Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Most neonatal lung diseases are characterized by increased alveolar surface tension causing atelectasis. To offset this, pressure is applied to the upper airway through either noninvasive or invasive techniques. Regardless of the support technique applied, mean airway pressure (mPaw) is a good measure of the amount of support that is applied to offset these surface forces and improve ventilation/perfusion matching and oxygenation.
There are multiple ways to provide noninvasive support. These include nasal cannula (NC) supplemental oxygen, high-flow nasal cannula (HFNC) which provides some level of positive pressure, nasal CPAP (NCPAP), and noninvasive ventilation modes, both synchronized and non-synchronized.
There are multiple modes of mechanical ventilation, but all ventilator breaths can be characterized by when they start, how large they are, and the duration of the breath. Modes that support every breath and which provide consistent tidal volume (V T ) appear to be the best for most infants.
High-frequency ventilation predominantly includes high-frequency jet ventilation (HFJV) and high-frequency oscillatory ventilation (HFOV). The mechanisms by which they provide gas exchange are complex, but the guidelines for adjusting them are simple. HFOV and HFJV are typically used for infants with severe lung disease.
Noninvasive assessment of the respiratory status of an infant includes a physical exam, chest radiographs, and pulse oximetry. For ventilated infants, assessment of ventilator graphics plays a key role in optimizing ventilator support.
Respiratory support for most infants with immature and/or abnormal lungs falls into two broad categories: provision of supplemental oxygen and provision of pressure. Supplemental oxygen is one of the oldest therapies in modern neonatal care, while pressure applied to the upper airways and transmitted to the alveolar level is more recent but is fundamental to offsetting lung pathology. The techniques for providing these therapies can be thought of as a continuum extending from simple nasal cannula oxygen to nasal continuous positive airway pressure (CPAP) and noninvasive ventilation, to sophisticated forms of invasive mechanical ventilation. Moving through this continuum allows for the support of infants with more severe lung disease but is associated with concomitant complexity.
Most neonatal lung diseases are characterized by atelectasis and ventilation/perfusion mismatch, resulting in impaired oxygenation. Other common neonatal conditions such as pulmonary hypertension and congenital cardiovascular anomalies can also result in right-to-left shunting and impaired oxygenation. Regardless of the cause, impaired oxygen delivery from the alveoli to the pulmonary capillary bed can lead to reduced oxygen content in the blood (hypoxemia) and tissue (hypoxia).
Administration of supplemental oxygen to newborns with lung disease has been part of routine neonatal care since the first part of the 20th century. An increased F I O 2 increases the alveolar O 2 tension (P A O 2 ) in both well-ventilated and partially ventilated areas of the lung. The resulting increase in the alveolar-arterial O 2 gradient drives O 2 from the alveoli into the pulmonary circulation and partially compensates for the hypoxemia associated with ventilation/perfusion mismatch. However, supplemental O 2 does not improve the underlying atelectasis which may be causing the hypoxemia.
The primary goal of supplemental oxygen is to maintain adequate oxygen availability to the tissues, especially to the central nervous system and the heart. However, achieving the optimal level of supplemental oxygen support requires carefully balancing the toxicity inherent in oxygen supplementation and the potential damage to end organs caused by high arterial P a O 2 levels against the potential damage caused by hypoxemia.
For infants with hypoxemia secondary to decreased ventilation/perfusion matching, correction of the ventilation/perfusion mismatch is at least as important as giving supplemental oxygen. In most acute neonatal lung diseases this means providing pressure to the upper airway to offset the surface forces leading to alveolar collapse and atelectasis. Thus, finding the ideal level of supplemental oxygen is almost always done in the context of simultaneously finding the ideal level of positive pressure support.
Early strategies to “normalize” oxygenation in preterm infants with the use of high F I O 2 resulted in high rates of retinopathy of prematurity (ROP) and blindness. The early, liberal use of high levels of supplemental oxygen was then followed by a period when F I O 2 was restricted, regardless of blood oxygenation level, and was associated with increased rates of neurologic damage and death. The introduction of continuous oxygen monitoring, first with transcutaneous oxygen levels (P TC O 2 ), then with saturation monitoring by pulse oximetry (S P O 2 ), led to finer control of oxygenation and attempts to keep infants within tighter oxygenation target ranges.
The ideal target ranges for oxygenation and ventilation are not certain and the ranges employed vary to some extent from center to center. General trends and guidelines are outlined below.
Infants, particularly preterm infants, have an inherently unstable respiratory system that makes it extremely difficult to maintain oxygenation saturation within a narrow range. In addition, preterm infants are easily destabilized by handling and procedures. Normal oxygen saturation for room air–breathing term or healthy preterm infants, after the immediate transition period, is greater than 93%, with P a O 2 levels above 70 mmHg. This is not surprising, since the mature and healthy infant lung has normal ventilation/perfusion matching and should be able to deliver “normal” amounts of oxygen to the blood.
