Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cancer is the second leading cause of death in the United States; only cardiovascular diseases exact a higher toll. Cancer is not one disease but many, all sharing a profound dysregulation of growth. Some types of cancer are curable, while others are virtually always fatal. Advances in diagnosis, treatment, and prognosis will depend on a deeper understanding of the molecular and cellular basis of each type of cancer.
This chapter deals with the basic biology of neoplasia—the nature of benign and malignant neoplasms and the molecular basis of neoplastic transformation. The host response to tumors and the clinical features of neoplasia are also discussed. Before turning to the defining characteristics of cancer cells and the mechanisms of carcinogenesis, we first summarize the fundamental and shared features of all cancers:
Cancer is a genetic disorder caused by DNA mutations. Pathogenic mutations may occur due to exposure to mutagens, may occur spontaneously due to error-prone processes in cells, or may be inherited. In addition, cancers frequently show epigenetic alterations (e.g., altered DNA methylation and histone modification). In concert, these genetic and epigenetic abnormalities alter the expression or function of key genes that regulate fundamental cellular processes, such as growth, survival, and senescence.
Genetic alterations in cancer cells are heritable, being passed to daughter cells upon cell division; as a result, cells harboring these mutations are subject to Darwinian selection (survival of the fittest). Cells bearing mutations that provide a growth or survival advantage outcompete their neighbors and thus come to dominate the population. At the time of tumor initiation, these selective advantages are conferred on a single cell and, as a result, individual tumors are clonal (i.e., the progeny of one cell). However, beyond the point of initiation Darwinian selection continues to shape the clonal evolution of cancers by favoring the proliferation of genetically distinct subclones with more aggressive characteristics, a concept referred to as tumor progression.
Mutations and epigenetic alterations impart to cancer cells a set of properties that are referred to collectively as cancer hallmarks. These properties produce the cellular phenotypes that dictate the natural history of cancers as well as their response to various therapies. The molecular underpinnings of each hallmark of cancer are discussed later.
Basic research has elucidated many of the cellular and molecular abnormalities that give rise to cancer and govern its behavior. These insights are in turn leading to a revolution in the diagnosis and treatment of cancer, an evolving triumph of biomedical science.
Neoplasia means “new growth,” and neoplastic cells are said to be “transformed” because they replicate incessantly as a result of resistance to the regulatory influences that control normal cells. Neoplasms therefore enjoy a degree of autonomy, but some important dependencies remain. All neoplasms, for example, depend on the host for their nutrition and blood supply, and neoplasms derived from hormone responsive tissues often require endocrine support. As we will discuss, such dependencies can sometimes be exploited therapeutically.
In common medical usage, a neoplasm is often referred to as a tumor, and the study of tumors is called oncology (from oncos, “tumor,” and logos, “study of”). Tumors are generally categorized as benign or malignant, an assessment that is central to the accurate prediction of a tumor’s behavior and prognosis.
A tumor is said to be benign when its microscopic and gross characteristics indicate that it will remain localized and is amenable to local surgical removal. Patients with benign tumors can generally be cured of their disease. However, not all benign tumors are easily excised, and some produce significant morbidity or are even lethal, particularly if they are located near a vital structure or organ.
Malignant, as applied to a neoplasm, implies that the lesion may be locally invasive and has the capacity to spread to distant sites (metastasize). Malignant tumors are collectively referred to as cancers, derived from the Latin word for “crab,” as they infiltrate and seize upon normal tissues in an obstinate manner, similar to a crab’s behavior. Not all cancers pursue an aggressive course, and, paradoxically, some of the most aggressive are also highly curable, but the designation malignant constitutes a red flag.
All tumors, benign and malignant, have two basic components: (1) parenchyma, made up of transformed or neoplastic cells and (2) stroma, the supporting host-derived, nonneoplastic connective tissue, inflammatory cells, and blood vessels. Even leukemias, neoplasms in which the malignant cells circulate in the blood ( Chapter 10 ), depend on stromal interactions to support the growth of the tumor cells. The parenchyma of the neoplasm largely determines its biologic behavior, and it is this component from which the tumor derives its name. However, the stroma is crucial to the growth of the neoplasm, since it provides the blood supply. Moreover, stromal cells and tumor cells carry on a two-way conversation that influences the behavior and growth pattern of tumor cells.
In general, benign tumors are designated by attaching the suffix - oma to the cell type from which the tumor arises. For example, a benign tumor of fibroblasts is a fibroma ; a benign cartilaginous tumor is a chondroma. More complex nomenclature is used for benign epithelial tumors.
Adenoma is applied to most benign epithelial neoplasms, including those that produce glandlike structures and those that do not.
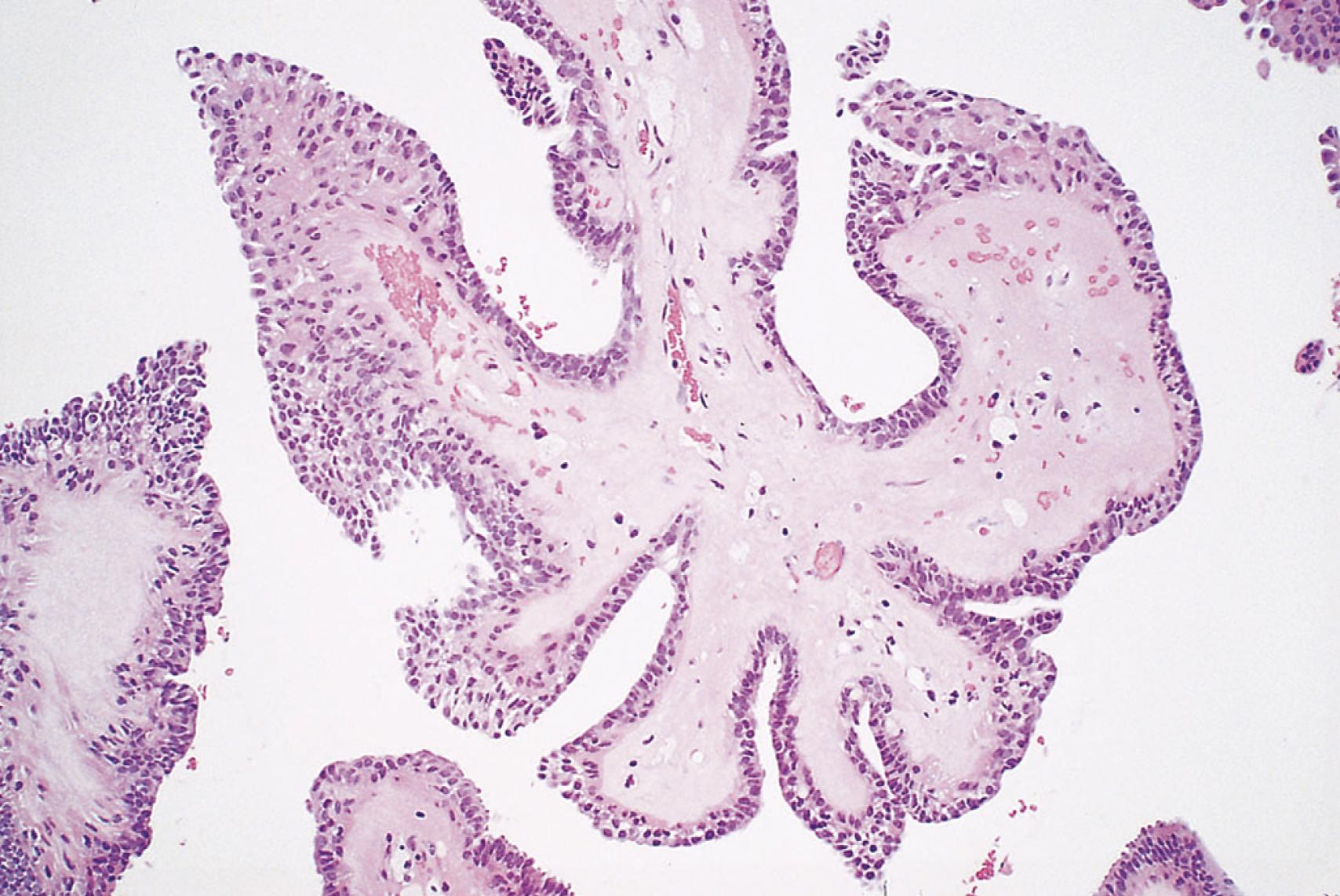
Papilloma refers to a benign epithelial neoplasm that produces microscopic or macroscopic fingerlike fronds ( eFig. 6.1 ).
Polyp refers to a mass that projects above a mucosal surface to form a macroscopically visible structure ( Fig. 6.1 ). Although this term is commonly used for benign tumors, some malignant tumors may grow as polyps, and other polyps (such as nasal polyps) are not neoplastic but inflammatory in origin.
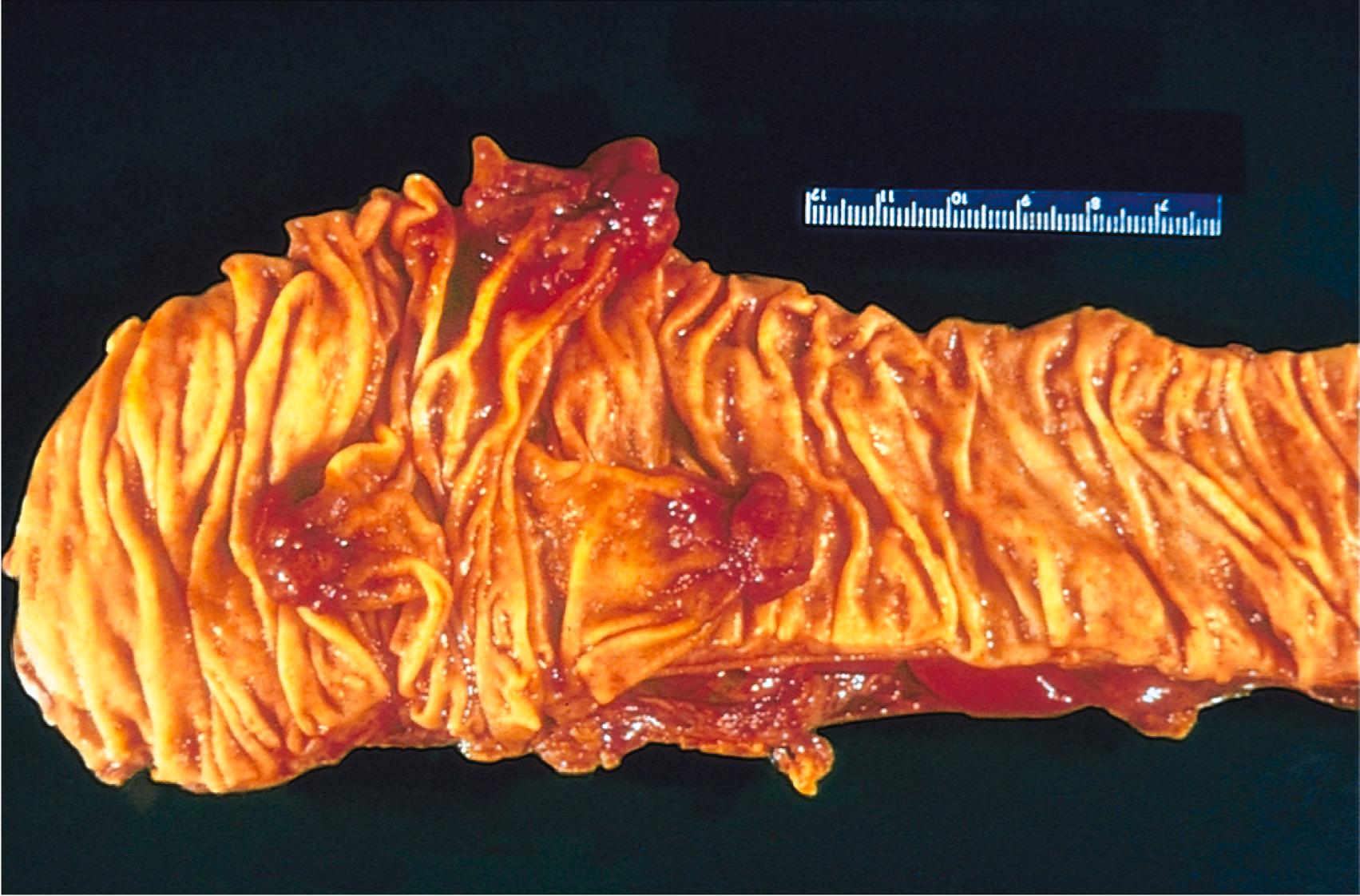
Cystadenoma refers to hollow cystic mass; these are most common in the ovary ( Chapter 17 ).
The nomenclature of malignant tumors essentially follows that of benign tumors, with certain additions and exceptions. Although the nomenclature of neoplasms is complex (and sometimes inconsistent), students must be familiar with it because it is the means by which a tumor’s nature and significance are conveyed by physicians.
Malignant neoplasms arising in “solid” mesenchymal tissue or its derivatives are named sarcomas, whereas those arising from the cells of the blood are called leukemias or lymphomas. Sarcomas are designated based on their principal cell type. Thus, a malignant neoplasm comprising fatlike cells is a lipo sarcoma, and a malignant neoplasm composed of chondrocyte-like cells is a chondro sarcoma.
Although epithelia may be derived from all three germ cell layers, malignant neoplasms of epithelial cells are called carcinomas regardless of the tissue of origin. Thus, malignant neoplasms arising in the renal tubular epithelium (mesoderm), the skin (ectoderm), or lining epithelium of the gut (endoderm) are all considered carcinomas.
Carcinomas are subdivided further. Carcinomas that grow in a glandular pattern are called adenocarcinomas, and those that produce squamous cells are called squamous cell carcinomas. Sometimes the tissue or organ of origin is specified, as in the designation of renal cell carcinoma. Tumors may show little or no differentiation; these are referred to as poorly differentiated or undifferentiated carcinoma.
