagnosis.
Both benign and malignant lesions can present as a mass in the neck, which makes a careful approach to these lesions important.
A complete history and physical examination as well as appropriate ancillary studies are important to arrive at the correct diagnosis.
Appropriate use of fine-needle aspiration biopsy, immunohistochemistry (p16), and endoscopy is critical for determining the presence of an upper aerodigestive tract malignancy.
Understanding the utility of different imaging modalities—such as magnetic resonance imaging, computed tomography scanning, positron emission tomography imaging, and angiography—can aid greatly in the evaluation and treatment of these differing pathologies.
Radiographic evidence is sufficient to make a diagnosis of paraganglioma.
Treatment of neoplasms of the neck depends critically on determination of a correct diagnosis and can include observation, surgery, radiation therapy, chemotherapy, or a combination of these modalities.
Neoplasms of the neck include those tumors that arise from, rather than metastasize to, lymphovascular structures of the neck and those tumors that originate from the soft tissues of the neck. These neoplasms may be either benign or malignant, and although they are less common than metastatic lesions to the neck, the clinician should always consider these tumors in the differential diagnosis of a neck mass. The emergence of human papillomavirus (HPV)–related malignancies of the head and neck region has influenced the approach to neck masses and resulted in an awareness that the majority of cystic neck masses in adults are metastatic from an HPV-associated malignancy arising within the oropharynx. The neck metastasis may be the only evidence clinically, radiographically, and/or histologically. Thus this “unknown primary” is a crucial part of the differential diagnosis for any clinician studying neoplasms of the neck. This chapter is divided into discussions of benign and malignant entities and comprises a comprehensive review of neoplasms that may originate in the neck.
The diagnosis of neoplasms of the neck involves the standard practice of the complete history and physical examination. The patient should be questioned regarding prior neoplasms, family history, and systemic signs and symptoms in addition to the risk factors for primary and metastatic lesions to this area. A comprehensive head and neck examination should include the ear and temporal bone, sinonasal cavity, nasopharynx, oropharynx, hypopharynx, and larynx and should also include a full cranial nerve examination in addition to examination of the neck. Specific information collected from the physical examination of the neck should include the character of the mass, its mobility, and any pulsation in addition to an accurate assessment of the neck level. For instance, immobility of a pulsatile mass in the craniocaudal direction may point to the presence of a carotid body tumor. If the neck mass is not pulsatile or vascular in nature, a fine-needle aspiration (FNA) should be performed; this can be done either before or after appropriate imaging. Only when the FNA is nondiagnostic or inadequate should excisional biopsy be undertaken, and at that time the surgeon should be prepared to proceed with appropriate neck dissection with margin analysis if necessary.
Computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography (US), and positron emission tomography (PET) or combinations of these studies may be useful in the evaluation of soft tissue tumors of the head and neck region. The particular advantages of each technique vary by type of tumor, location, regional disease, and proximity to vital structures. The goal of imaging neck masses is to determine (1) characteristics that may narrow the differential diagnosis, (2) the extent of disease and/or the presence of perineural invasion, (3) the mass's association with neurovascular structures, and (4) the presence of nodal disease. Although radiographic findings may provide indications to the diagnosis within the differential, accurate diagnosis cannot be ascertained without histologic evaluation.
Contrasted CT imaging is considered the gold standard for imaging the neck for the workup of most neck masses. CT imaging is particularly useful in evaluating bone detail and calcification within the tumor, and the addition of contrast improves the diagnostic utility of the soft tissue examination. CT angiography is sometimes useful as an adjunct in identifying the integrity, degree of tumor involvement, and/or compression of the carotid artery in neck neoplasms. Balloon test occlusion studies can be combined with angiography to evaluate the intracranial circulation in cases where carotid compromise may be expected from tumor growth or surgical resection. CT-directed biopsy of difficult-to-access tumors such as those of the parapharyngeal space may provide diagnostic tissue.