The search for the ideal S P O 2 target range in infants can be divided into three broad areas, each with its own body of research. The first is in delivery room resuscitation where recent research has focused on the rate at which the newborn’s S P O 2 should transition from the low intrauterine levels to higher postnatal levels. The second is the debate about the ideal S P O 2 for infants with BPD, particularly to avoid or treat the pulmonary hypertension that often accompanies severe BPD. Both newborn resuscitation and BPD are addressed in more detail elsewhere in this textbook. The third area of research on ideal S P O 2 target ranges is in preterm infants during the acute, post-delivery period. This has largely come down to a delicate balance between avoiding the higher S P O 2 ranges which are associated with an increased risk of ROP and avoiding the lower S P O 2 ranges which may be associated with increased mortality. Early data showed that ROP severity was associated with the duration of hyperoxemia and emphasized the importance of oxygen monitoring. Higher rates of ROP and a more severe respiratory course appeared to occur in neonatal centers that tolerated higher S P O 2 levels, and observational data indicated that decreasing the S P O 2 target range could reduce the rates of ROP. Subsequent randomized trials to compare the effects of targeting an S P O 2 of 85% to 89% versus 91% to 95% had mixed results, with some studies suggesting an increased mortality in infants in the lower S P O 2 range, and others not finding a difference between the ranges. Taken together, these studies suggest that there is an increased risk of ROP but a decreased incidence of death or NEC in the higher target S P O 2 range. However, the composite outcome of death or major disability does not differ between the groups. The most recent European Consensus Guidelines for the Management of RDS suggest targeting S P O 2 90% to 94%, with alarm limits set between 89% and 95%.
Regardless of the exact target S P O 2 range desired, maintaining infants within this range is often a challenge. Immature respiratory drive and a compliant chest wall, which increases the risk of atelectasis, frequently combine to make oxygenation inherently unstable. Preterm infants receiving supplemental oxygen appear to spend only half of the time with S P O 2 within their target range. The picture is also complicated because caregivers may tolerate higher than ideal S P O 2 levels with the purpose of reducing the frequency of desaturation spells or attenuating their severity. While S P O 2 changes rapidly in response to the infant’s oxygenation status, adjustments in F I O 2 and/or level of positive pressure support may lead to gradual changes in gas exchange. Thus, there is always a risk of responding too slowly to changes in S P O 2 and a simultaneous risk of over-correcting. In clinical practice, many infants experience hyperoxemia because F I O 2 is not quickly returned to the basal level when a hypoxemic “spell” resolves. In the end, optimal management of an individual infant’s oxygenation status is usually a combination of clearly defined policies, careful S P O 2 monitoring, and the judgment of experienced clinicians about how to respond to infants who stray from their desired target S P O 2 range.
Policies of oxygenation monitoring should clearly identify both the intended range and the alarm limits of S P O 2 . Setting S P O 2 alarm limits near the prescribed S P O 2 target range can increase the proportion of time premature infants spend within the target range. However, narrow alarm limit ranges will increase the number of times the monitor alarms and can lead to “alarm fatigue.” Staff compliance with S P O 2 alarms, and their response to them plays an important role in keeping infants in the target S P O 2 range. For this reason, staff education and communication are important components of maintaining infants within a target S P O 2 range.
Automated systems to adjust F I O 2 to maintain S P O 2 within a target range have been investigated in multiple trials and show consistent improvement in the maintenance of S P O 2 within the target range as well as reductions in oxygen exposure and staff workload. While theoretically attractive, this technology is not yet widely available for clinical use.
Tolerance to higher carbon dioxide levels may reduce the need for support and reduce the duration of ventilation. However, the results of clinical trials of permissive hypercapnia have been inconsistent. Initial trials suggested faster weaning of infants from mechanical ventilation when higher PCO 2 levels were tolerated. On the other hand, there are data suggesting that hypercapnia impairs cerebral autoregulation in the newborn premature infant, suggesting a need for caution in tolerating acute hypercapnia. The most recent large study of hypercapnia found no difference in the rate of death or BPD, and showed no difference in adverse sequelae at follow-up. Taken together, these studies suggest that the optimal safe range of PCO 2 has yet to be determined. In clinical practice, most centers tolerate some degree of hypercapnia (e.g., PCO 2 in the 50s) in acute lung disease and tolerate a greater degree of compensated hypercapnia in infants with more chronic disease.
General guidelines:
Rapid changes in PCO 2 should always be avoided. In particular, there is concern about the impact of PCO 2 changes on cerebral blood flow.