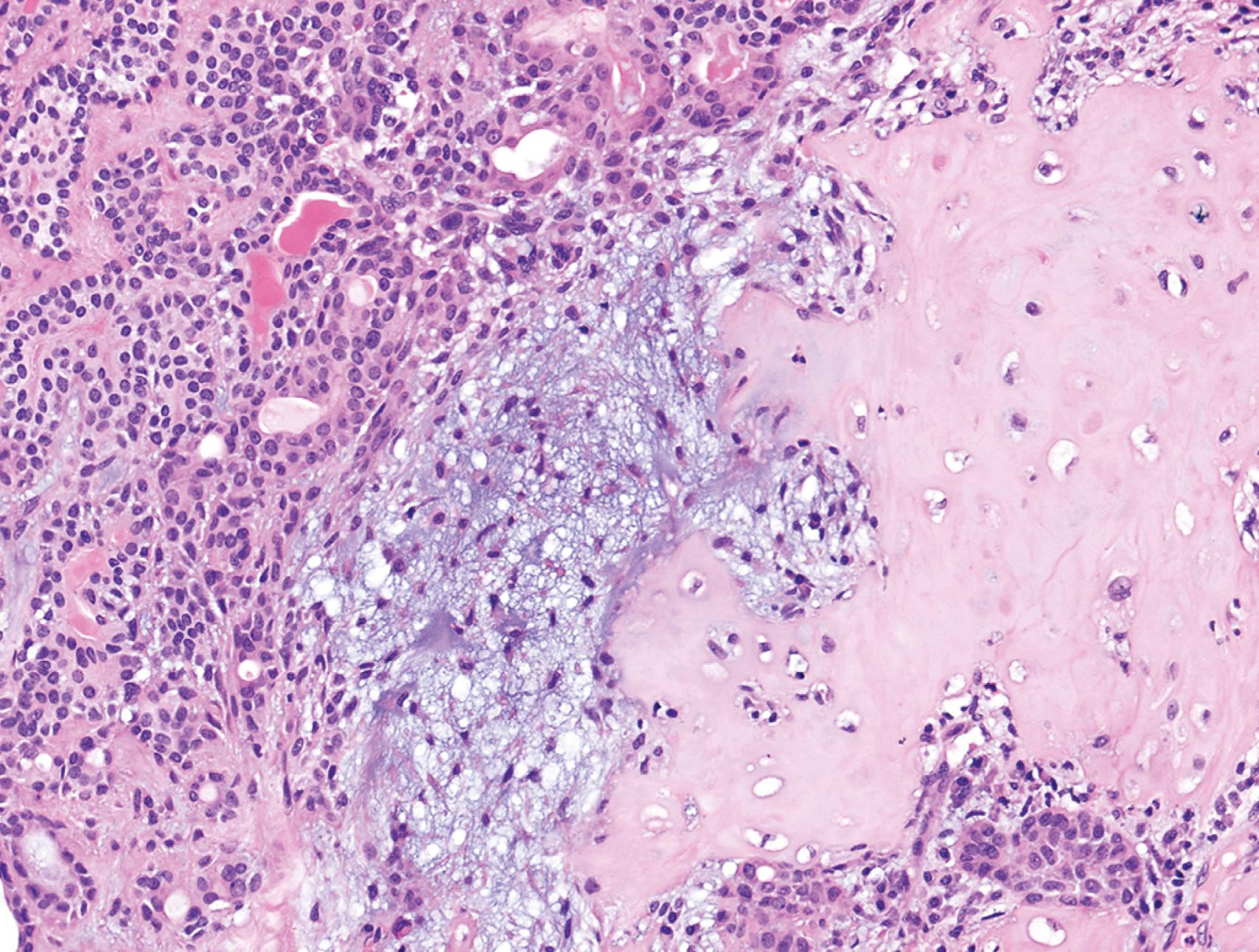
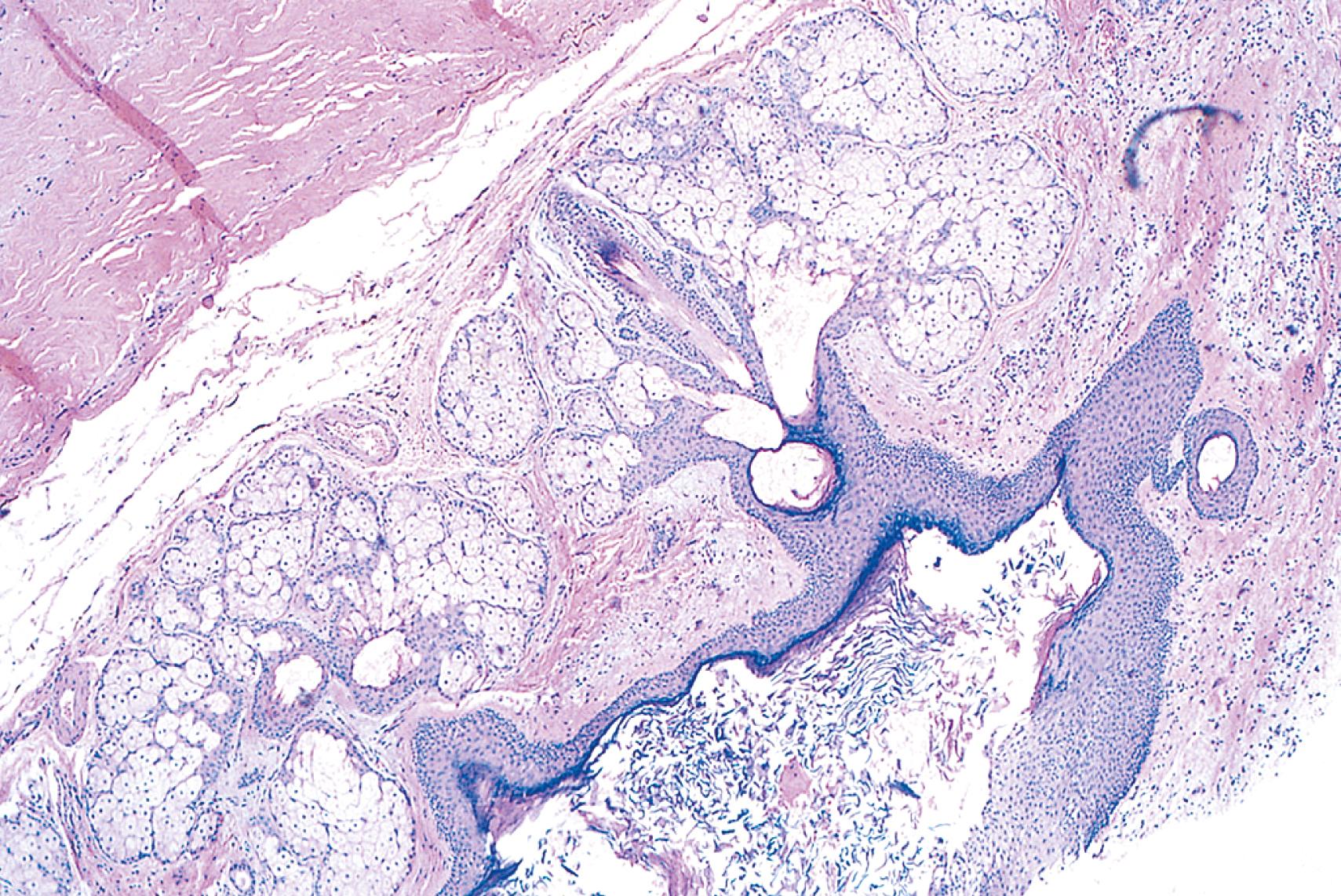
The neoplastic cells in a tumor, whether benign or malignant, usually resemble each other, consistent with their origin from a single transformed progenitor cell. In some unusual instances, however, the tumor cells undergo divergent differentiation, creating so-called “mixed tumors.” Mixed tumors are clonal, but the progenitor cell in such tumors has the capacity to differentiate along more than one lineage. One example is mixed tumor of the salivary gland, commonly referred to as pleomorphic adenoma . This benign tumor has epithelial components dispersed throughout a fibromyxoid stroma, sometimes harboring islands of cartilage or bone ( Fig. 6.2 ). Teratoma is a special type of mixed tumor that contains recognizable mature or immature cells or tissues derived from more than one germ cell layer, and sometimes all three. Teratomas originate from totipotent germ cells, which normally reside in the ovary and testis and may be present in midline embryonic rests. Germ cells have the capacity to differentiate into any of the cell types found in the adult body; therefore, they may give rise to neoplasms that contain elements resembling bone, epithelium, muscle, fat, nerve, and other tissues, mixed together in a helter-skelter fashion ( eFig. 6.2 ).
The specific names of the more common neoplasms are presented in Table 6.1 . Some glaring inconsistencies may be noted. For example, the terms lymphoma, mesothelioma, melanoma, and seminoma are used for malignant neoplasms. Unfortunately for students, these exceptions are firmly entrenched in medical terminology. There are also other instances of confusing terminology:
Hamartoma is a mass of disorganized tissue resembling the involved site, such as the lung or the liver. Although historically thought of as developmental malformations, hamartomas have clonal chromosomal aberrations that are acquired through somatic mutations and are best considered unusual benign neoplasms.
Choristoma is a congenital anomaly consisting of a heterotopic nest of cells. For example, a small nodule of pancreatic tissue may be found in the submucosa of the stomach, duodenum, or small intestine. The designation - oma, connoting a neoplasm, gives these lesions an undeserved gravity, as they are usually trivial.
| Tissue of Origin | Benign | Malignant |
|---|---|---|
| Tumors Composed Predominantly of a Single Cell Type | ||
| Connective tissue and derivatives | Fibroma | Fibrosarcoma |
| Lipoma | Liposarcoma | |
| Chondroma | Chondrosarcoma | |
| Osteoma | Osteosarcoma | |
| Endothelium and related cell types | ||
| Blood vessels | Hemangioma | Angiosarcoma |
| Lymph vessels | Lymphangioma | Lymphangiosarcoma |
| Mesothelium | Mesothelioma | |
| Brain coverings | Meningioma | Invasive meningioma |
| Blood cells and related cell types | ||
| Hematopoietic cells | Leukemias | |
| Lymphoid tissue | Lymphomas | |
| Muscle | ||
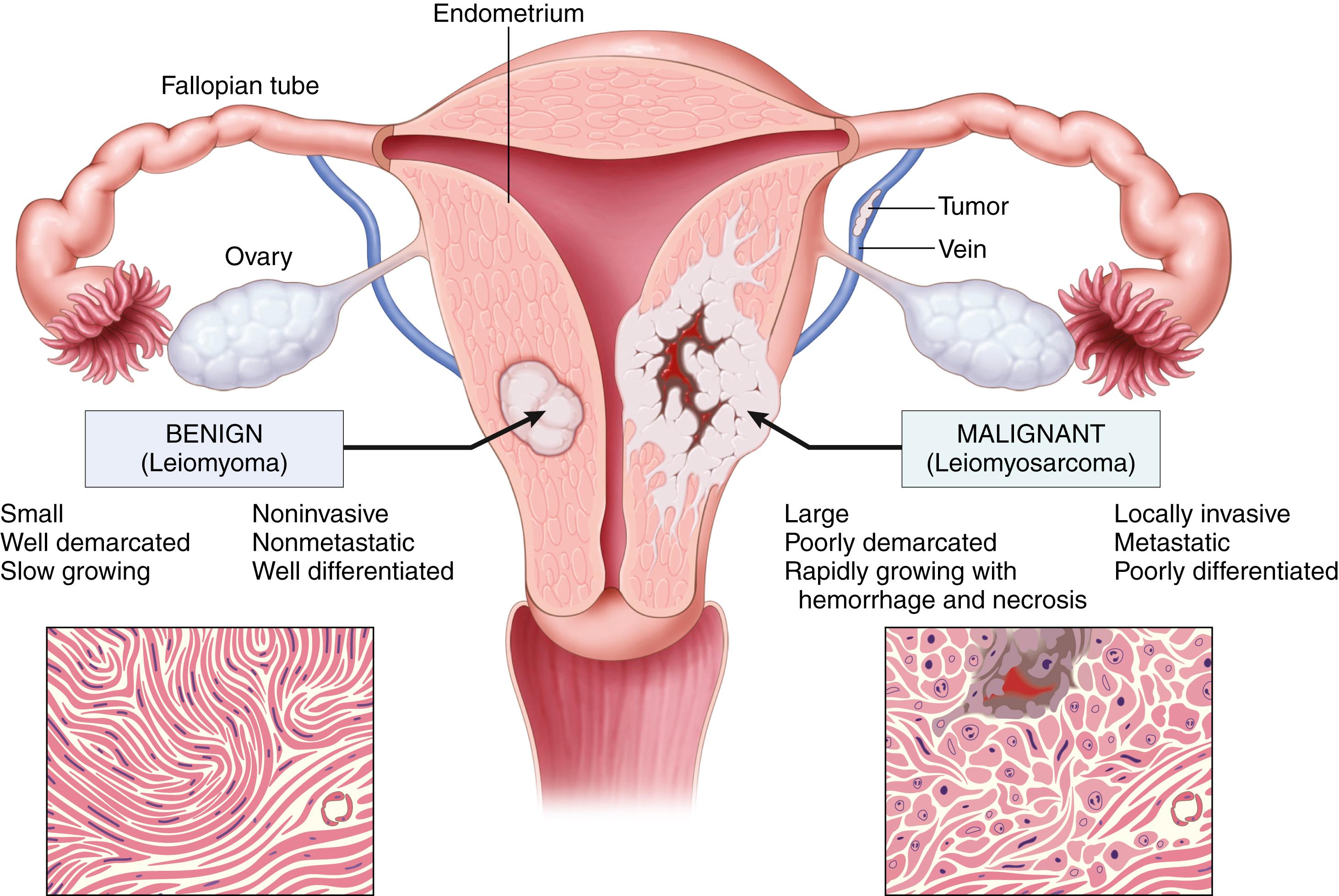
| Smooth | Leiomyoma | Leiomyosarcoma |
| Striated | Rhabdomyoma | Rhabdomyosarcoma |
| Skin | ||
| Stratified squamous | Squamous cell papilloma | Squamous cell or epidermoid carcinoma |
| Basal cells of skin or adnexa | Basal cell carcinoma | |
| Tumors of melanocytes | Nevus | Melanoma |
| Epithelial lining of glands or ducts | Adenoma | Adenocarcinoma |
| Papilloma | Papillary carcinomas | |
| Cystadenoma | Cystadenocarcinoma | |
| Lung | Bronchial adenoma | Bronchogenic carcinoma |
| Kidney | Renal tubular adenoma | Renal cell carcinoma |
| Liver | Hepatic adenoma | Hepatocellular carcinoma |
| Bladder | Urothelial papilloma | Urothelial carcinoma |
| Placenta | Hydatidiform mole | Choriocarcinoma |
| Testicle | Seminoma Embryonal carcinoma |
|
| Ovary | Serous cystadenoma, mucinous cystadenoma | Serous cystadenocarcinoma, mucinous cystadenocarcinoma |
| Tumors Composed of Multiple Cell Types Normally Derived From the Same Germ Cell Layer | ||
| Salivary glands | Pleomorphic adenoma (mixed tumor of salivary gland) | Malignant mixed tumor of salivary gland |
| Renal anlage | Wilms tumor | |
| Tumors Composed of Multiple Cell Types Normally Derived From More Than One Germ Cell Layer | ||
| Totipotential cells in gonads or in embryonic rests | Mature teratoma, dermoid cyst | Immature teratoma, teratocarcinoma |
Three features can be used to distinguish between most benign and malignant tumors: differentiation and anaplasia; local invasion; and metastasis. In general, rapid growth also signifies malignancy, but some malignant tumors grow slowly and as a result growth rate is not a reliable discriminator. Although some neoplasms defy easy characterization, in most instances the determination of benign versus malignant is made with remarkable accuracy using long-established criteria.
Differentiation refers to the extent to which neoplasms resemble their cells of origin; lack of differentiation is called anaplasia . In general, benign neoplasms are composed of well-differentiated cells that closely resemble their normal counterparts. A lipoma is made up of mature fat cells laden with cytoplasmic lipid vacuoles, and a chondroma is made up of mature cartilage cells that synthesize cartilaginous matrix—evidence of morphologic and functional differentiation ( eFig. 6.3 ). In well-differentiated benign tumors, mitoses are usually rare and are of normal configuration.