MRI has advantages over CT in evaluating soft tissue extent and relationships in the head and neck region; however, image quality may be degraded by patient motion. MRI is recommended for tumors adjacent to vital structures of this region, primarily for nasopharyngeal, sinonasal, and parotid lesions, and is superior to CT in assessing for the presence of perineural invasion. Similar to CT, MRI should be performed with contrast enhancement using gadolinium agents. Fat suppression is helpful for postcontrast and T2-weighted sequences; however, nonenhanced T1-weighted images without fat suppression should always be obtained because they frequently provide the best delineation of the normal anatomic structures and the extent of pathologic processes.
PET with 18-fluorodeoxyglucose with fused CT images (PET/CT) has evolved as a well-established imaging modality in the management of patients with head and neck cancer. At the time of initial workup, PET/CT is often used to assess (1) the extent of the primary tumor, (2) the presence of regional and distant metastatic disease, and (3) the presence of second primary tumors such as lung or esophageal cancers. Also, PET/CT has an expanding role in the search for an unknown primary. It also has a role in the decision making for neck treatment following definitive chemoradiation.
PET/CT is useful in the evaluation of known or suspected malignancy of the neck. PET has a high sensitivity for malignancy (it detected 93.9% of a series of 212 sarcomas with a sensitivity of 93.7% for soft tissue sarcomas and 94.6% for osseous sarcomas); however, a significant overlap remains in standardized uptake values between low- and high-grade sarcomas, and differentiation of the grade based on PET avidity remains imprecise. PET/CT with 18-fluorodeoxyglucose is of limited utility for most benign neck tumors; however, it can occasionally be useful in the management of patients with head and neck paragangliomas (PGLs) to screen for the presence of metastases or for other sites of disease. Other radionuclides, such as 18F-fluorodopa, may have a higher sensitivity. It is also clinically relevant, not only to identify malignant disease within the neck but also to provide information on regional nodal metastatic disease and potential distant disease; thus these modalities assist with accurate staging and treatment. In high-grade malignancies, it is often important to use PET or PET-CT to provide overall staging information prior to embarking on aggressive treatment.
Ultrasound imaging has become a valuable tool in the head and neck surgeon's diagnostic repertoire. It has the ability to allow the addition of imaging to the in-office examination of the neck as an adjunct to the physical examination and allows the streamlining of services in skilled hands. Examination of a neck mass with ultrasound allows for characterization of the borders and vascularity of the mass and associated structures; determining whether the mass is a lymph node or not; and, if so, whether it has suspicious features for malignancy (loss of fatty hilum, necrosis, microcalcifications, hypoechoic, abnormal vasculature). It allows not only the characterization of lymph nodes and neoplasms in the neck but also image-guided FNA biopsy of masses or nodes of interest. US as a primary prognostic tool in the neck can help decide whether elective neck dissection is necessary. Although ultrasound and ultrasound-guided FNA can decrease the likelihood of occult metastasis in oral cavity cancer, its sensitivity has been reported to range between 50% and 73% in a clinically node-negative neck. As a result, elective neck dissection is still considered the gold standard for staging the neck.
Benign neoplasms of the neck are often misdiagnosed as infectious in etiology (lymphadenitis) or congenital (branchial cleft cyst) on initial presentation. Thus the diagnosis of all neck masses requires a vigilant approach using the history, physical examination, radiologic studies, and FNA biopsy. All neck masses in an adult must be considered malignancies until proven otherwise, nearly always histologically. In particular, with the emergence of the unknown primary cystic HPV-associated squamous cell carcinoma, all patients with a cystic neck mass in the Level II or III region should have imaging dedicated to the oropharynx and neck and FNA to evaluate cytologic characteristics. This will also include immunohistochemistry (IHC) for p16, a surrogate marker of HPV status. Because benign tumors are uncommon, a review of the diagnostic approach is vital to the clinician's evaluation and management of each case. Benign primary neoplasms of the neck include vascular tumors such as PGLs, peripheral nerve neoplasms such as schwannomas or neurofibromas, lipomas, and salivary gland tumors. The following discussion of the more common benign neoplasms is categorized by tissue of origin.
PGLs comprise the most common class of benign vascular neoplasms of the neck and arise from extra-adrenal paraganglionic cells derived from the neural crest.