Hypocarbia (P a CO 2 < 35) should always be avoided. In most infants, hypocarbia represents excessive minute ventilation, with the associated risks of lung damage and cardiovascular compromise from excessive pressure and/or volume. Hypocarbia has also been associated with neurodevelopmental morbidity in multiple studies. While hypocarbia was at one time used as a standard therapy for decreasing pulmonary vascular resistance in infants with pulmonary artery hypertension, that approach has almost disappeared with the ready availability of inhaled nitric oxide (iNO) as a far more safe and effective way to decrease pulmonary vascular resistance.
The degree of hypercarbia which can be tolerated probably varies with post-natal age and with the degree of compensatory metabolic alkalosis. Most centers are comfortable tolerating PCO 2 levels in the 60s (with an acceptable pH) in infants with chronic lung disease.
The target pH for most infants is usually around 7.25 to 7.35. Higher pH values typically represent hyperventilation and hypocarbia, whereas pH values less than 7.20 to 7.25 raise concerns about the potential impact of acidosis on cellular function, including adverse effects on myocardial function and cardiac output.
In neonates, supplemental O 2 can be administered by itself or in conjunction with positive pressure techniques. The simplest ways of providing O 2 without positive pressure include the head box or tent, mask, and low-flow nasal cannula (NC). Other types of support, including high-flow nasal cannula (HFNC), NCPAP, noninvasive ventilation (NIV), and invasive mechanical ventilation all provide some level of positive pressure.
With a head box or tent, gas at the desired F I O 2 is delivered to a box or tent which is placed over the infant’s head. The gas is typically heated and humidified and must be delivered at a flow rate that assures rapid exchange of gas in the hood and prevents accumulation of exhaled CO 2 . This technique is simple but cumbersome, and somewhat limits access to the infant. Although widely used in previous years, O 2 supplementation via head box or tent has been almost entirely replaced by nasal canula oxygen.
NC devices deliver a constant flow of blended gas at a set F I O 2 to the nostrils. Typically, the F I O 2 is set with a blender that mixes 100% O 2 with room air, and the flow is set with a simple meter that can be adjusted to the desired rate, measured in L/min. At low flows, usually defined as less than 1 or 2 L/min, the gas is typically not heated or humidified. With low-flow NCs, the effective F I O 2 is determined by the relative contribution to each breath of the gas from the NC and the amount of room air that the infant entrains or breaths from around the NC. During mouth breathing or crying, the infant is breathing exclusively room air and derives no supplementation from the NC. For any baby with a given inspiratory flow, increasing the NC flow will increase the amount of gas that the baby inhales from the NC and decrease the amount that is entrained from the surrounding room air, so that at some NC flow rate the baby’s F I O 2 is essentially the same as the F I O 2 of the NC. However, the point at which the NC flow rate is sufficient to make the baby’s F I O 2 equal to the NC F I O 2 is dependent upon multiple factors and is not easily measured. In clinical practice, the exact inhaled F I O 2 is not as important as the baby’s oxygenation status, typically measured by pulse oximeter (S P O 2 ). It is important to note that a NC at low flow rates does not provide any pressure to the lungs, so is most suited to infants who do not need some level of positive pressure.
Depending on the size of the baby, cannula flows greater than 1 to 2 L/min can produce significant positive pressure at the nose, essentially providing a form of NCPAP. In general, NC flows above 2 L/min are considered HFNC, and the maximal HFNC flow used in NICUs is usually around 8 L/min. With HFNC most, or all, inspired gas reaching the baby is from the cannula rather than entrained room air. For this reason, HFNC systems should use gas that has been conditioned to avoid drying of the nose and mucosal damage. Conditioning of the gas includes heating and humidification, leading some to refer to this technique as HHHFNC (heated, humidified, high-flow nasal cannula).
HFNC support is adjusted by varying the flow rate and F I O 2 . With HFNC, the effective pressure applied to the infant’s upper airway increases as the HFNC flow increases. Depending on the HFNC flow rate, the size of the nasal cannula prongs, and the size of the infant, significant or even excessive levels of NCPAP can be delivered. The major advantage of HFNC is the simple nasal interface, typically with loose-fitting nasal prongs. This makes a system of support that is easy to manage and is generally perceived as comfortable for the infant. However, the significant disadvantage of HFNC as a method of providing NCPAP is that the level of NCPAP is not measured and is dependent on multiple factors. This leads to potentially under- or over-estimating the level of NCPAP support the HFNC is providing. HFNC may “wash out” CO 2 from the hypopharynx, so it can be effective in PCO 2 control if high or consistent levels of NCPAP are not required.
Despite the widespread use of HFNC in preterm infants, the data on its efficacy compared to NCPAP is equivocal. The broad use of HFNC is largely due to ease of use, less risk of injury to nares, and perceived increased patient comfort with the use of an HFNC. In large trials comparing HFNC to NCPAP for support following extubation, there are no significant differences. The data on the use of HFNC as a primary mode of support is mixed. Several trials have suggested that there is no difference between HFNC and NCPAP. On the other hand, several large trials comparing HFNC to NCPAP as the initial mode of support concluded that HFNC has a significantly higher failure rate. As suggested by recent reviews, the reduced nasal irritation seen with HFNC makes it an attractive option despite evidence that it is less efficient than NCPAP in preventing intubation.