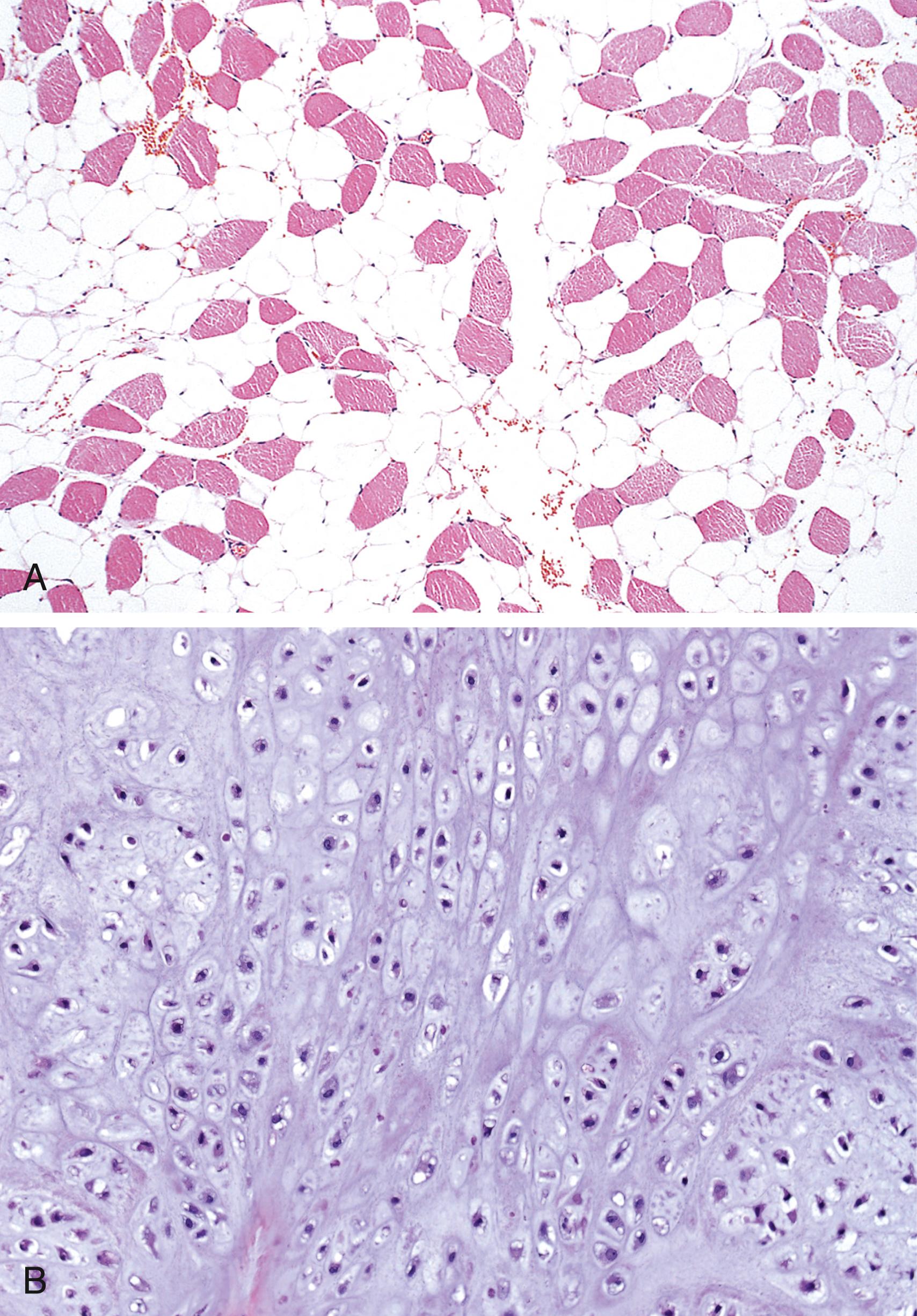
By contrast, most malignant neoplasms exhibit morphologic alterations that demonstrate their malignant nature. In well-differentiated cancers ( Fig. 6.3 ), these features may be quite subtle. For example, well-differentiated adenocarcinoma of the thyroid gland may contain normal-appearing follicles, its malignant potential being revealed only by invasion into adjacent tissues or metastasis. In addition, cancers may induce stromal responses that are not seen in benign tumors. For example, certain cancers induce a dense, abundant fibrous stroma (desmoplasia), making them hard, “scirrhous” tumors.
Tumors composed of undifferentiated cells are said to be anaplastic, a feature that is a reliable indicator of malignancy. The term anaplasia literally means “backward formation”—implying dedifferentiation, or loss of the structural and functional differentiation of normal cells. In some instances, dedifferentiation of apparently mature cells occurs during carcinogenesis. However, other cancers arise from stem cells in tissues; in these tumors, failure of differentiation of transformed stem cells, rather than dedifferentiation of specialized cells, accounts for their anaplastic appearance. Anaplastic cells often display the following features:
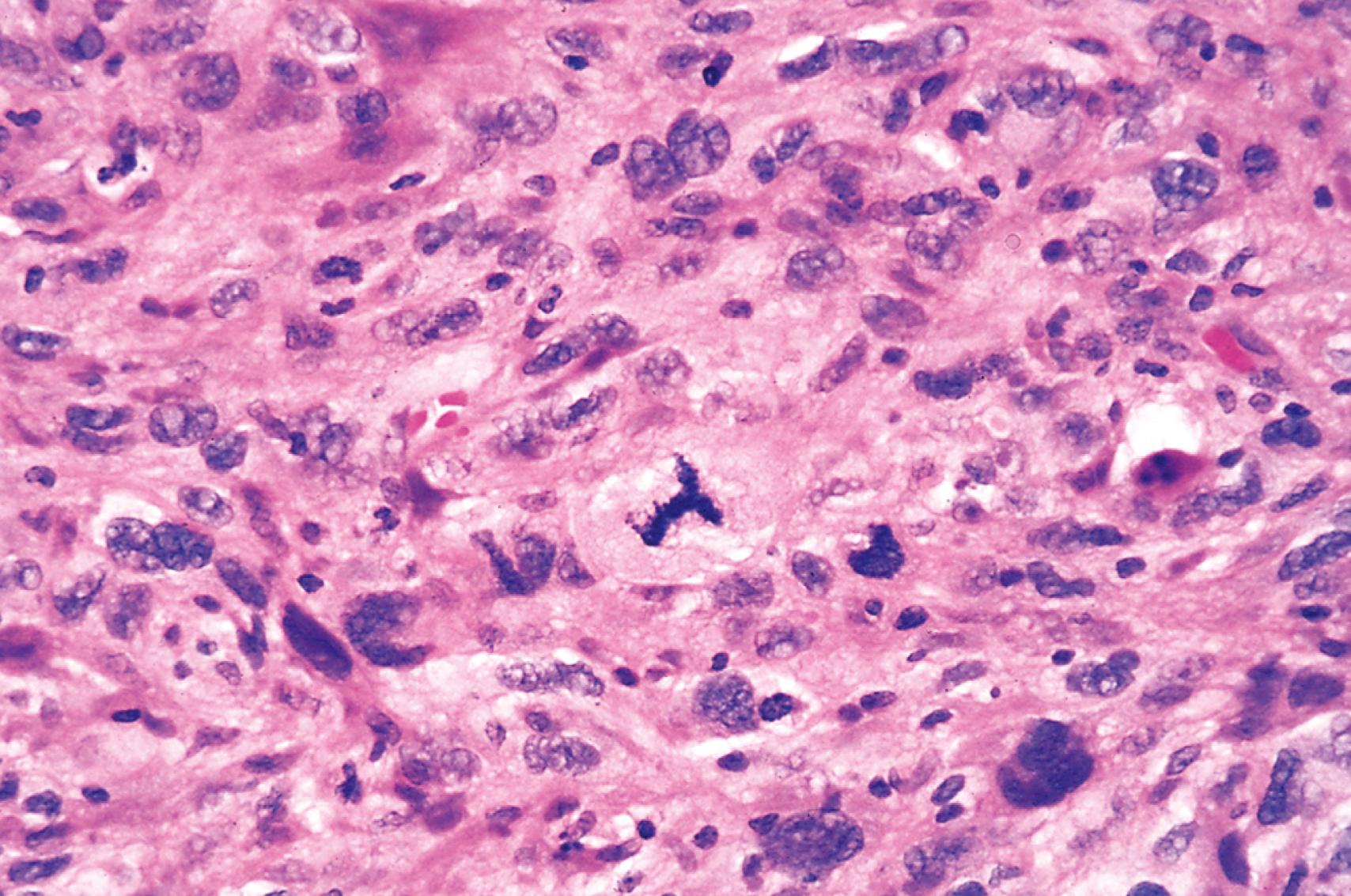
Cellular and nuclear pleomorphism . Tumor cells and their nuclei show great variation in shape and size ( Fig. 6.4 ). In addition nuclei show hyperchromasia (dark staining), or unusually prominent single or multiple nucleoli. Enlargement of nuclei may result in an increased nuclear-to-cytoplasmic ratio that approaches 1 : 1 instead of the normal 1 : 4 or 1 : 6. Nucleoli may attain astounding sizes, sometimes approaching the diameter of normal lymphocytes.
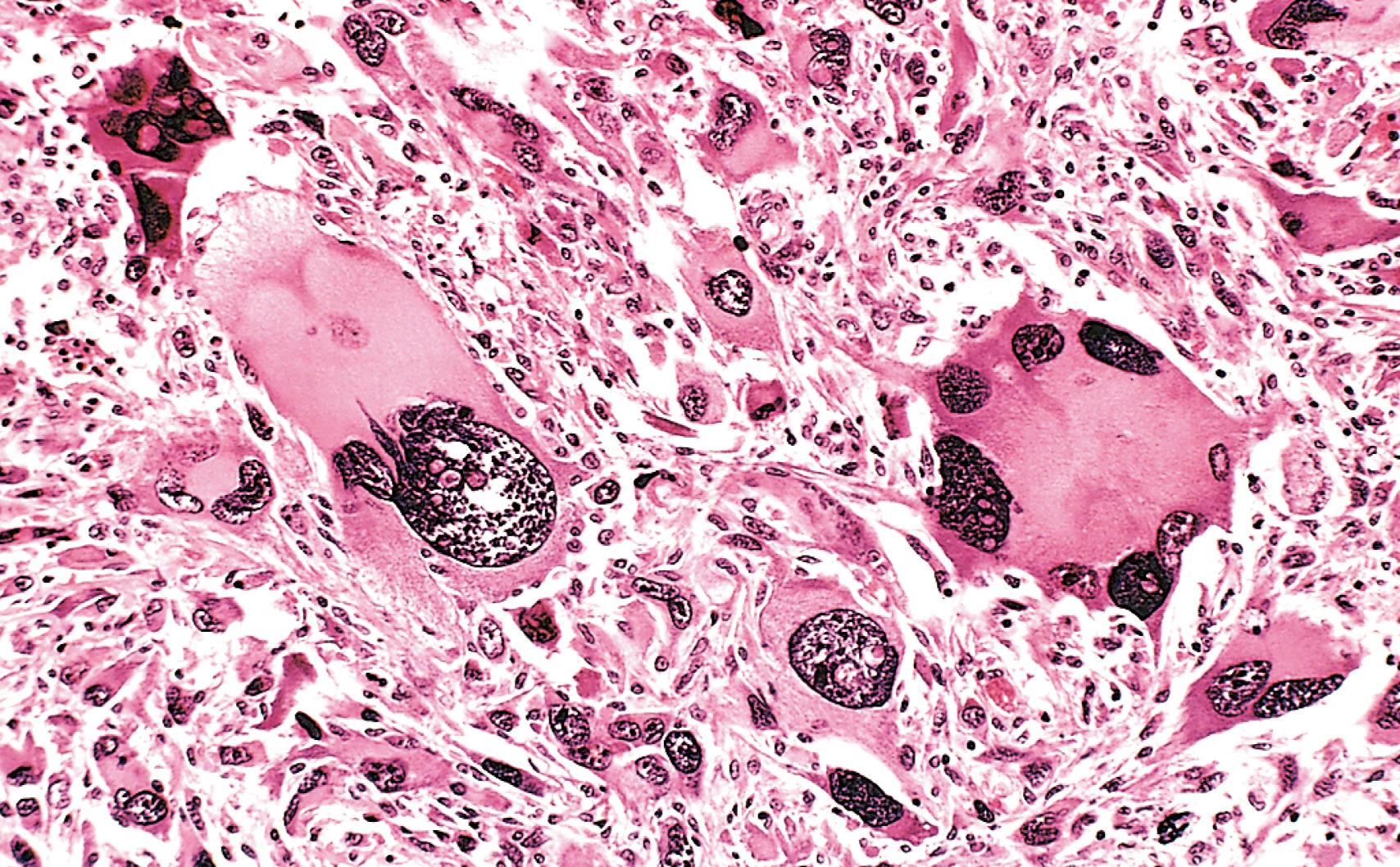
Tumor giant cells may be formed. These are considerably larger than neighboring cells and may possess either one enormous nucleus or several nuclei (see Fig. 6.4 ).
Atypical mitoses, which may be numerous. Multiple spindles may produce tripolar or quadripolar mitotic figures ( Fig. 6.5 ).
Loss of polarity, such that cells grow in sheets, with loss of normal orientation and absence of identifiable growth patterns, such as glands or stratified squamous architecture
Well-differentiated tumor cells are likely to retain the functional capabilities of their normal counterparts, whereas anaplastic tumor cells are much less likely to have specialized functional activities. For example, benign neoplasms and even well-differentiated cancers of endocrine glands frequently elaborate the hormones characteristic of their cell of origin. Similarly, well-differentiated squamous cell carcinomas produce keratin (see Fig. 6.3 ), just as well-differentiated hepatocellular carcinomas secrete bile. In other instances, unanticipated functions emerge. Some cancers express fetal proteins not produced by comparable cells in the adult. Cancers of nonendocrine origin may also produce so-called “ectopic hormones.” For example, certain lung carcinomas secrete adrenocorticotropic hormone (ACTH), parathyroid hormone–like hormone, insulin, glucagon, and others. More is said about these so-called “paraneoplastic” phenomena later.
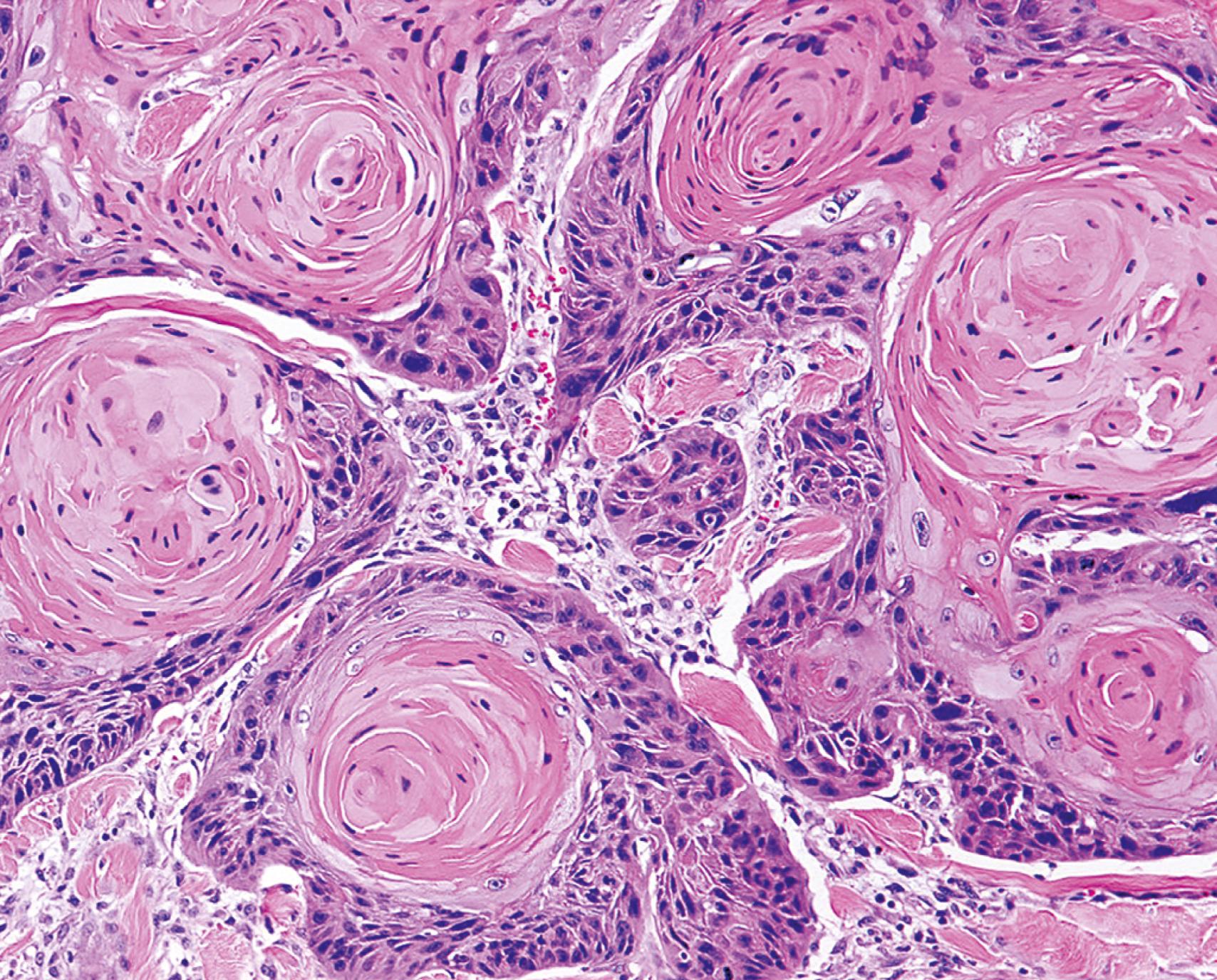
Also relevant in the discussion of differentiation and anaplasia is dysplasia, referring to disorderly proliferation. Dysplastic epithelium is recognized by loss of uniformity of individual cells and disturbed architectural orientation. Dysplastic cells exhibit pleomorphism and often possess abnormally large, hyperchromatic nuclei. Mitotic figures are more abundant than usual and frequently appear in the superficial epithelium, an abnormal location. In addition, there is architectural disarray. For example, the usual progressive maturation of tall cells in the basal layer to flattened squames on the surface of squamous epithelium may be lost, such that the epithelium consists of a disordered mixture of dark basal-appearing cells. When dysplastic changes are severe and involve the entire thickness of the epithelium, the lesion is referred to as carcinoma in situ, a preinvasive stage of cancer ( Fig. 6.6 ).