The paraganglion system denotes a collection of neuroectoderm-derived chromaffin cells in extra-adrenal sites. The system is vital as a source of catecholamines in fetal development prior to the formation of the adrenal medulla. Normal paraganglia contain two types of cells:
Type 1, chief cells or granular cells
Type 1 cells contain dense-core granules filled with catecholamines, a property that places them in the amine precursor and uptake decarboxylase system.
Type 1 cells predominate and are arranged in an organized, nested pattern known as Zellballen, surrounded by sustentacular cells in a fibrous stroma. Type 1 chief cells tend to be polygonal with abundant granular eosinophilic cytoplasm.
Type 2, supporting or sustentacular cells
Type 2, or sustentacular cells, are elongated cells that closely resemble Schwann cells. Their function is not entirely clear. They peripherally surround type 1 cells, are difficult to identify by light microscopy, and appear as spindle-shaped basophilic cells.
Nuclear pleomorphism and cellular hyperchromatism are common in PGLs and should not be considered evidence of malignancy. Immunohistochemistry aids the diagnosis of these neoplasms. Type 1 cells stain positively with neuron-specific enolase, chromogranin A, and synaptophysin. Type 2 cells stain with S-100 and focally with glial fibrillary acidic protein.
The controversy over the proper nomenclature of PGLs has been confusing. Historically, they have been referred to as glomus tumors, chemodectomas, and nonchromaffin tumors. The correct terminology of PGLs is based on location (i.e., carotid, jugulotympanic, and vagal PGLs). Nevertheless, the terms carotid body tumor, glomus tympanicum, and glomus jugulare persist. Other terms—such as chemodectoma, glomus tumor, and nonchromaffin tumor —are less accurate and should be avoided. Chemodectoma is an inaccurate term to describe all PGLs of the head and neck because the carotid body is the only known paraganglia of the head and neck that behaves as a chemoreceptor. The term glomus tumor more accurately describes benign cutaneous tumors that arise from the neuromyoarterial cells that surround arteriovenous anastomoses. The term nonchromaffin tumor relates to histologic staining characteristics. An early histologic staining technique using the chromaffin reaction failed to show the presence of catecholamines; PGLs were, therefore, described as nonchromaffin tumors. Newer techniques, however, have detected catecholamines in small quantities. The chromaffin reaction is a highly insensitive method by which to classify these tumors.
Approximately 90% of tumors that arise from the paraganglion system are in the adrenal gland. These tumors are termed pheochromocytomas. The remaining 10% arise from extra-adrenal sites: 85% of these arise in the abdomen, 12% in the thorax, and the remaining 3% in the head and neck area. The most common PGL of the head and neck is the carotid PGL, followed by jugulotympanic and, less frequently, vagal PGLs. Other rare sites include the larynx, nasal cavity, orbit, and trachea. It has been estimated that PGLs comprise 1 in 30,000 head and neck tumors. However, the true incidence of PGLs may be unknown because previous reports have confused PGLs with neuroendocrine tumors. Further complicating an accurate estimate is the multicentricity of these tumors, seen particularly in familial PGLs.
Carotid PGLs are the most common type of head and neck PGL and serve as the template of discussion in regard to the history, physiology, and etiologic factors relevant to PGLs.
The anatomist von Haller first described the carotid body in 1743; its function, however, was unknown at the time. Histologic studies of the carotid body revealed glandular acini, so the carotid body was renamed the carotid gland. Von Luschka first described a tumor of the carotid body in 1862. In 1880, Reigner performed the first resection of a carotid body tumor, but the patient did not survive. Six years later, Maydl resected a carotid body tumor, and the patient survived but had postoperative hemiplegia and aphasia. In 1889, Albert was the first surgeon to successfully resect a carotid body without ligating the carotid vessels. The first successful removal of a carotid body tumor in the United States was reported by Scudder in 1903. In the same year, the term paraganglion was first used by histologist Kohn to describe the carotid body. This term was most appropriate because cells of the carotid body originate from the neural crest and migrate in close association with autonomic ganglion cells, hence the term paraganglionic . In 1950, Mulligan described a neoplastic degeneration of the carotid body in a dog as a chemodectoma because of the chemoreceptor function of the carotid body.