In most cases, the choice between HFNC and “real” NCPAP comes down to a balance between infant comfort, simplicity, and the ability to accurately measure and control the level of NCPAP. Given concerns about the effectiveness of HFNC in preventing intubation, it is probably most suited to infants at relatively low risk of requiring intubation or re-intubation.
The care of preterm infants with respiratory distress syndrome (RDS) was revolutionized with the introduction of NCPAP in 1971. Because RDS is characterized by decreased alveolar compliance and atelectasis, positive pressure applied to the upper airway will at least partially offset the surface forces leading to alveolar collapse. The prevention of alveolar collapse preserves functional residual capacity (FRC) and preserves lung compliance. This both improves ventilation/perfusion matching and decreases work of breathing. In addition to its effect at the alveolar level, NCPAP provides positive pressure to the entire airway. In patients with laryngomalacia, tracheomalacia, or bronchomalacia, positive pressure can also stabilize the “floppy airway” by preventing airway collapse during expiration. This is an important role for NCPAP in babies with congenital upper airway anomalies, as well as in babies with distal airway abnormalities associated with severe BPD.
There are several nasal interfaces for NCPAP, each with their own advantages and disadvantages. Probably the most common approach uses double nasal prongs which extend only a short distance into the nose, allowing relatively little leak and good transmission of pressure. NCPAP can also be delivered through a mask that fits tightly over the nose, leaving the mouth uncovered. In many centers, alternating between the tight-fitting short prongs and the nasal mask is seen as an ideal compromise for effectively delivering the measured pressure, and providing an interface that does not cause nasal irritation or skin breakdown.
The positive pressure for NCPAP can be generated in several ways. With “ventilator CPAP,” the CPAP circuit is attached to a conventional ventilator that is set to deliver a fixed CPAP level—essentially the same as ventilation with a frequency of 0 breaths/ min. The level of CPAP support and the F I O 2 are set at the ventilator, and inspiratory gas heating and humidification are accomplished with a standard in-line humidifier. With “bubble CPAP,” the pressure is generated by using a water column as an adjustable pop-off valve at the end of the expiratory limb of the respiratory circuit. The level of CPAP pressure is adjusted by the height of the water column above the end of the expiratory limb. This is an extremely simple system for monitoring the integrity of the CPAP device (lack of bubbles suggests there is an upstream leak or other problem) and for adjusting the level of CPAP (by adjusting the height of the water column). However, the depth of the water column is not an accurate predictor of the level of NCPAP delivered at the nasal interface. There are commercial integrated bubble CPAP systems that incorporate heating and humidification and the “bubble” component. However, simple bubble CPAP systems can be easily assembled from standard NICU supplies.
One of the intriguing aspects of bubble NCPAP is the possibility that the bubbling at the end of the expiratory limb is actually advantageous. Bubbling results in small oscillations in pressure in the expiratory limb of the circuit which are transmitted “upstream” to the nasal interface and then to the airways. It is not clear whether this oscillation has an actual clinical impact.
In most North American NICUs, the typical levels of NCPAP for preterm infants range from 5 to 8 cmH 2 O. There is some evidence that the higher end of this range is more effective in preventing extubation failure in preterm infants who still need supplemental oxygen at the time of extubation.
A variation of NCPAP provides two alternating levels of NCPAP support, with the theoretical advantage of optimizing lung recruitment and minimizing atelectasis without causing overdistension. This approach is frequently used in adults and is termed BiPAP (biphasic positive airway pressure). In infants, it is usually referred to as SiPAP and can be thought of as providing alternating levels of CPAP, or as providing CPAP with intermittent sigh breaths, usually only 1 to 3 cmH 2 O above the lower baseline CPAP level. Although SiPAP/BiPAP has a set rate with alternating upper and lower pressures, it typically does not provide ventilation in terms of a set respiratory rate or tidal volume. Rather, it provides varying levels of NCPAP, with the patient breathing spontaneously above these NCPAP levels. There is conflicting evidence about whether BiPAP can increase CO 2 elimination and oxygenation compared with NCPAP. There is also conflicting evidence about whether BiPAP improves respiratory outcomes. There is some evidence that synchronized BiPAP (done using a Graseby capsule, described below) decreases the duration of respiratory support compared to NCPAP. However, un-synchronized BiPAP does not appear to be superior to NCPAP in sustaining extubation.