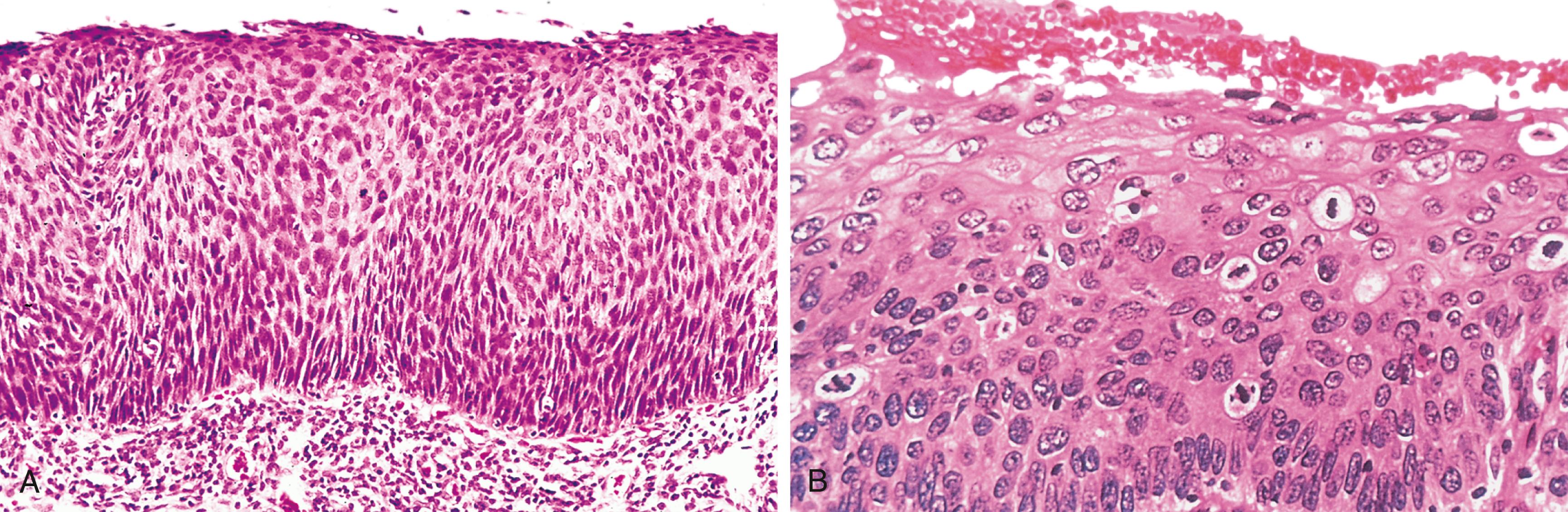
It is important to appreciate that dysplasia is not synonymous with cancer. Mild to moderate dysplasia sometimes regresses completely, particularly if inciting causes are removed. However, dysplasia is often present adjacent to frankly malignant neoplasms (e.g., in lung carcinomas arising in the context of cigarette smoking) and as a general rule marks a tissue as being at increased risk for cancer development.
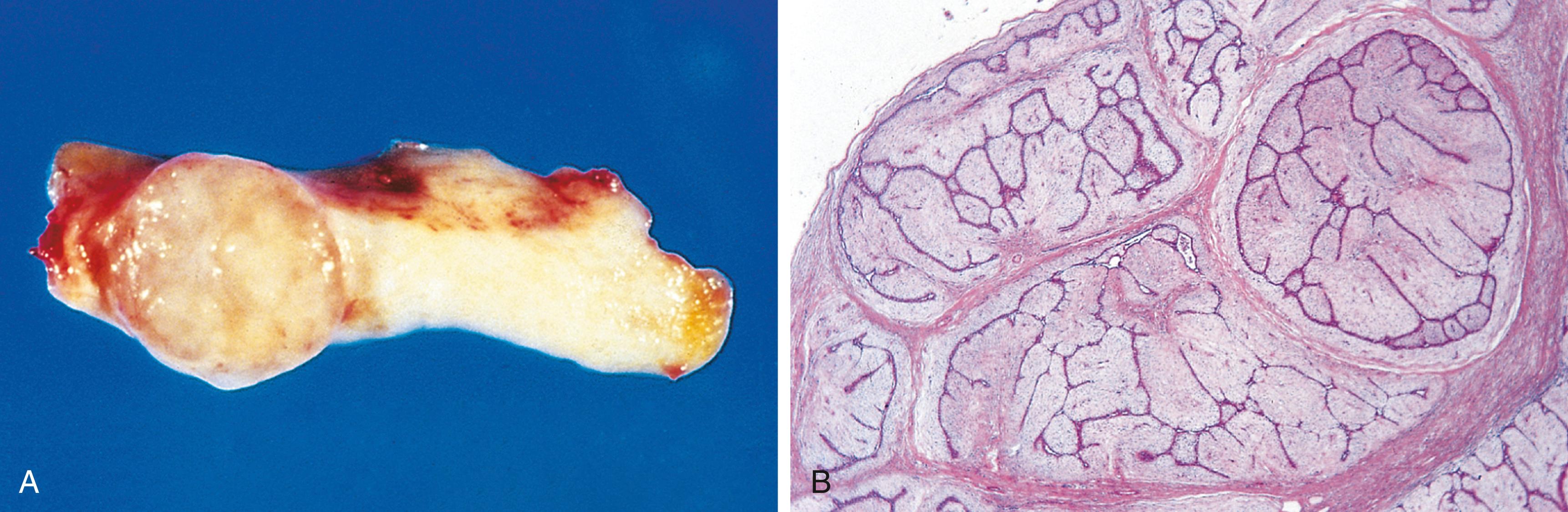
The growth of cancers is accompanied by progressive infiltration, invasion, and destruction of surrounding tissues, whereas most benign tumors grow as cohesive expansile masses that remain localized. Because benign tumors grow and expand slowly, they usually develop a rim of compressed fibrous tissue, often called the capsule ( Fig. 6.7 ). The capsule consists of extracellular matrix that is deposited by stromal cells such as fibroblasts, which may be activated by the mechanical stress created by compression of normal tissue by the expanding tumor. Encapsulation creates a tissue plane that makes the tumor discrete, moveable (nonfixed), and readily excisable by surgical enucleation. However, not all benign neoplasms are encapsulated. For example, uterine leiomyoma is discretely demarcated from the surrounding smooth muscle by a zone of compressed and attenuated normal myometrium but lacks a capsule. A few benign tumors are neither encapsulated nor discretely defined; lack of demarcation is particularly likely in benign vascular neoplasms such as hemangiomas, which may be difficult to excise. These exceptions are pointed out only to emphasize that, although encapsulation is the rule in benign tumors, the lack of a capsule does not mean a tumor is malignant.
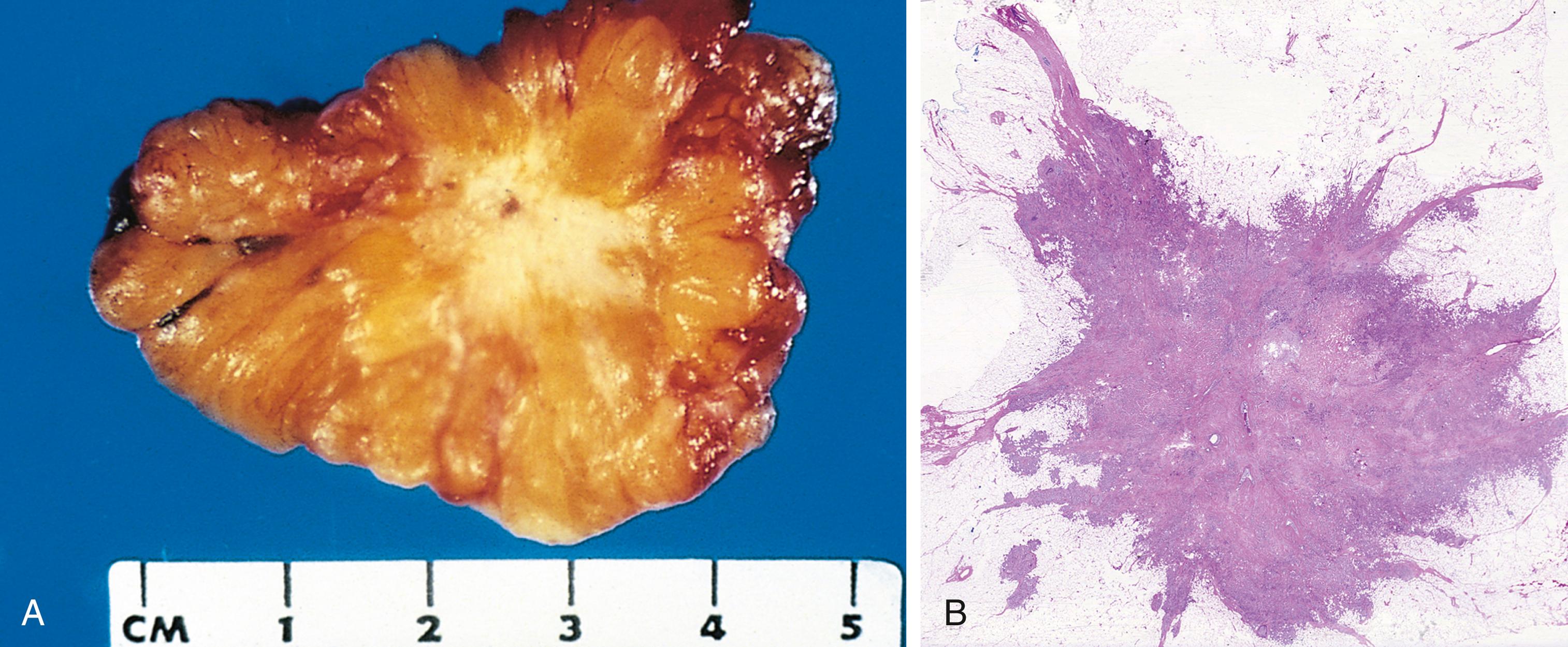
Next to the development of metastases, invasiveness is the feature that most reliably distinguishes cancers from benign tumors ( Fig. 6.8 ). Cancers lack well-defined capsules. There are instances in which a slowly growing malignant tumor, such as follicular carcinoma of thyroid, appears to be encased by a capsule, but careful microscopic examination reveals tiny tongues of tumor penetrating the margin and infiltrating adjacent structures. This infiltrative mode of growth makes it necessary to remove a wide margin of surrounding normal tissue when complete excision of a malignant tumor is attempted. Pathologists carefully examine the margins of resected tumors to ensure they are devoid of cancer cells (clean surgical margins).
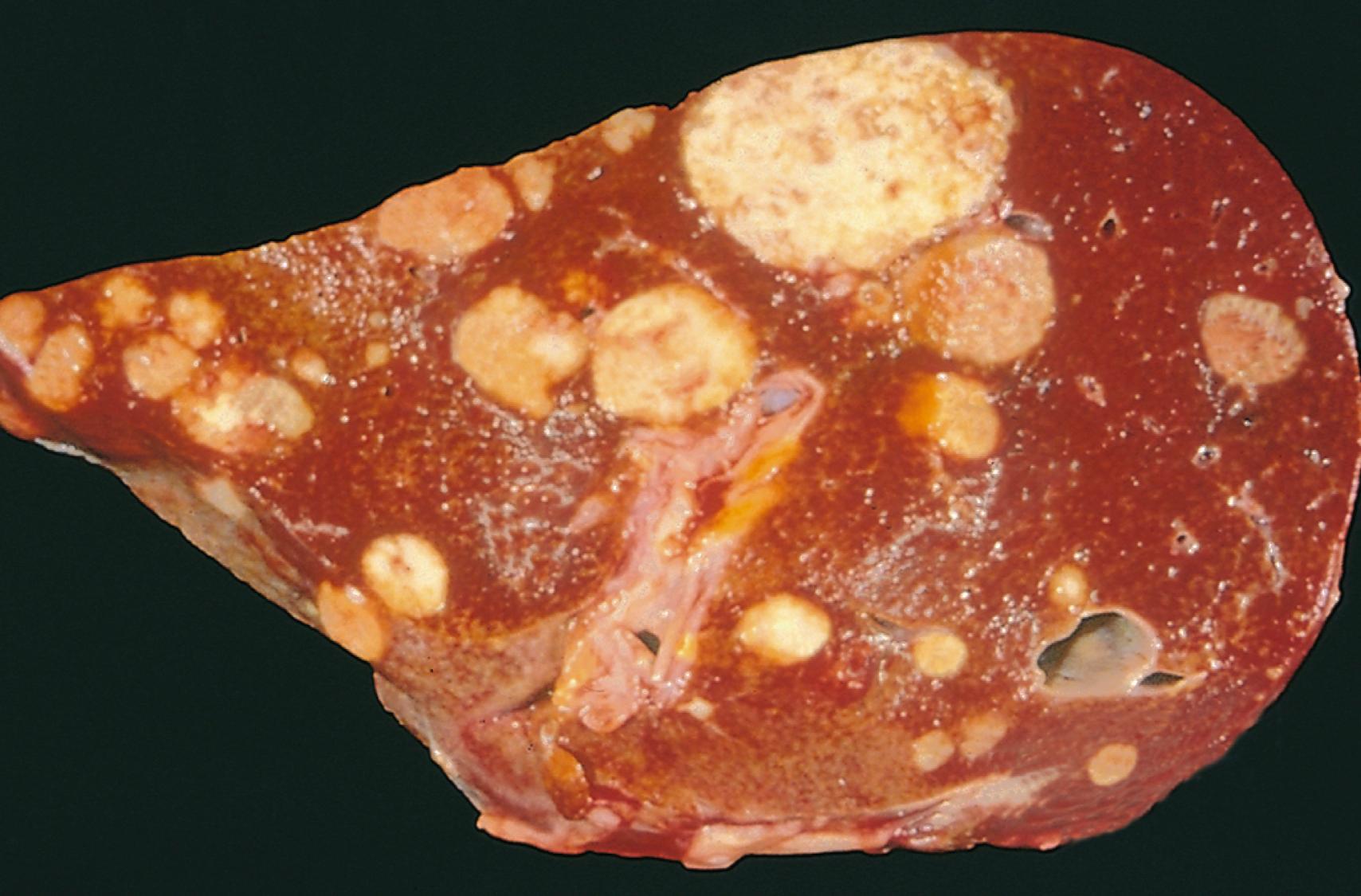
Metastasis is defined by the spread of a tumor to sites that are physically discontinuous with the primary tumor and is one of the hallmarks of malignant tumors. The invasiveness of cancers permits them to penetrate blood vessels, lymphatics, and body cavities, providing opportunities for spread ( Fig. 6.9 ). Overall, approximately 30% of patients with newly diagnosed solid tumors (excluding skin cancers other than melanomas) present with clinically evident metastases, and an additional 20% have occult metastases at the time of diagnosis.
In general, large anaplastic neoplasms are more likely to have metastasized at diagnosis, but there are exceptions. Extremely small cancers may metastasize; conversely, some large and ominous-looking lesions may not. While all malignant tumors can metastasize, some do so very infrequently. For example, basal cell carcinomas of the skin and most primary tumors of the central nervous system are locally invasive but rarely metastasize. It is evident then that the properties of local invasion and metastasis are separable.