The carotid body is located in the adventitia of the posteromedial aspect of the bifurcation of the common carotid artery. The normal carotid body measures 3 to 5 mm in diameter but is often larger in people who live at higher altitudes. The average weight of the normal adult gland is 12 mg, with a wide range previously reported as 1 to 47 mg. During surgical removal, the typical finding is a small reddish-brown to tan ovoid structure attached to the carotid vessels at the bifurcation by the Mayer ligament, through which the feeding vessels run, primarily from the external carotid artery. Blood flow and oxygen consumption of the carotid body, gram for gram, exceed those of the brain or thyroid gland. Sensory innervation is from the Hering nerve, a branch of the glossopharyngeal nerve that originates approximately 1.5 cm distal to the jugular foramen.
The carotid body has a chemoreceptor role by modulating respiratory and cardiovascular function in response to fluctuations in arterial pH, oxygen, and carbon dioxide tension. Acidemia, hypoxia, and hypercapnia stimulate the carotid body to initiate an autonomic reflex, which leads to increased respiratory rate and depth along with increased heart rate, blood pressure, and cerebral cortical activity. It is this close association with respiratory drive and the sympathetic nervous system response that has prompted investigation of the carotid body's role in disease processes such as obstructive sleep apnea and sudden infant death syndrome. The intermittent hypoxia caused by obstructive sleep apnea is sensed by the carotid body, which mediates the hypertensive response and respiratory rate. It has also been demonstrated that some children who died of sudden infant death syndrome had either small carotid bodies or a decreased ratio of mature type 1 to type 2 cells, which is hypothesized to attenuate the child's response to a hypoxic crisis.
The etiology of PGLs is multifactorial, but most (60%) are solitary. Multiple pheochromocytomas and PGLs are seen in familial syndromes, mainly multiple endocrine neoplasia types 2A and 2B. Other syndromes associated with PGLs are neurofibromatosis type 1 and von Hippel–Lindau disease, which is characterized by retinal angiomas and cerebellar hemangioblastomas. A Carney triad demonstrates the association of PGL, pulmonary chondroma, and gastric leiomyosarcoma. In addition to these associations, a syndrome of familial PGLs characterized by multiple PGLs, especially in the head and neck region, has been described and occurs in up to 20% to 25% of cases; it is associated with germline alterations in the succinate dehydrogenase gene. The familial nature of carotid PGLs was first suggested by Chase in 1933 in his description of two sisters with carotid body tumors.
Genetic mutations responsible for the hereditary form of PGL have been identified in genes that code for succinate dehydrogenase subunit D (SDHD) , B (SDHB) , and C (SDHC) , which map to chromosomes 11, 1, and 1, respectively. Succinate dehydrogenase is an enzyme complex within the inner mitochondrial matrix that is responsible for the oxidation of succinate to fumarate in the Krebs cycle as well as the reduction of ubiquinone to ubiquinol within the respiratory chain. Hereditary PGL syndrome has been classified genetically into four entities: PGL1, PGL2, PGL3, and PGL4. Germline mutations in SDHD, SDHB , and SDHC have been identified in PGL1, PGL4, and PGL3, respectively. More recently PGL2 has been associated with a mutation in SDHAF2 . Individuals with hereditary PGL syndrome have earlier onset of tumors and a higher frequency of bilateral and/or multiple tumors than do those with sporadic disease. Past reports suggest familial PGL to be rare. Recent literature, however, challenges this assertion and suggests that the association of PGLs with germline genetic mutations is likely to be significantly higher, generally around 25% to 30% of cases, with SDH mutations making up the majority, followed by VHL , RET , and NF1 .
PGL1 is caused by mutations in the D subunit of the succinate dehydrogenase gene and is the most common inherited genetic abnormality in families with a history of PGLs. All three syndromes follow an autosomal pattern of inheritance, but the inheritance pattern for PGL1 is autosomal dominant modified by genomic imprinting. Genomic imprinting in PGLs was described by van der Mey and colleagues after reviewing data from 15 large Dutch pedigrees. The imprintable gene is transmitted in a Mendelian manner, but the expression of the gene is determined by the sex of the transmitting parent. With PGLs, the gene results in the development of a tumor when it is paternally inherited. Offspring of male carriers were observed to demonstrate a 50% incidence of tumors, whereas children of female carriers never developed tumors. PGL1 is associated most commonly with head and neck tumors but also confers a risk for pheochromocytoma. It is estimated that 75% of individuals with a gene mutation will develop a tumor by age 40, with multifocal tumors arising in 56% of cases.