The use of a nasal interface, rather than an endotracheal tube, to provide ventilatory support dates to the early days of neonatal mechanical ventilation and remains an active area of research. It is an attractive option because it may provide a higher level of support than NCPAP while avoiding the potential complications of an endotracheal tube. It is particularly attractive for less mature infants whose respiratory status is compromised by a combination of RDS, immature and collapsible chest wall, decreased respiratory drive and apnea, and decreased respiratory muscle strength and endurance.
The nomenclature of NIV can be confusing, reflecting the fact that there are multiple ways to apply a mechanical ventilatory breath to a nasal interface. The acronyms NIMV (nasal intermittent mandatory ventilation or noninvasive mechanical ventilation) and NIPPV (nasal intermittent positive pressure ventilation) are sometimes used interchangeably with NIV.
In preterm infants, the cycling positive pressure of NIV at the upper airway produces an intermittent respiratory stimulus which could decrease central apnea. In addition to this stimulatory effect, it is possible that NIV also decreases apnea by facilitating lung recruitment and decreasing the work of breathing. At least one study has suggested that unsynchronized NIV can significantly reduce apnea. Compared to NCPAP, synchronized NIV appears to decrease the work of breathing in infants with RDS.
Clinical trials comparing NIV to NCPAP for prevention of extubation failure have shown mixed results, probably because some studies have used an unsynchronized form of NIV and others have looked at synchronized NIV. Synchronized NIV has been shown in small studies to reduce extubation failure compared to NCPAP. However, the largest trial of NIV vs NCPAP combined synchronized and non-synchronized NIV modes and showed only a marginal decrease in the rate of post-extubation failure. Recent meta-analysis suggests that NIV may be superior to NCPAP for preventing extubation failure, but does not reduce BPD or death, and that synchronization may be important for the successful application of NIV.
NIV, both synchronized and unsynchronized, has also been used in early RDS to decrease the need for intubation. Synchronized NIV appears to be superior to NCPAP in reducing the need for intubation. The data from trials comparing unsynchronized NIV to NCPAP are mixed, with some showing a decrease in the need for intubation, and others showing no difference.
The practical use of NIV is limited by several important factors. The nose and upper airway act as a filter so that pressures set at the ventilator are attenuated at the alveolar level. In essence, what is delivered to the lung may be significantly less than what is set at the ventilator and cannot be easily monitored. With unsynchronized NIV breaths delivered through small nasal cannula prongs (e.g., RAM cannula), there appears to be no significant transmission of tidal volume to the infant, suggesting that the main effect of NIV may be a simple increase in mean airway pressure.
The simplest form of NIV is conventional time-cycled pressure-limited ventilation that uses the same circuits and gas-conditioning devices used for invasive ventilation. This approach, sometimes termed NIMV (nasal intermittent mandatory ventilation) has the advantage of simplicity, but the lack of synchrony with the infant makes it a poor form of support. The non-synchronized breaths are frequently delivered as the infant is attempting to exhale, leading to increased work of breathing and inefficient pressure delivery.
Synchronization of NIV is limited by the synchronization method. Many of the older studies of synchronized NIV used a small pressure sensor (Graseby pressure capsule) which was placed on the abdomen to detect abdominal movement at the onset of inspiration. However, this technique is not available with commercially produced ventilators in the US. Other approaches to synchronizing NIV which rely on flow sensing are not ideal because of the problems with leaking around the nasal interface leading to undetected breaths and/or “auto-cycling” of the ventilator.
Neurally adjusted ventilatory assist (NAVA) is a mode by which the ventilator pressure is adjusted in proportion to the electrical activity of the diaphragm, as measured by electrodes embedded within a special nasogastric or orogastric tube. Although used for both invasive and noninvasive ventilation, NAVA is a particularly attractive way to provide synchronized NIV. With NAVA, the triggering of breaths is very sensitive to spontaneous inspiratory effort and is not affected by the leaks that make flow-synchronized NIV problematic. It also has the advantage of adjusting the size of the mechanical breath to the infant’s respiratory effort. In clinical trials, NAVA appears to be an effective technique for synchronizing both invasive and noninvasive support.
Several investigators have explored delivering high-frequency oscillatory ventilation (HFOV) through a nasal interface. It is unclear whether nasal HFOV is superior to NCPAP in terms of CO 2 exchange. At least one study suggests that it is not superior to NIV in reducing extubation failure, but is associated with less feeding intolerance. A review of European NICU practices suggests that this technique has been used in a number of European NICUs. In the US, it remains an intriguing investigational technique.
With any form of NIV, the nasal interface employed is usually short prongs or a nasal mask. NCPAP prongs are attached to tubing that provides both an inspiratory and expiratory limb. Prongs should fit snugly into the nares without blanching the surrounding tissue. If a nasal cannula is used, there is no expiratory limb and the prongs used must be smaller than those used with NCPAP to provide for exhalation around the nasal prongs. The cannula thus delivers significantly less pressure than is measured at the ventilator. The risks of nasal damage and obstruction sometimes observed during the use of NCPAP are also present with NIV. Proper application and maintenance of the nasal interface and avoidance of excessive force on the nasal septum are important to avoid these complications.