A special circumstance involves so-called “blood cancers,” the leukemias and lymphomas. These tumors are derived from blood-forming cells that normally have the capacity to enter the bloodstream and travel to distant sites; as a result, with only rare exceptions, leukemias and lymphomas are assumed to be disseminated at diagnosis and are always considered to be malignant.
Malignant neoplasms disseminate by one of three pathways: (1) seeding within body cavities; (2) lymphatic spread; or (3) hematogenous spread. Spread by seeding occurs when neoplasms invade a body cavity. This mode of dissemination is particularly characteristic of cancers of the ovary, which often cover the peritoneal surfaces widely yet may not invade the underlying tissues. In this instance, the ability to implant and grow at distant sites seems to be separable from the capacity to invade. Neoplasms of the central nervous system, such as a medulloblastoma or ependymoma, may penetrate the cerebral ventricles and be carried by the cerebrospinal fluid until they implant on the meningeal surfaces surrounding the brain or spinal cord.
Lymphatic spread is more typical of carcinomas, whereas hematogenous spread is favored by sarcomas. There are numerous interconnections, however, between the lymphatic and vascular systems, so all forms of cancer may disseminate through either or both systems. The pattern of lymph node involvement depends on the site of the primary neoplasm and the pathways of local lymphatic drainage. Lung carcinomas arising in the respiratory passages metastasize first to the regional bronchial lymph nodes and then to the tracheobronchial and hilar nodes. Carcinoma of the breast usually arises in the upper outer quadrant and spreads first to the axillary nodes; however, medial breast lesions may drain to the nodes along the internal mammary artery. Thereafter, in both instances, the supraclavicular and infraclavicular nodes may be seeded. In some cases, cancer cells travel in lymphatic channels through the immediately proximal nodes only to be trapped in subsequent lymph nodes, producing so-called “skip metastases.” The cells may also traverse all the lymph nodes and reach the vascular compartment by way of the thoracic duct.
A “sentinel lymph node” is the first regional lymph node that receives lymph flow from a primary tumor. It can be identified by injection of dyes or radiolabeled tracers near the primary tumor. Biopsy of sentinel lymph nodes allows determination of the extent of tumor spread and can be used to plan treatment. Of note, although enlargement of nodes near a primary neoplasm should raise concern of metastatic spread, it does not always indicate cancerous involvement. The necrotic cells of the neoplasm and tumor antigens often evoke immunologic responses in draining nodes that lead to reactive hyperplasia (lymphadenitis). Thus, biopsy is necessary to determine if an enlarged lymph node is involved by tumor.
Hematogenous spread of cancers is most likely to occur through the penetration of thin-walled veins instead of thick-walled arteries. With venous invasion, the bloodborne cells often arrest in the first capillary bed they encounter. Since the portal system flows to the liver and caval blood flows to the lungs, the liver and lungs are the most frequent sites of hematogenous dissemination. Cancers arising in organs near the vertebral column such as the thyroid and prostate often embolize through the paravertebral plexus, possibly explaining the proclivity of these cancers to spread to the spine. Other carcinomas, particularly renal cell carcinoma and hepatocellular carcinoma, have a propensity to grow within veins in a snakelike fashion, sometimes extending up the inferior vena cava to the right side of the heart. Remarkably, such intravenous growth may not be accompanied by widespread dissemination.
In addition to the stepwise spread of cancers to the next draining lymph node or capillary bed, other cancers frequently spread to noncontiguous organs. For example, prostatic carcinoma preferentially spreads to bone, bronchogenic carcinoma tends to involve the adrenal glands and the brain, neuroblastoma spreads to the liver and bones, and uveal melanoma spreads to the liver. Conversely, other tissues such as skeletal muscle, although rich in capillaries, are rarely sites of tumor metastases. The molecular basis of such tissue-specific homing of tumor cells is discussed later.
Thus, numerous features of tumors (summarized in Fig. 6.10 ) usually permit the differentiation of benign and malignant neoplasms.
Major insights into the causes of cancer have been obtained by identifying associations between particular environmental, hereditary, or cultural influences and specific neoplasms. The well-established causal association of cigarette smoking with lung cancer arose primarily from epidemiologic studies. A comparison of the incidence rates for colon cancer and dietary patterns in the Western world and in Africa led to the recognition that dietary fat and fiber content may contribute significantly to the causation of this cancer. Certain diseases associated with an increased risk for cancer also provide clues to cancer pathogenesis. Next, we first summarize the overall magnitude of cancer incidence and then review factors relating to the patient and the environment that influence the predisposition to cancer.
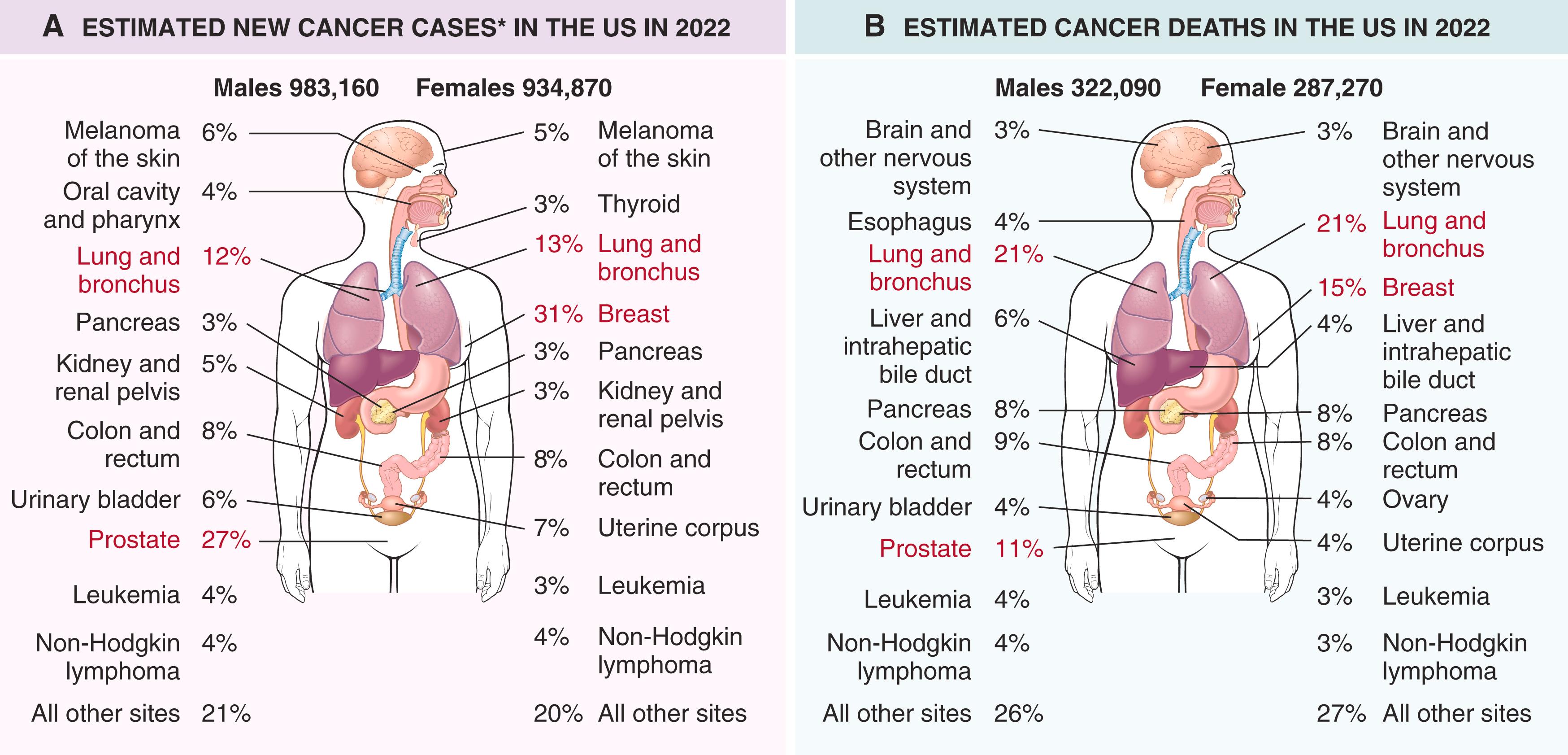
For the year 2018, it is estimated that there were over 17 million cases of cancer and 9.6 million deaths due to cancer worldwide (approximately 26,300 deaths per day). Moreover, due to increasing population size, it is projected that the numbers of cancer cases and deaths worldwide will increase to 24 million and 14.6 million, respectively, by the year 2035. Additional perspective on the prevalence of specific cancers can be gained from national incidence and mortality data. In the United States, it is estimated that the year 2022 will be marked by 1.9 million new cases of cancer and 609,000 cancer deaths. Recent incidence data for the most common forms of cancers in the United States, with the major killers identified, are presented in Fig. 6.11 .
The death rates for certain cancers in the United States have changed over several decades. Since 1995, the cancer death rate has decreased by roughly 20% in men and 10% in women. Among men, 80% of the decrease is attributed to lower death rates for cancers of the lung, prostate, and colon; among women, nearly 60% of the decrease is due to reductions in death rates from breast and colorectal cancers. Decreased use of tobacco products is responsible for the reduction in lung cancer deaths, while improved detection and treatment are responsible for the decrease in death rates for colorectal, female breast, and prostate cancers.
The last half-century has also seen a sharp decline in death rates from cervical cancer and gastric cancer in the United States. The decrease in cervical cancer is directly attributable to widespread use of the Papanicolaou (Pap) test for early detection of this tumor and its precursor lesions. The widespread deployment of the human papillomavirus (HPV) vaccine may nearly eliminate this cancer in coming years. The cause of the decline in death rates for cancer of the stomach is obscure; it may be related to decreasing exposure to unknown dietary carcinogens.
Environmental exposures are dominant risk factors for many common cancers, suggesting that many cancers are preventable. This notion is supported by the geographic variation in death rates from specific cancers, which is thought to stem mainly from differences in environmental exposures. For instance, death rates from breast cancer are about four to five times higher in the United States and Europe than in Japan. Conversely, the death rate for gastric carcinoma in men and women is about seven times higher in Japan than in the United States. Hepatocellular carcinoma is the most lethal cancer in many parts of Africa. Most evidence suggests that these geographic differences have environmental origins. For example, Nisei (second-generation Japanese living in the United States) have mortality rates for certain forms of cancer that are intermediate between those in natives of Japan and in Americans who have lived in the United States for many generations. The two rates come closer with each passing generation.
There is no paucity of environmental factors that contribute to cancer. They are present in the ambient environment, in the workplace, in food, and in personal practices. Some are universal (e.g., sunlight) whereas others are largely restricted to urban settings (e.g., asbestos) or particular occupations ( Table 6.2 ). The most important environmental exposures linked to cancer include the following:
Diet. Certain features of diet have been implicated as predisposing influences. More broadly, obesity, currently epidemic in the United States and other parts of the world, is associated with a modestly increased risk for developing many cancers.
Smoking, particularly of cigarettes, is linked to cancer of the mouth, pharynx, larynx, esophagus, pancreas, bladder, and, most significantly, the lung, as 90% of lung cancer deaths are related to smoking.
Alcohol consumption. Excess alcohol intake is an independent risk factor for cancers of the oropharynx, larynx, esophagus, breast, and (due to alcoholic cirrhosis) liver. Moreover, alcohol and tobacco smoking act synergistically to increase the risk for developing cancers of the upper airways and upper digestive tract.
Reproductive history. There is strong evidence that lifelong cumulative exposure to estrogen stimulation, particularly if unopposed by progesterone, increases the risk for developing cancers of the endometrium and breast, both of which are estrogen-responsive tissues.
Infectious agents are estimated to cause approximately 15% of cancers worldwide (discussed later).
| Agents or Groups of Agents | Human Cancers for Which Reasonable Evidence Is Available | Typical Use or Occurrence |
|---|---|---|
| Arsenic and arsenic compounds | Lung carcinoma, skin carcinoma | By-product of metal smelting; component of alloys, electrical and semiconductor devices, medications and herbicides, fungicides, and animal dips |
| Asbestos | Lung carcinoma, mesothelioma | Formerly used for many applications because of fire, heat, and friction resistance; still found in existing construction as well as fire-resistant textiles, friction materials (i.e., brake linings), underlayment and roofing papers, and floor tiles |
| Benzene | Acute myeloid leukemia | Principal component of light oil; despite known risk, many applications exist in printing and lithography, paint, rubber, dry cleaning, adhesives and coatings, and detergents; formerly widely used as solvent and fumigant |
| Cadmium and cadmium compounds | Prostate carcinoma | Uses include yellow pigments and phosphors; found in solders; used in batteries and as alloy and in metal platings and coatings |
| Chromium compounds | Lung carcinoma | Component of metal alloys, paints, pigments, and preservatives |
| Nickel compounds | Lung and oropharyngeal carcinoma | Nickel plating; component of ferrous alloys, ceramics, and batteries; by-product of stainless-steel arc welding |
| Radon and its decay products | Lung carcinoma | From decay of minerals containing uranium; potentially serious hazard in quarries and underground mines |
| Vinyl chloride | Hepatic angiosarcoma | Refrigerant; monomer for vinyl polymers; adhesive for plastics; formerly inert aerosol propellant in pressurized containers |
In general, the frequency of cancer increases with age. Most cancer deaths occur between 55 and 75 years of age; the rate declines, along with the population base, after 75 years of age. The rising incidence with age is explained by the accumulation of somatic mutations in cells that drive the emergence of malignant neoplasms (discussed later), and by the decline in immune surveillance that accompanies aging.
Although cancer preferentially affects older adults, it is also responsible for slightly more than 10% of all deaths among children younger than 15 years of age ( Chapter 5 ). The major lethal cancers in children are tumors of the central nervous system, leukemias, lymphomas, and soft tissue and bone sarcomas. As discussed later, study of childhood tumors such as retinoblastoma has provided fundamental insights into the pathogenesis of malignant transformation.
Acquired conditions that predispose to cancer include chronic inflammatory disorders, immunodeficiency states, and precursor lesions. Many chronic inflammatory conditions create a fertile “soil” for the development of malignant tumors ( Table 6.3 ). Tumors arising in the context of chronic inflammation are mostly carcinomas but also include mesothelioma and several kinds of lymphoma. By contrast, immunodeficiency states mainly predispose to virus-induced cancers, including specific types of lymphoma and carcinoma and some sarcoma-like proliferations.
| Pathologic Condition | Associated Neoplasm(s) | Etiologic Agent |
|---|---|---|
| Asbestosis, silicosis | Mesothelioma, lung carcinoma | Asbestos fibers, silica particles |
| Inflammatory bowel disease | Colorectal carcinoma | |
| Lichen sclerosis | Vulvar squamous cell carcinoma | |
| Pancreatitis | Pancreatic carcinoma | Chronic alcohol use, germline mutations (e.g., in the trypsinogen gene) |
| Chronic cholecystitis | Gallbladder cancer | Bile acids, bacteria, gallbladder stones |
| Reflux esophagitis, Barrett esophagus | Esophageal adenocarcinoma | Gastric acid |
| Sjögren syndrome, Hashimoto thyroiditis | Extranodal marginal zone lymphoma | |
| Opisthorchis, cholangitis | Cholangiocarcinoma, colon carcinoma | Liver flukes (Opisthorchis viverrini) |
| Gastritis/ulcers | Gastric adenocarcinoma, extranodal marginal zone lymphoma | Helicobacter pylori |
| Hepatitis | Hepatocellular carcinoma | Hepatitis B and/or C virus |
| Osteomyelitis | Carcinoma in draining sinuses | Bacterial infection |
| Chronic cystitis | Bladder carcinoma | Schistosomiasis |
Precursor lesions are characterized by disturbances of epithelial differentiation that are associated with an elevated risk of carcinoma. They may arise secondary to chronic inflammation or hormonal imbalances (in endocrine-sensitive tissues) or occur spontaneously. Molecular analyses have shown that precursor lesions often possess some of the same genetic lesions that are found in their associated cancers (discussed later). However, progression to cancer is not inevitable, and it is important to recognize precursor lesions because their removal or reversal lowers cancer risk.