Germline mutations in SDHB (PGL4) are less common than their D-subunit counterparts. However, they are associated with a later onset of disease (40% penetrance by age 40) and a higher likelihood of unifocal disease; they also harbor a higher risk of chest or abdominal PGLs and pheochromocytomas as well as a 33% chance of malignancy. Identification of these individuals is crucial in the management of the at-risk family members to ensure that proper screening for pheochromocytoma and other PGLs takes place. Several cases of renal cell carcinoma and papillary thyroid carcinoma in carriers of these mutations have also been reported. The role of genetic counseling and testing in this population of patients cannot be overemphasized. Germline mutations in SDHC (PGL3) are rare and account for about 4% of head and neck PGLs. Few families that carry this mutation have been identified. There is a lower rate of malignancy than SDHB mutations and a lower rate of multicentricity than in SDHD mutations.
Because of the apparent high rate of mutations in patients with a history consistent with sporadic PGLs, consideration of genetic counseling and testing should be given to every patient with a carotid body tumor or other PGLs. Other risk factors for head and neck PGLs include living at a high altitude, which is associated with chronic hypoxemia. There has been reported a dose-dependent relationship between altitude and the incidence of paraganglionomas.
The characteristic feature of carotid PGL is a slow growth rate, which is reflected clinically by the delay between the first symptoms and the diagnosis, which averages between 4 and 7 years. A carotid body tumor usually presents as a lateral cervical mass, which is mobile laterally but less mobile in the craniocaudal direction because of its adherence to the carotid arteries. This physical finding has been called a positive Fontaine sign. Alternatively, a carotid PGL may present as a parapharyngeal mass. Many carotid PGLs are pulsatile by transmission from the carotid vessels or, less commonly, expand themselves, which reflects their extreme intrinsic vascularity. Sometimes a bruit may be heard by auscultation, but it can disappear with carotid compression. The consistency varies from soft and elastic to firm, and these tumors are generally nontender. As they enlarge, progressive symptoms of dysphagia, odynophagia, hoarseness, and other cranial nerve deficits (IX through XII) appear.
Carotid sinus syndrome syncope has been described in association with carotid PGLs. The syndrome involves a loss of consciousness accompanied by a reflex bradycardia and hypertension. Inciting stimuli include spontaneous movement of the head or digital pressure applied to the tumor.
Rarely, PGL of the head and neck may present as a functional neuropeptide-secreting tumor. The capacity for catecholamine synthesis in head and neck PGLs, however, does not always translate to clinical findings. Although all PGLs have neurosecretory granules, only 1% to 3% are considered functional. In 1962, Glenner and coworkers first described a functional carotid body tumor secreting norepinephrine. Patients should be asked about signs and symptoms indicating elevated catecholamines. Complaints of headaches, palpitations, flushing, and perspiration should be evaluated. In these patients, a 24-hour urine collection is examined for norepinephrine and its metabolites, including vanillylmandelic acid and normetanephrine. Alternatively, plasma metanephrine may be assessed. Excess epinephrine should prompt suspicion of an adrenal pheochromocytoma, because head and neck PGLs lack the enzyme to convert norepinephrine to epinephrine (phenylethanolamine-N-methyltransferase). If a tumor is found to be functional preoperatively, α- and β-adrenergic blocking is undertaken; this decreases the risk for sudden catecholamine release, which may occur with tumor manipulation in surgery. Routine screening for urinary metanephrines and vanillylmandelic acid and serum catecholamines is indicated only for multiple or familial PGLs or in the presence of catecholamine-related symptoms.
The carotid PGL is the most common PGL in the head and neck, and the most frequent combination of multiple tumors is with bilateral carotid body tumors. The overall incidence of multiple tumors in sporadic cases is classically reported in the literature to be approximately 10% (the so-called rule of 10); some of these may be unrecognized familial genetic mutations. If a familial pattern is recognized, the incidence of multiple tumors is reported to be between 30% and 50%, depending on the specific germline mutation (see earlier sections).