The complications of NCPAP or NIV are related to the gas pressure that is applied to the alveoli and to direct contact of the interface with the nose. With any of the noninvasive modalities that provide positive pressure, there is the risk of providing too much pressure and over-inflating the lung, both increasing the work of breathing and potentially causing both acute and chronic lung damage. Excessive pressure can also reduce venous return, increase pulmonary vascular resistance, and reduce cardiac output.
If nasal prongs are too large or are applied with too much pressure over the nasal septum, they can produce erosions or pressure necrosis that sometimes requires the interruption of NCPAP or NIV. Avoiding these complications while keeping the nasal prongs in place is a task that requires careful observation and skill.
During the application of NCPAP or NIV, there is also a chance of gas being pushed into the stomach. A nasogastric catheter is often used to avoid excessive gas accumulation and consequent gastric distention.
Noninvasive support can be useful in preventing progressive atelectasis and respiratory decompensation, both early in the neonatal course and following extubation. While NCPAP is the standard against which other techniques are measured, there may be advantages to HFNC and/or synchronized NIV in selected patients. HFNC is an attractive way of providing support because it is less likely to cause nasal injury than NCPAP. However, it has the significant disadvantage of not providing easily measured and adjusted levels of NCPAP, so it is probably most useful in post-extubation infants, and as a primary form of support for infants who are at relatively little risk of needing intubation. Synchronized NIV may offer advantages over NCPAP or un-synchronized NIV in preventing intubation and reducing extubation failure, although data are not yet available on its impact on long-term outcomes such as mortality or BPD.
HFNC and various forms of NIV are likely to become a larger part of NICU respiratory support as neonatal caregivers continue to search for the best ways to support infants with non-invasive techniques. As with most techniques in the NICU, well-designed protocols, rigorous training of staff, and meticulous attention to detail may well be as important, or more important, than the specific modality employed.
There are multiple approaches to weaning a baby from noninvasive support with supplemental oxygen to being unsupported in room air. While the most conservative approach would be a gradual progression from NIV to NCPAP to HFNC to NC to no support, this approach is probably not necessary for all infants. An argument can be made that preterm infants, with their compliant chest walls and tendency to atelectasis, should remain on some form of positive pressure support as long as they have any ventilation/perfusion mismatch and need for supplemental oxygen. With this strategy, infants would remain on NIV, NCPAP, or HFNC until F I O 2 = 0.21, then be transitioned to room air without support.
There is relatively little published data to guide the decision of when to wean from positive pressure (HFNC, NCPAP, NIV) to supplemental oxygen via low-flow NC, or to room air. One randomized study examined strategies for managing infants who were stable on NCPAP 4 to 6 cmH 2 O with F I O 2 less than 0.25, and concluded the optimal strategy was to discontinue NCPAP, but resume it for at least 48 hours if the infant failed the transition, with failure defined as increased work of breathing, increased apnea/bradycardia, F I O 2 greater than 0.25, or PCO 2 greater than 65. However, a recent study suggested that continuing NCPAP for 2 weeks beyond the point where infants who were stable on NCPAP 4 to 6 cmH 2 O and F I O 2 less than 0.25 resulted in significantly improved FRC through hospital discharge. This would suggest that the possible advantages of improving lung volume should be carefully weighed against the difficulty of continuing NCPAP in a maturing infant.
There are also multiple approaches to weaning from NCPAP, including “sprints” on and off NCPAP, a gradual decrease in NCPAP pressure, and abrupt discontinuation of NCPAP. All of these approaches work, and there does not appear to be a major impact of strategy on the outcome. A strategy of abrupt discontinuation of NCPAP may lead to earlier discontinuation than a strategy of gradual weaning.
Regardless of the strategy used in transitioning an infant from noninvasive positive pressure support, it is essential to observe the infant closely for changes in gas exchange and work of breathing.
For some infants, supplemental oxygen and the positive pressure of noninvasive support are not sufficient to compensate for the degree of lung disease. For these infants, invasive (via an endotracheal tube) ventilation is required. Mechanical ventilation is designed to achieve two things: oxygenation (via optimal mean airway pressure) and CO 2 removal (via minute ventilation) while causing minimal lung injury. This can be accomplished with multiple types of “conventional” ventilators which mimic normal tidal breathing, and with high-frequency ventilators which cause very efficient mixing of gas between the alveoli and the airway without tidal breathing.