Many different precursor lesions have been described; among the most common are the following:
Squamous metaplasia and dysplasia of bronchial mucosa, seen in habitual smokers—a risk factor for lung carcinoma ( Chapter 11 )
Endometrial hyperplasia and dysplasia, seen in women with unopposed estrogenic stimulation, is a risk factor for endometrial carcinoma ( Chapter 17 )
Leukoplakia of the oral cavity, vulva, and penis, which may progress to squamous cell carcinoma ( Chapters 13 , 16 , and 17 )
Villous adenoma of the colon is associated with a high risk for progression to colorectal carcinoma ( Chapter 13 )
In this context it also may be asked, “What is the risk for malignant change in a benign neoplasm?”—or, stated differently, “Are benign tumors precancers?” In general, the answer is no but, inevitably, there are exceptions; it may be better to say that each type of benign tumor is associated with a particular level of risk, ranging from high to virtually nonexistent. For example, adenomas of the colon undergo malignant transformation in up to 50% of cases, whereas malignant change is extremely rare in uterine leiomyomas.
Certain cancers are hereditary, usually due to germline mutations that affect the function of a gene that suppresses cancer (a so-called “tumor suppressor gene,” discussed later). What then can be said about the influence of heredity on “sporadic” malignant neoplasms, which constitute roughly 95% of the cancers in the United States? While the evidence suggests that sporadic cancers are largely attributable to environmental factors or acquired predisposing conditions, it may be difficult to tease out hereditary and genetic contributions because environmental and genetic factors often interact. Such interactions may be particularly complex when tumor development is affected by small contributions from multiple genes. Furthermore, genetic factors may alter the risk for developing environmentally induced cancers. Instances where this holds true often involve inherited variation in enzymes such as components of the cytochrome P-450 system that metabolize procarcinogens to active carcinogens. Conversely, environmental factors can influence the risk for developing cancer, even in individuals who inherit well-defined “cancer genes.”
Cancer is a disease caused by mutations that alter the function of a finite subset of the 20,000 or so human genes. For simplicity, we will refer to these genes as cancer genes. Cancer genes can be defined as genes that are recurrently affected by genetic aberrations in cancers, presumably because they contribute directly to the malignant behavior of cancer cells. Causative mutations that give rise to cancer genes may be acquired by the action of environmental agents (such as chemicals, radiation, or viruses), may occur spontaneously, or may be inherited in the germline. If such mutations drive carcinogenesis, a key prediction is that each cell in an individual tumor should share mutations that were present in the founding cell at the time of transformation. This expectation has been realized in all tumors that have been systematically analyzed by genomic sequencing, providing strong support for the hypothesis that cancer is at its root a genetic disease.
Cancer genes number in the hundreds and new ones are still being discovered. They fall into one of four major functional classes:
Oncogenes induce a transformed phenotype when expressed in cells by promoting increased cell growth. Oncogenes are mutated or overexpressed versions of normal cellular genes, which are called proto-oncogenes. Most oncogenes encode transcription factors, factors that trigger progrowth signaling pathways, or factors that enhance cell survival. They are considered dominant genes because a mutation involving a single allele is sufficient to produce an oncogenic effect.
Tumor suppressor genes normally prevent uncontrolled growth and, when mutated or lost from a cell, allow the transformed phenotype to develop. In most instances, both normal alleles of tumor suppressor genes must be damaged or silenced for transformation to occur. Tumor suppressor genes can be placed into two general groups: those that act as important brakes on cellular proliferation and those that are responsible for sensing genomic damage. The latter genes may initiate and choreograph a complex “damage control response” that leads to the cessation of proliferation or, if the damage is too great to be repaired, induce apoptosis.
Genes that regulate apoptosis primarily influence cell survival, rather than stimulating proliferation. Understandably, genes of this class that protect against apoptosis are often overexpressed in cancer cells, whereas those that promote apoptosis tend to be underexpressed or functionally inactivated by mutations.
To this list may now be added genes that regulate interactions between tumor cells and host cells, as these genes are also recurrently mutated or functionally altered in certain cancers. Particularly important are genes that enhance or inhibit recognition of tumor cells by the host immune system.
In most instances, the specific mutations that give rise to cancer genes are acquired during life and are confined to the cancer cells. However, specific causative mutations are sometimes inherited in the germline and are therefore present in every cell in the body, placing the affected individual at high risk for particular cancers ( Table 6.4 ). We will touch on important familial cancer syndromes and associated genes and cancers later in this chapter.
| Inherited Predisposition | Gene(s) |
|---|---|
| Autosomal Dominant Cancer Syndromes | |
| Retinoblastoma | RB |
| Li-Fraumeni syndrome (various tumors) | TP53 |
| Melanoma | CDKN2A |
| Familial adenomatous polyposis/colon cancer | APC |
| Neurofibromatosis 1 and 2 | NF1, NF2 |
| Breast and ovarian tumors | BRCA1, BRCA2 |
| Multiple endocrine neoplasia 1 and 2 | MEN1, RET |
| Hereditary nonpolyposis colon cancer | MSH2, MLH1, MSH6 |
| Nevoid basal cell carcinoma syndrome | PTCH1 |
| Autosomal Recessive Syndromes of Defective DNA Repair | |
| Xeroderma pigmentosum | Diverse genes involved in nucleotide excision repair |
| Ataxia-telangiectasia | ATM |
| Bloom syndrome | BLM |
| Fanconi anemia | Diverse genes involved in repair of DNA cross-links |
Presented next is a discussion of the varied genetic lesions that underlie altered cancer gene expression and function.
The genetic changes found in cancers vary from point mutations involving single nucleotides to abnormalities large enough to produce gross changes in chromosome structure. In certain neoplasms, genetic abnormalities are nonrandom and highly characteristic. Specific chromosomal abnormalities have been identified in many leukemias and lymphomas and in an increasing number of nonhematopoietic tumors, while other tumors are characterized by particular point mutations. These recurrent genetic changes alter the activity of one or more cancer genes in a fashion that gives the affected cells a selective advantage, presumably by contributing to one or more of the hallmarks of cancer.
Driver mutations are mutations that alter the function of cancer genes and thereby directly contribute to the development or progression of cancer. They are usually acquired, but as mentioned earlier, occasionally inherited. By contrast, passenger mutations are acquired mutations that are neutral in terms of fitness and do not affect cellular behavior. Because they occur at random, passenger mutations are sprinkled throughout the genome, whereas driver mutations tend to be tightly clustered within cancer genes. It is now appreciated that passenger mutations may greatly outnumber driver mutations, particularly in cancers caused by carcinogen exposure, such as melanoma and smoking-related lung cancer.
Although apparently innocuous in nature, passenger mutations are important in several ways:
In carcinogen-associated cancers, analysis of passenger mutations has provided definitive evidence that most genomic damage is directly caused by the carcinogen in question. For example, before sequencing of melanoma genomes, the causative role of sun exposure in this cancer was debated. This is no longer so, as most melanomas have thousands of passenger mutations of a type that is specifically linked to damage caused by ultraviolet light.
One effect of passenger mutations is that they create genetic variants that, while initially neutral, may provide tumor cells with a selective advantage in the setting of therapy. The evidence for this comes from DNA sequence analyses of tumors at the time of recurrence after drug therapy. In many instances, mutations that lead directly to drug resistance are found in most tumor cells, generally in the target of the drug (e.g., a tyrosine kinase like BCR-ABL, described later). Generally, the same resistance mutations can also be found before therapy, but only in a very small fraction of cells. In such instances, it appears that the selective pressure of therapy “converts” a neutral passenger mutation into a driver mutation, contributing to tumor progression.
Passenger mutations produce altered proteins that elicit host immune responses. The significance of this will become apparent when we discuss cancer immunity and immunotherapy.
Point mutations can either activate or inactivate the protein products of the affected genes depending on their precise position and consequence. Point mutations that convert proto-oncogenes into oncogenes generally produce a gain of function by altering amino acid residues in a domain that normally holds the protein’s activity in check. A cardinal example is point mutations that convert the proto-oncogene RAS into a cancer gene, one of the most common events in human cancers. By contrast, point mutations in tumor suppressor genes reduce or disable the function of the encoded protein. The tumor suppressor gene that is most commonly affected by point mutations in cancer is TP53, a prototypical tumor suppressor gene (discussed later).
Gene rearrangements may be produced by chromosomal translocations, inversions, deletions, or other more complex genetic events. Specific gene rearrangements are highly associated with certain malignancies, particularly neoplasms derived from hematopoietic cells and other kinds of mesenchymal cells. These rearrangements can activate proto-oncogenes in two ways:
Some gene rearrangements result in overexpression of proto-oncogenes by removing them from their normal regulatory elements and placing them under control of a highly active promoter or enhancer. Two different types of B-cell lymphoma provide illustrative examples of this mechanism. In more than 90% of cases of Burkitt lymphoma, the cells have a balanced reciprocal translocation, usually between chromosomes 8 and 14, that leads to overexpression of the MYC gene on chromosome 8 by juxtaposition of MYC with immunoglobulin heavy chain gene regulatory elements on chromosome 14 ( Fig. 6.12 ). In follicular lymphoma, a balanced reciprocal translocation between chromosomes 14 and 18 leads to overexpression of the antiapoptotic gene BCL2 on chromosome 18, also driven by immunoglobulin gene regulatory elements.
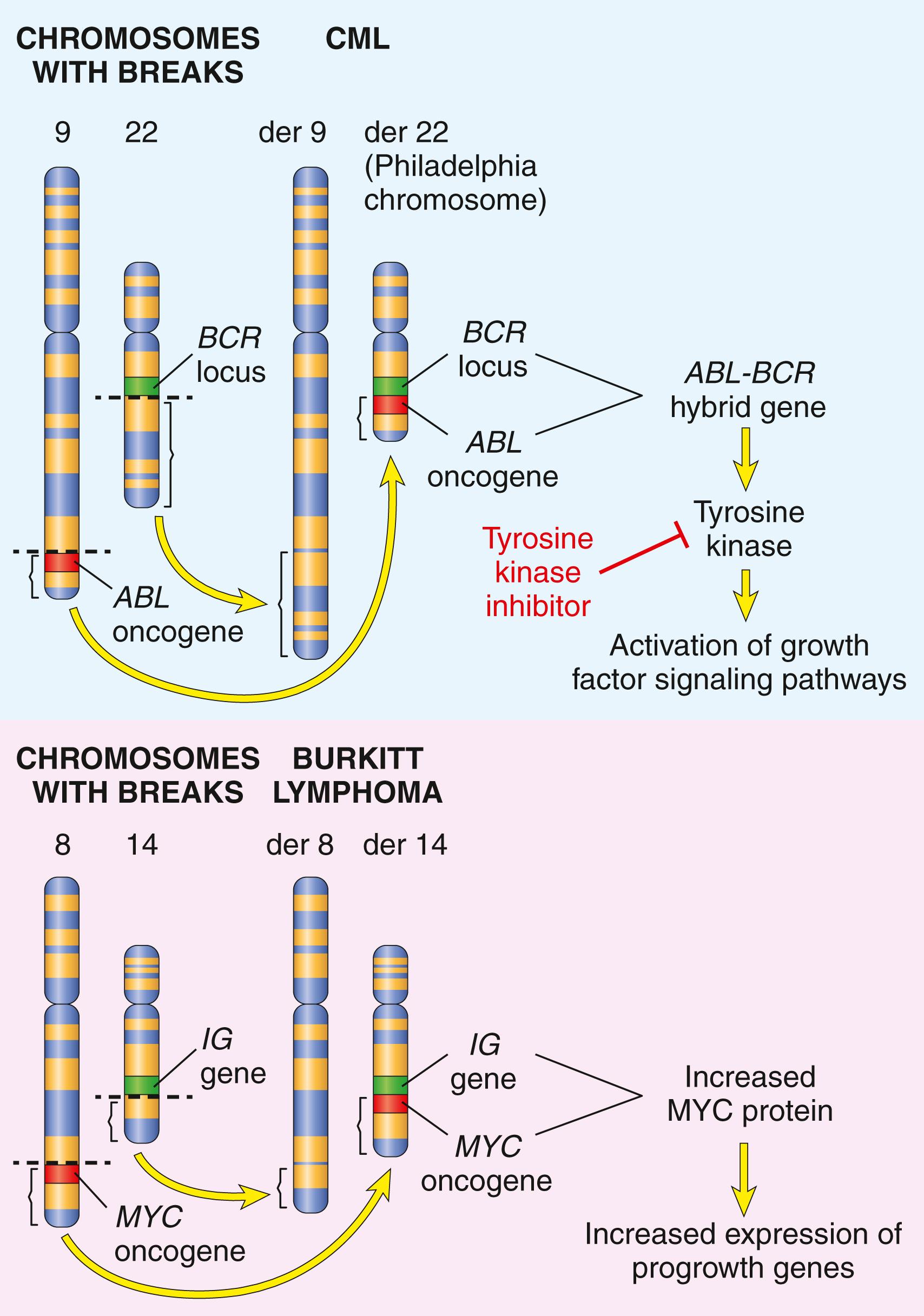
Other oncogenic gene rearrangements create fusion genes encoding novel chimeric proteins. Most notable is the Philadelphia (Ph) chromosome in chronic myeloid leukemia ( Chapter 10 ), which is usually created by a balanced reciprocal translocation between chromosomes 9 and 22 (see Fig. 6.12 ). This cytogenetic change is seen in more than 90% of cases of chronic myeloid leukemia and results in the fusion of portions of the BCR gene on chromosome 22 and the ABL gene on chromosome 9. The few Philadelphia chromosome–negative cases harbor cryptic (karyotypically invisible) BCR-ABL fusion genes, the presence of which is the sine qua non of chronic myeloid leukemia. As discussed later, the BCR-ABL fusion gene encodes a novel tyrosine kinase with potent transforming activity.
Lymphoid tumors are most commonly associated with recurrent gene rearrangements. This relationship exists because normal lymphocytes express special enzymes that are intended to introduce DNA breaks during the processes of immunoglobulin or T-cell receptor gene recombination. Repair of these DNA breaks is error prone, and the resulting mistakes sometimes result in gene rearrangements that activate proto-oncogenes. Two other types of mesenchymal tumors, myeloid neoplasms (acute myeloid leukemias and myeloproliferative neoplasms) and sarcomas, also frequently possess gene rearrangements. Unlike lymphoid neoplasms, the cause of the DNA breaks that lead to gene rearrangements in myeloid neoplasms and sarcomas is unknown. In general, the rearrangements that are seen in myeloid neoplasms and sarcomas create fusion genes that encode either hyperactive tyrosine kinases (akin to BCR-ABL) or novel oncogenic transcription factors. A well-characterized example of the latter is the (11;22)(q24;q12) translocation in Ewing sarcoma ( Chapter 19 ) that creates a fusion gene encoding a chimeric oncoprotein composed of portions of two different transcription factors called EWS and FLI1.
Identification of pathogenic gene rearrangements in carcinomas has lagged because karyotypically evident translocations and inversions (which point to the location of important oncogenes) are rare in carcinomas. However, widespread sequencing of cancer genomes has revealed recurrent cryptic pathogenic gene rearrangements in carcinomas as well. As with hematologic malignancies and sarcomas, gene rearrangements in carcinoma contribute to carcinogenesis either by increasing expression of an oncogene or by generation of a novel fusion gene. Examples will be discussed in other chapters. As with a fusion gene such as BCR-ABL, some of the proteins encoded by fusion genes in carcinomas are also drug targets (e.g., EML-ALK in lung cancer; Chapter 11 ).