Harrington and Dockerty attempted to classify malignant tumors of the carotid body with criteria for malignancy including mitoses with giant cells, nuclear pleomorphism, and capsular invasion. Using these criteria, 50% of the 20 tumors studied would be considered malignant. However, it has since been determined that increased mitotic rate and capsular invasion should not be considered determinants of malignancy, particularly since nearly all carotid body tumors demonstrate some degree of capsular invasion. Malignancy is therefore determined solely by the presence of metastasis, which must be proven with biopsy because PGLs may exhibit multicentricity. The diagnosis of malignancy should be made by evidence of spread to regional lymph nodes or distant sites, most commonly the lung and bones.
Malignant PGLs have been reported in 6% of carotid body PGLs by Batsakis. Orbital and laryngeal PGLs have the highest rate of malignancy at 25%, followed by vagal PGLs (10%) and then jugulotympanic paragangliomas (5%). Accurate 5-year survival rates are difficult to obtain because of the low malignancy rate of this uncommon tumor. Data from the National Cancer Data Base suggest an overall 5-year survival rate of 60%. Distant metastases had a worse prognosis, with a 5-year survival rate of 11.8%, whereas those with regional spread of disease fared much better, with a 5-year survival rate of 78%.
Various diagnostic imaging modalities are available in the workup of carotid body tumors. Noninvasive duplex US demonstrates a hypervascular mass and the tumor's relationship to the carotid artery. US may also delineate any intrinsic carotid artery disease.
CT with intravenous contrast is usually the first imaging study obtained and demonstrates a hypervascular mass with avid contrast enhancement, similar to normal vessels, at the carotid bifurcation, which splays the internal and external carotid arteries. Flow voids may be seen. CT angiography may be performed to demonstrate the relationship of the carotid vessels to the enhancing neck mass.
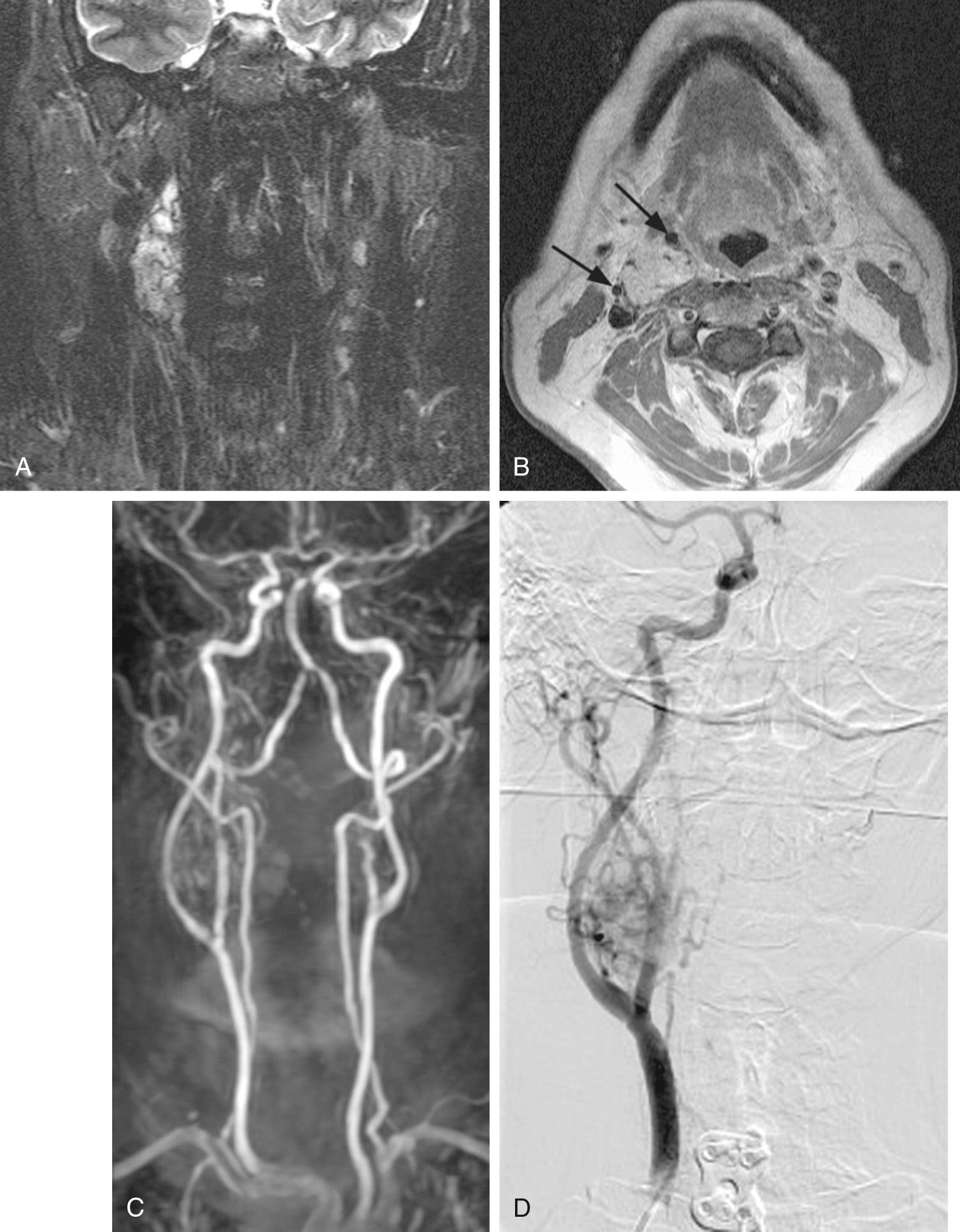
MRI with gadolinium may be the most useful imaging study for evaluating carotid body tumors ( Fig. 115.1A and B ) because, compared with CT scanning, it offers superior soft tissue contrast without the need for ionizing radiation. It should be obtained for nearly all PGLs to evaluate associated soft tissue and vascular involvement. MRI is sensitive for tumors as small as 0.8 cm. On T2-weighted images, PGLs larger than 2 cm in diameter typically demonstrate internal flow voids, dark lines, and dots (salt-and-pepper appearance) corresponding to