Neonatal intubation is a technically challenging procedure that requires significant time to master, often requires multiple attempts, and is associated with a significant risk of complications. Although usually a quick procedure when performed by a skilled practitioner, it is still uncomfortable for the infant and associated with significant autonomic changes, including blood pressure, oxygenation, and cerebral perfusion pressure. Both the US and Canadian recommendations for all non-emergent intubations include the use of analgesia or a combination of analgesia and muscle relaxation; simple sedation is not sufficient. Even skilled practitioners find that paralysis increases success and decreases the potential trauma of intubation. For this reason, some centers perform all elective intubations, including for surfactant administration, after what is essentially a rapid sequence anesthesia induction. In this case, infants need to be supported until they are fully recovered from the medications, usually within 20 to 60 minutes.
Although not directly addressed in the US and Canadian guidelines, the recommendation for analgesia with each intubation could be extrapolated to other laryngoscopic procedures such as surfactant instillation with intubate/surfactant/extubate (INSURE) or less invasive surfactant administration (LISA) techniques. The discomfort and attendant physiologic changes of intubation are largely associated with the laryngoscopy and stimulation of the laryngeal area, not just the placement of an endotracheal tube (ETT). However, providing adequate levels of analgesia for INSURE or LISA decrease the ability to immediately transition to noninvasive support following the surfactant instillation.
A significant advance in intubation techniques is the introduction of more sophisticated laryngoscopes into the NICU. These allow visualization of the upper airway with a small camera at the tip of the laryngoscope and allow members of the team other than the one performing the intubation to see the anatomy and the procedure. This is useful both for teaching and for instrumentation of the difficult airway.
Despite the wide range of ventilator devices and strategies available, the fundamental physiology of mechanical ventilation follows a few simple rules. Most neonatal lung diseases are characterized by atelectasis, so providing optimal pressure to counteract surface forces is paramount for optimizing both oxygenation and ventilation. Basic principles and guidelines include the following:
Oxygenation is impaired by the ventilation/perfusion mismatch which results from atelectasis. Assuming the lung is not over-distended, increasing mean airway pressure (sometimes abbreviated MAP, but more appropriately mPaw) will decrease atelectasis and improve oxygenation.
Oxygenation can also be impaired by an over-inflated lung which reduces venous return to the heart.
CO 2 exchange is impaired by inadequate minute ventilation which results from inadequate tidal volume and/or inadequate respiratory rate. Assuming adequate inspiratory and expiratory times (T insp and T exp ), increasing minute ventilation will increase CO 2 exchange. For most infants with acute lung disease a V T of approximately 5 mL/kg is appropriate.
Synchrony between the infant and the ventilator will decrease the work of breathing and make both patient breaths and mechanical breaths more efficient. Synchronizing the ventilator with the patient includes matching the onset of the ventilator breath with patient inspiration, as well as matching the duration of the ventilator breath (T insp ) with the onset of patient exhalation. In lung diseases that are restrictive and without a significant obstructive component (e.g., RDS) the lung time constant (R × C L ) is small, meaning there is rapid transmission of pressure from the upper airway to the alveolus, so short T insp and short T exp are effective. In small infants with significant RDS, T insp as low as 0.2 to 0.3 seconds may be effective.
There are multiple neonatal ventilators on the market, each with multiple modes of ventilation, and each of these modes has multiple parameters which can be adjusted. To make matters more confusing, some modes have similar names but different mechanisms of action, or different names but similar modes of action. Although this leads to what seems like an unlimited number of choices about how to best ventilate an infant, these choices can be simplified by understanding the basic physiology and nomenclature of modern ventilation. Regardless of the ventilator brand or mode of ventilation, it is helpful to think of a ventilator breath as characterized by several components which can be used to describe any conventional tidal mechanical breath ( Fig. 40.1 ). Ventilator breaths can be described by the following:
Onset of the breath, termed the trigger . Breaths that are initiated because a clock in the ventilator has determined it is time to deliver a breath are termed time-triggered breaths. Breaths that are initiated by the patient are termed patient-triggered breaths.
Size of the breath, termed the limit . Historically most adult ventilators delivered a set volume with each breath ( volume-limited breath) and most neonatal ventilators delivered a set inspiratory pressure with each breath ( pressure-limited breath). More sophisticated neonatal ventilators can deliver both volume- and pressure-limited breaths. In addition, with some ventilator modes, the size of the breath is determined by constantly adjusting combinations of volume, pressure, and flow. There is good evidence that this technique of volume-targeted ventilation, compared to pressure-limited or volume-limited ventilation, reduces death, intraventricular hemorrhage (IVH), and BPD.
Duration of the breath or when the breath ends, termed the cycle . A breath that lasts for a fixed inspiratory time (T insp ) is termed time-cycled . A breath that is terminated when inspiratory flow drops below a certain point is termed flow-cycled . Because the decrease in inspiratory flow is dependent on lung compliance and respiratory effort, the duration of flow-cycled breaths is partially controlled by the infant.