Recurrent deletion of specific regions of chromosomes in cancer cells usually results in the loss of particular tumor suppressor genes. Tumor suppressive functions generally require inactivation of both alleles before antioncogenic activities are lost. A common mechanism is for an inactivating point mutation to occur in one allele and a deletion to occur in the other. As discussed later, deletions involving chromosome 13q14, the site of the RB gene, are associated with retinoblastoma, and deletion of chromosome 17p is associated with loss of TP53, perhaps the most important tumor suppressor gene.
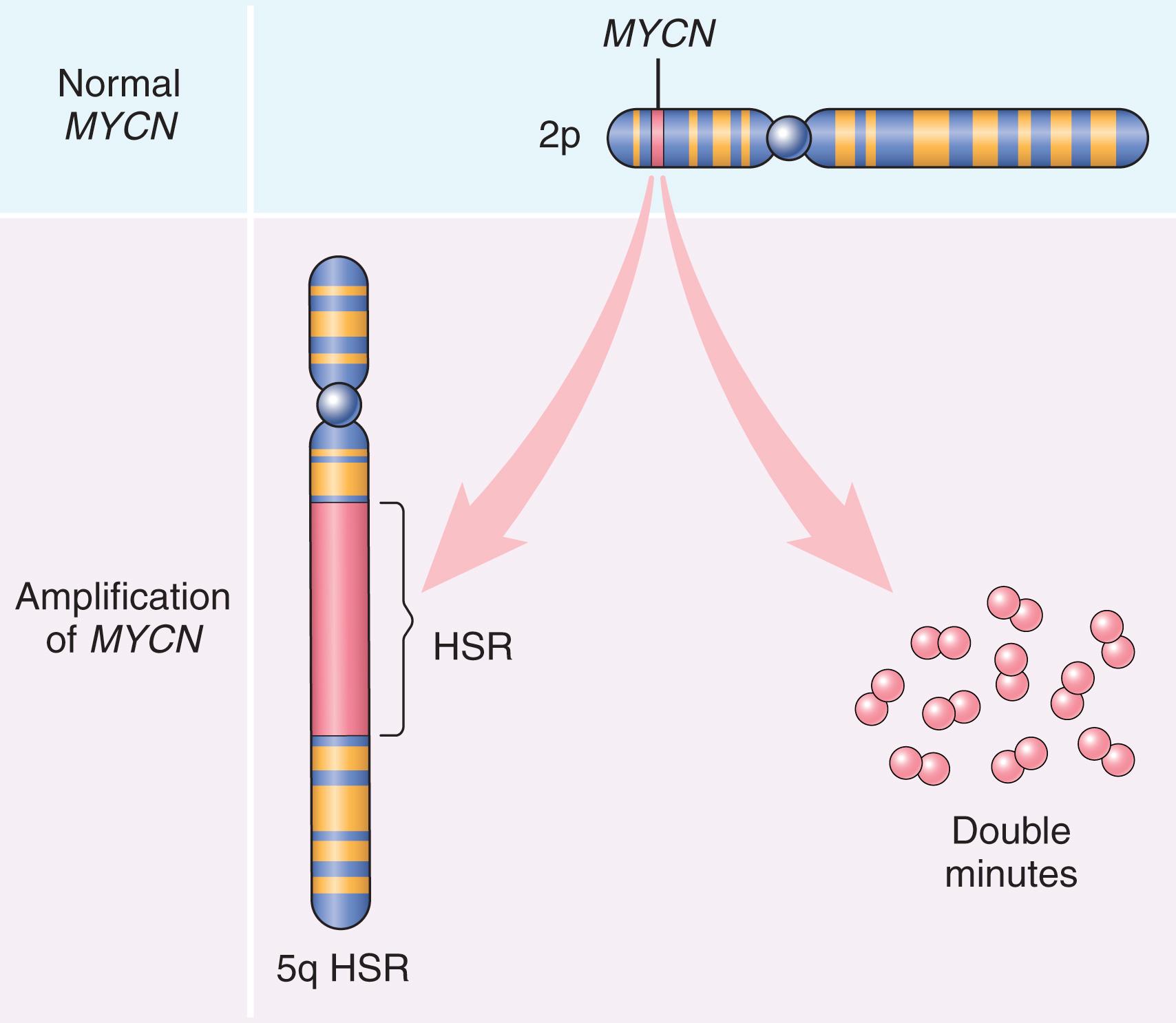
Proto-oncogenes may be converted to oncogenes by gene amplification, with consequent overexpression and hyperactivity of otherwise normal proteins. Such amplification may produce up to several hundred copies of a gene, a change in copy number that is readily detected by molecular hybridization with appropriate DNA probes. In some cases, the amplified genes produce chromosomal changes that can be identified microscopically. Two patterns are seen: (1) multiple small, extrachromosomal structures called double minutes and (2) homogeneously staining regions . The latter derive from the insertion of the amplified genes into new chromosomal locations, which may be distant from the normal location of the involved genes. Because regions containing amplified genes lack normal banding, they have a homogeneous staining pattern in a G-banded karyotype. Two clinically important examples of amplification involve the MYCN gene in neuroblastoma and the HER2 gene in breast cancers. In 25% to 30% of neuroblastomas, MYCN is amplified, a feature that is associated with poor prognosis ( Fig. 6.13 ). HER2 (also known as ERBB2 ) amplification occurs in about 20% of breast cancers, and therapy directed against the receptor encoded by the HER2 gene is highly effective in this subset of tumors.
Aneuploidy is defined as a number of chromosomes that is not a multiple of the haploid state; for humans, that is any chromosome number that is not a multiple of 23. Aneuploidy is remarkably common in cancers and was proposed as a cause of carcinogenesis over 100 years ago. It frequently results from errors of the mitotic checkpoint, a major cell cycle control mechanism that prevents mistakes in chromosome segregation by inhibiting the transition to anaphase until all of the replicated chromosomes have attached to spindle microtubules.
Mechanistic data establishing aneuploidy as a cause of carcinogenesis, rather than a consequence, have been difficult to generate. However, detailed analysis of cancer cells suggests that aneuploidy increases the copy number of key oncogenes and decreases the copy number of potent tumor suppressors. For example, chromosome 8, which is almost never lost and is often present in increased copies in tumor cells, is where the MYC oncogene is located. By contrast, portions of chromosome 17, where the TP53 gene is located, are often lost and infrequently gained. Thus, tumor development and progression may be molded by changes in chromosome numbers that enhance the dosage of oncogenes or decrease the dosage of tumor suppressor genes.
As discussed in Chapter 4 , microRNAs (miRNAs) are short, noncoding, single-stranded RNAs that function as negative regulators of genes. They inhibit gene expression posttranscriptionally by repressing translation or, in some cases, by promoting the cleavage of messenger RNA (mRNA). In view of their important functions in control of cell growth, differentiation, and survival, it is not surprising that miRNAs can also contribute to carcinogenesis. Specifically, if the target of a miRNA is a tumor suppressor gene, then overactivity of the miRNA can reduce the activity of the encoded tumor suppressor protein. Such miRNAs are sometimes referred to as oncomirs. Conversely, if a miRNA normally inhibits the translation of an oncogene, a reduction in the quantity or function of that miRNA will lead to overproduction of the oncogene product. Such relationships have been established by miRNA profiling of several human tumors. For example, downregulation or deletion of certain miRNAs in some leukemias and lymphomas results in increased expression of BCL2, an antiapoptotic gene. Dysregulation of other miRNAs that control the expression of the RAS and MYC oncogenes has also been detected in lung tumors and in certain B-cell leukemias, respectively.
You will recall from Chapter 4 that epigenetics refers to reversible, heritable changes in gene expression that occur without mutation. Such changes involve posttranslational modifications of histones and DNA methylation, both of which affect gene expression. In normal, differentiated cells, a large portion of the genome is not expressed. These regions of the genome are silenced by DNA methylation and histone modifications. On the other hand, cancer cells are characterized by global DNA hypomethylation and selective promoter-localized hypermethylation. Indeed, it has become evident that tumor suppressor genes are sometimes silenced by hypermethylation of promoter sequences, rather than by mutation. In addition, genome-wide hypomethylation has been shown to cause chromosomal instability and to induce tumors in mice. Thus, epigenetic changes may influence carcinogenesis in many ways. As an added wrinkle, deep sequencing of cancer genomes has identified mutations in genes that regulate epigenetic modifications in many cancers. Thus, certain genetic changes in cancers may be selected because they lead to epigenetic alterations (e.g., DNA methylation and histone modifications) that favor cancer growth and survival.
Carcinogenesis is a multistep process resulting from the accumulation of multiple genetic alterations that collectively give rise to the transformed phenotype and all of its associated hallmarks, discussed later. As mentioned earlier, the presence of driver mutations in some nonneoplastic precursor lesions suggests the need for additional mutations for transition to a full-blown cancer and thus supports this model.
Beyond tumor initiation from a single founding cell, it is important to recognize that cancers continue to undergo Darwinian selection and therefore continue to evolve ( Fig. 6.14 ). It is well established that during their course cancers generally become more aggressive and acquire greater malignant potential, a phenomenon referred to as tumor progression. At the molecular level, tumor progression most likely results from mutations that accumulate independently in different cells. Some of these mutations may be lethal, but others may affect the function of cancer genes, thereby making the affected cells more adept at growth, survival, invasion, metastasis, or immune evasion. Due to this selective advantage, subclones that acquire these mutations may come to dominate one area of a tumor, either at the primary site or at sites of metastasis. As a result of continuing mutation and Darwinian selection, even though malignant tumors are monoclonal in origin they are typically genetically heterogeneous by the time of their clinical presentation. In advanced tumors exhibiting genetic instability, the extent of genetic heterogeneity may be enormous.
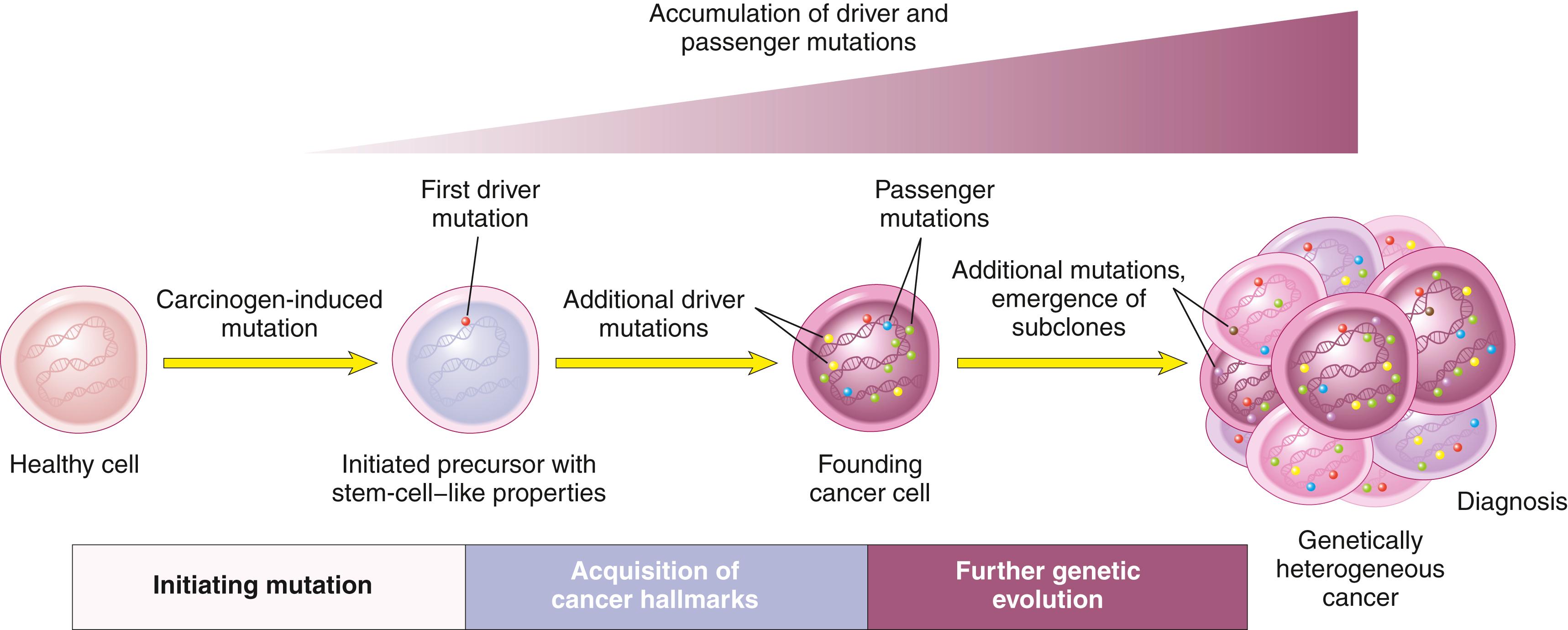
Genetic evolution shaped by Darwinian selection can explain the two most pernicious properties of cancers: the tendency over time for cancers to become both more aggressive and less responsive to therapy. Thus, genetic heterogeneity has implications not only for cancer progression but also for response to therapy. Experience has shown that when tumors recur after chemotherapy, the recurrent tumor is almost always resistant to the original drug regimen if it is given again. Experimental data suggest that this acquired resistance stems from the outgrowth of subclones that have, by chance, mutations (or epigenetic alterations) that confer drug resistance.
As mentioned earlier, bona fide cancer genes number in the hundreds, at a minimum. While it is traditional to describe the function of cancer genes one gene at a time, the blizzard of mutated genes emerging from the sequencing of cancer genomes has blanketed the landscape and revealed the limitations of trying to grasp the fundamental properties of cancer gene by gene. A more tractable and conceptually satisfying way to think about the biology of cancer is to consider the common phenotypic and biologic properties of cancer cells. It appears that all cancers display several fundamental changes in cell physiology, which are considered the hallmarks of cancer. These changes, illustrated in Fig. 6.15 , consist of the following:
Self-sufficiency in growth signals
Insensitivity to growth-inhibitory signals
Altered cellular metabolism
Evasion of apoptosis
Limitless replicative potential (immortality)
Sustained angiogenesis
Invasion and metastasis
Evasion of immune surveillance
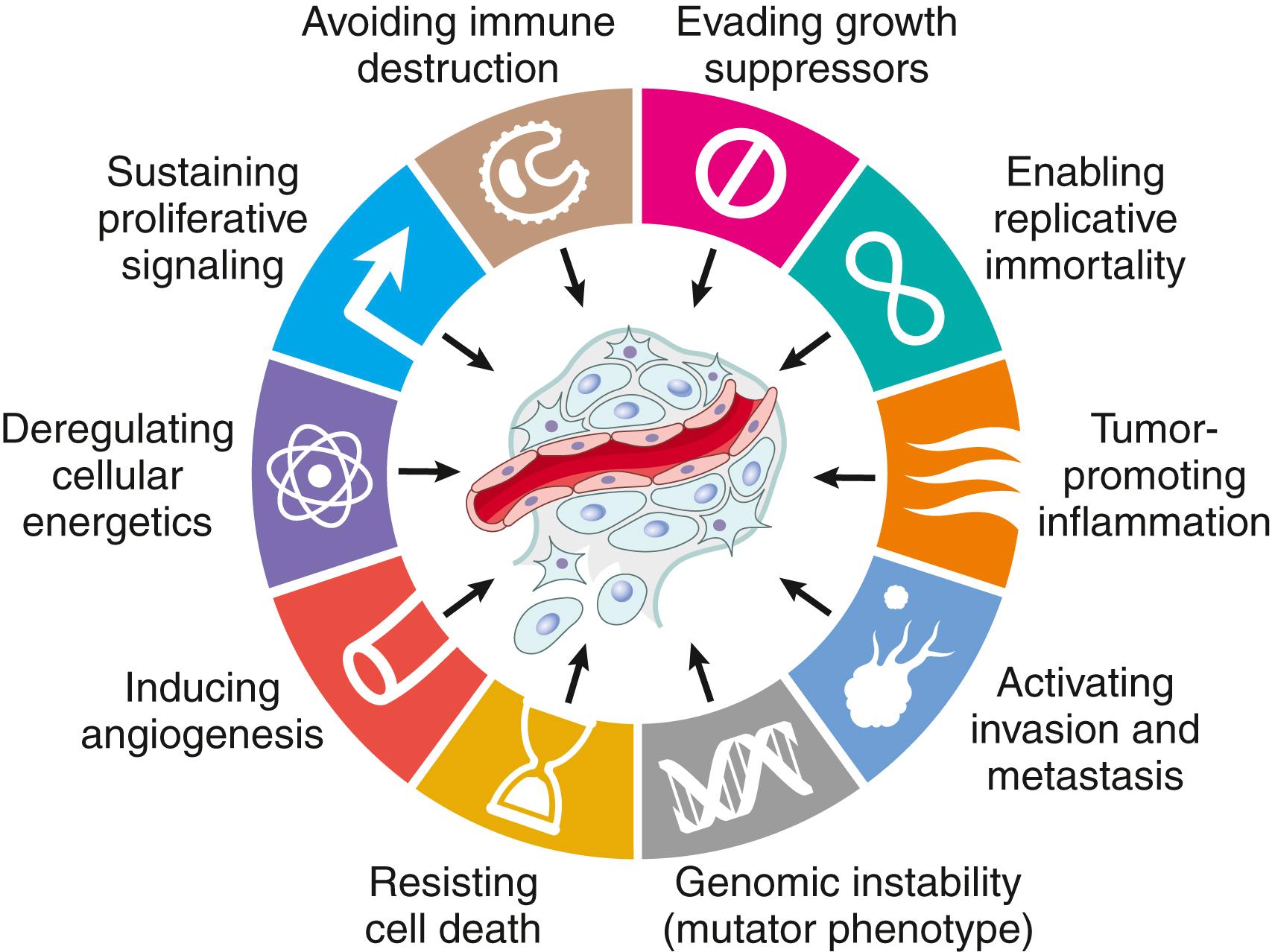
The acquisition of the genetic and epigenetic alterations that confer these hallmarks may be accelerated by cancer-promoting inflammation and by genomic instability . These are considered enabling characteristics because they aid and abet cellular transformation and subsequent tumor progression.