vascular structures. This, however, is not always present with carotid PGLs. PGLs typically have a faster uptake as well as washout of gadolinium than other tumors of the carotid sheath, such as schwannomas, which have a more steady uptake. Carotid body tumors demonstrate a characteristic lyre sign described as a bowing and displacing of the internal and external carotid arteries, as shown in Fig. 115.1B and C . Radiographic evaluation should be sufficient to make the diagnosis of carotid body PGL. Carotid angiography has been replaced by MRI (including magnetic resonance angiography [MRA]; see Fig. 115.1C ) or formal angiography.
The high density of somatostatin receptors in PGLs enables newer nuclear medicine functional imaging techniques that include metaiodobenzylguanidine and octreotide scanning. These are becoming more useful for screening for metastasis or pheochromocytoma but are still not considered standards of care. Octreotide scanning uses the indium-111-labeled somatostatin analogue octreotide to diagnose primary tumors of the amine precursor and uptake decarboxylase system as well as their metastases. 18F FDOPA (fluorodopa) PET/CT is a newer radionuclide that has a high sensitivity and specificity. These functional imaging studies have been recommended as possible screening tests for familial PGLs in patients at risk. They also enable the detection of additional tumors when a malignant PGL is suspected or the examination of patients at high risk for multifocality or malignancy, such as those with SDHB mutations.
Although not universally adopted in the literature on carotid body PGLs, a classification system has previously been proposed for carotid body tumors. In 1971, while he was a surgery resident at the Mayo Clinic, Shamblin and his coworkers described a classification system used to grade the difficulty of resection in carotid body tumors and the likelihood of resultant permanent cranial nerve functional deficits; this system still remains applicable and is widely used today.
Group I tumors are defined as localized, relatively small, and minimally attached to the carotid vessels. Surgical excision is generally described as carried out without difficulty in this group. Group II encompassed tumors adherent to or partially surrounding the vessels, with moderate arterial attachment. These tumors are described as amenable to careful surgical removal. Group III carotid body tumors completely encase the carotids. Shamblin and colleagues recommended approaching these tumors with great care and with consideration for vessel replacement.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here