Mutations in genes that regulate some or all these cellular traits are seen in every cancer; accordingly, these traits form the basis of the following discussion of the molecular origins of cancer. Of note, by convention, gene symbols are italicized but their protein products are not (e.g., RB gene and RB protein, TP53 and p53, MYC and MYC).
The self-sufficiency in growth that characterizes cancer cells generally stems from gain-of-function mutations that convert proto-oncogenes to oncogenes. Oncogenes encode proteins called oncoproteins that promote cell growth even in the absence of normal growth-promoting signals. To appreciate how oncogenes drive inappropriate cell growth, it is helpful to review the sequence of events that characterize normal cell proliferation. Under physiologic conditions, signals that drive cell proliferation can be resolved into the following steps:
Binding of a growth factor to its specific receptor on the cell membrane
Transient and limited activation of the growth factor receptor, which in turn activates several signal-transducing proteins on the inner leaflet of the plasma membrane
Transmission of the transduced signal across the cytosol to the nucleus by second messengers or a cascade of signal transduction molecules
Induction and activation of nuclear regulatory factors that initiate and regulate DNA transcription and thus the biosynthesis of other cellular components that are needed for cell division, such as organelles, membrane components, and ribosomes
Entry and progression of the cell into the cell cycle, resulting ultimately in cell division
The mechanisms that endow cancer cells with the ability to proliferate can be grouped according to their role in the growth factor–induced signal transduction cascade and cell cycle regulation. Indeed, each one of the steps listed above is susceptible to perturbation in cancer cells.
Cancers may secrete their own growth factors or induce stromal cells in the tumor microenvironment to produce growth factors. Most growth factors are made by one cell type and act on a neighboring cell of a different type to stimulate proliferation (paracrine action). Normally, cells that produce the growth factor do not express that factor’s receptor, preventing the formation of positive feedback loops within the same cell. This “rule” is broken by certain cancers in several ways.
Some cancer cells acquire the ability to synthesize the same growth factors to which they are responsive. For example, many glioblastomas secrete platelet-derived growth factor (PDGF) and have amplifications of the PDGF receptor gene, and many sarcomas secrete transforming growth factor-α (TGF-α) and express its receptor. Similar autocrine loops are fairly common in other types of cancer as well.
In other cancers, the tumor cells send signals that induce normal cells in the supporting stroma to produce growth factors that feedback to stimulate tumor growth.
Next in the sequence of progrowth signaling is growth factor receptors, many of which have intrinsic kinase activity when activated by growth factor binding. Many of the myriad growth factor receptors function as oncoproteins when they are mutated or if they are overexpressed. The best-documented examples of overexpression involve the epidermal growth factor (EGF) receptor family. ERBB1, the EGF receptor, is overexpressed in 80% of squamous cell carcinomas of the lung, 50% or more of glioblastomas, and 80% to 100% of epithelial tumors of the head and neck. As mentioned earlier, the gene encoding a related receptor, HER2 (ERBB2), is amplified in approximately 20% of breast cancers and in a smaller fraction of adenocarcinomas of the lung, ovary, stomach, and salivary glands. The significance of HER2 in the pathogenesis of breast cancers is illustrated dramatically by the clinical benefit derived from blocking the extracellular domain of this receptor with anti-HER2 antibodies, an elegant example of “bench to bedside” medicine. In other instances, the tyrosine kinase activity of the receptors is stimulated by point mutations or small insertions or deletions that lead to subtle but functionally important changes in protein structure, or by gene rearrangements that create fusion genes encoding chimeric receptors. In each of these cases, the mutated receptors are either constitutively active, delivering mitogenic signals to cells even in the absence of growth factors, or are hyperresponsive to growth factors.
Cancer cells often acquire growth autonomy as a result of mutations in genes that encode components of signaling pathways downstream of growth factor receptors. The signaling proteins that couple growth factor receptors to their nuclear targets are activated by ligand binding to growth factor receptors. Two particularly important oncogenic signaling molecules are RAS and ABL, which are discussed next.
RAS is the most commonly mutated oncogene in human tumors. Approximately 20% of all human tumors contain mutated RAS genes, and the frequency is even higher in specific cancers (e.g., pancreatic adenocarcinoma). RAS is a member of a family of small G proteins that bind guanosine nucleotides (guanosine triphosphate [GTP] and guanosine diphosphate [GDP]). The activity of RAS is regulated by the relative binding of GDP and GTP.
Normally, RAS flips back and forth between an excited signal-transmitting state and a quiescent state. RAS is inactive when bound to GDP; stimulation of cells by growth factors such as EGF and PDGF leads to exchange of GDP for GTP and subsequent conformational changes that activate RAS ( Fig. 6.16 ). This excited signal-emitting state is short lived, however, because an intrinsic guanosine triphosphatase (GTPase) activity of activated RAS hydrolyzes GTP to GDP, releasing a phosphate group and returning the protein to its quiescent GDP-bound state. The GTPase activity of activated RAS is magnified dramatically by a family of GTPase-activating proteins (GAPs) that act as molecular brakes that prevent uncontrolled RAS activation by favoring hydrolysis of GTP to GDP.
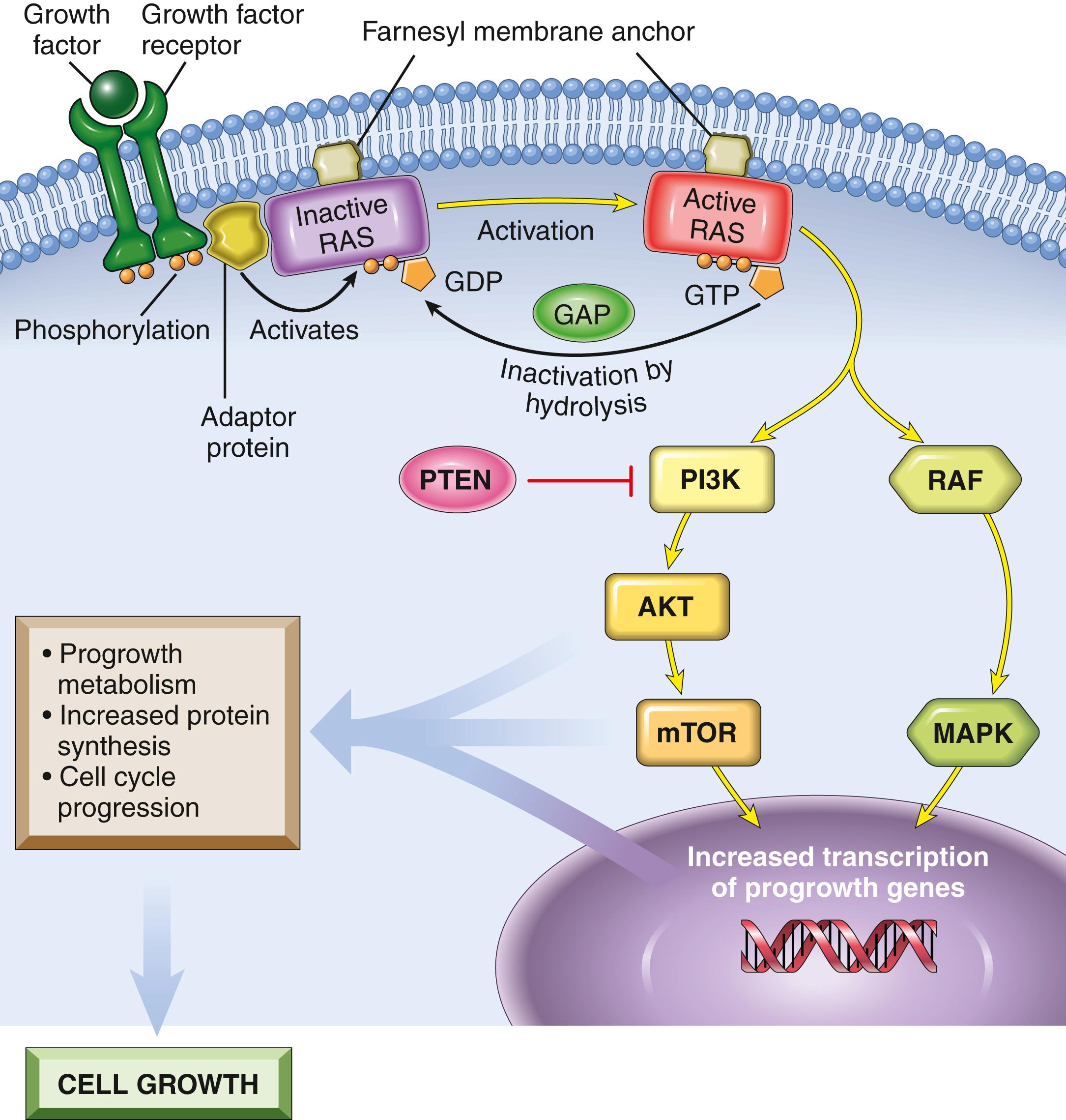
Activated RAS stimulates downstream regulators of proliferation by several interconnected pathways that converge on the nucleus and alter the expression of genes that regulate growth, such as MYC. While details of the signaling cascades (some illustrated in Fig. 6.16 ) downstream of RAS are not discussed here, an important point is that mutational activation of these signaling intermediates mimics the growth-promoting effects of activated RAS. For example, BRAF, which is part of the so-called “RAF/ERK/MAP kinase pathway,” is mutated in more than 60% of melanomas and is associated with unregulated cell proliferation. Mutations of phosphatidylinositol-3 kinase (PI3 kinase) in the PI3K/AKT pathway also occur with high frequency in some tumor types, with similar consequences.
RAS most commonly becomes constitutively activated as a result of point mutations in amino acid residues that are either within the GTP-binding pocket or in the enzymatic region that carries out GTP hydrolysis. Both types of mutations interfere with the breakdown of GTP so RAS stays in the GTP-bound active form and the cell receives continuous progrowth signals. It follows logically that the consequences of activating mutations in RAS should be mimicked by loss-of-function mutations in GAPs, which would also lead to decreased GTP hydrolysis. Indeed, the GAP neurofibromin-1 (NF1) is mutated in the cancer-prone familial disorder neurofibromatosis type 1 ( Chapter 20 ) and is a bona fide tumor suppressor.
Several non–receptor tyrosine kinases function as signal transducing molecules. In this group, ABL is the best defined with respect to carcinogenesis.
The ABL proto-oncoprotein has tyrosine kinase activity that is dampened by internal negative regulatory domains. As discussed earlier (see Fig. 6.12 ), in chronic myeloid leukemia and certain acute leukemias, part of the ABL gene is translocated from its normal site on chromosome 9 to chromosome 22, where it fuses with part of the breakpoint cluster region (BCR) gene. This fusion gene encodes a BCR-ABL hybrid protein that contains the ABL tyrosine kinase domain and a BCR domain that self-associates, an event that mimics the effect of ligand binding and results in constitutive tyrosine kinase activity. Of interest, the BCR-ABL protein activates all the signals that are downstream of RAS, making it a potent stimulator of cell growth.
The crucial role of BCR-ABL in cancer has been confirmed by the dramatic clinical response of patients with chronic myeloid leukemia to BCR-ABL kinase inhibitors. The prototype of this kind of drug, imatinib mesylate, galvanized interest in the design of drugs that target specific molecular lesions found in various cancers (so-called “targeted therapy”). BCR-ABL is also an example of the concept of oncogene addiction, wherein a tumor is profoundly dependent on a single signaling molecule. BCR-ABL fusion gene formation is an early, perhaps initiating, event that drives leukemogenesis. Development of leukemia probably requires other collaborating mutations, but the transformed cell continues to depend on BCR-ABL for signals that mediate growth and survival. BCR-ABL signaling can be likened to a central pillar around which the transformed state is “built.” If the pillar is removed by inhibition of the BCR-ABL kinase, the structure collapses. In view of this level of dependency, it is not surprising that acquired resistance of tumors to BCR-ABL inhibitors is often due to the outgrowth of a subclone with a mutation that prevents binding of the drug to the BCR-ABL protein.
The ultimate consequence of signaling through oncoproteins such as RAS or ABL is inappropriate and continuous stimulation of nuclear transcription factors that drive the expression of growth-promoting genes. A host of oncoproteins, including products of the MYC, MYB, JUN, FOS, and REL oncogenes, function as transcription factors that regulate the expression of growth-promoting genes. Of these, MYC is involved most commonly in human tumors.
Dysregulation of MYC promotes tumorigenesis by simultaneously promoting the progression of cells through the cell cycle and enhancing alterations in metabolism that support cell growth. MYC primarily functions by activating the transcription of other genes, including several growth-promoting genes, such as cyclin-dependent kinases (CDKs), whose products drive cells into the cell cycle (discussed next), and genes that control metabolic pathways that produce the building blocks (e.g., amino acids, lipids, nucleotides) that are needed for cell growth and division. As mentioned earlier (see Fig. 6.12 ), in Burkitt lymphoma dysregulation of MYC results from a (8;14) translocation. In breast, colon, lung, and many other cancers MYC is amplified, while in neuroblastomas and small cell cancers of the lung, the related MYCN and MYCL genes are amplified, respectively.
The ultimate outcome of growth-promoting stimuli is the entry of quiescent cells into the cell cycle, a complex process that regulates cellular proliferation. In addition to mutations in growth factor signaling pathways (discussed earlier), cancer cells are often freed of the normal requirements for growth factors by mutations or other alterations in genes that encode components of the cell cycle machinery. To understand how these alterations contribute to carcinogenesis, we must first briefly review the cell cycle and its key regulators.
Following entry into the cell cycle, normal cells undergo a tightly choreographed sequence of events that lead to DNA replication and ultimately cell division. These events occur in four distinct phases called G 1 (Gap1), S (synthesis), G 2 (Gap2), and M (mitosis); quiescent cells that are not actively proliferating are in the G 0 state. Cells can enter G 1 either from the G 0 quiescent cell pool or after completing a round of mitosis. Each stage requires completion of the previous step as well as the activation of stage-specific factors (described next); the failure to complete DNA replication or a deficiency of essential cofactors results in the arrest of cells at the various transition points between cell cycle phases.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here