Inspiratory flow pattern which is either a fixed inspiratory flow (constant-flow) or can vary with patient demand (demand-flow) . With constant flow ventilators, there is the possibility that the flow is not sufficient to meet the patient’s need during early inspiration, resulting in “flow starvation” which increases work of breathing and patient discomfort.
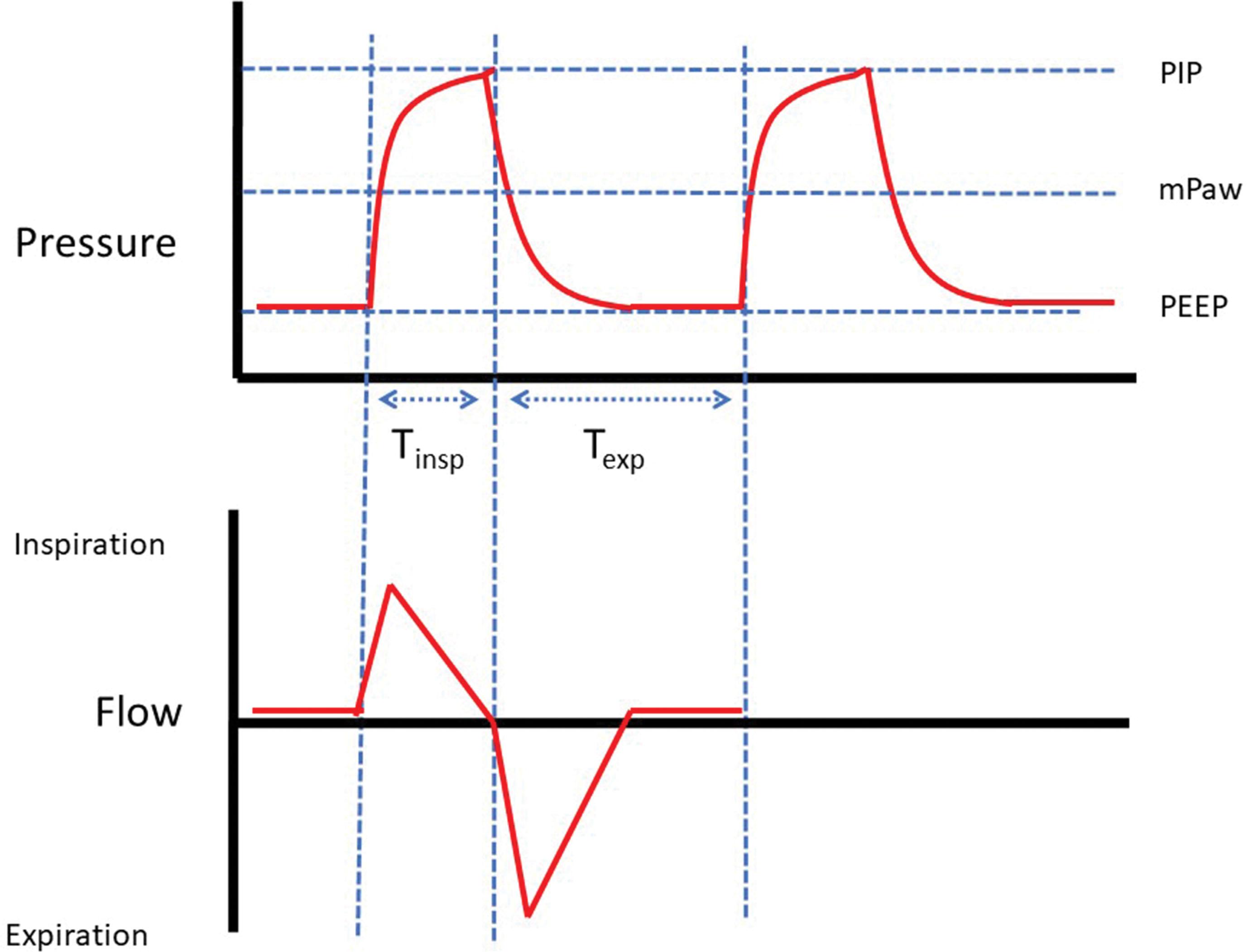
Below are some of the more commonly used neonatal ventilation modes, presented in the general order of development and clinical adoption.
Time-cycled pressure-limited intermittent mandatory ventilation (TCPL IMV) is sometimes referred to as TCPL, IMV, or IPPV (intermittent positive pressure ventilation). It is the oldest form of neonatal mechanical ventilation, and is conceptually very simple, although of limited utility in most modern NICUs. Characteristics of TCPL IMV include:
The size of the breath is pressure-limited, as the ventilator delivers a peak inspiratory pressure (PIP), then returns to end-expiratory pressure (PEEP). In general, adjusting PEEP affects FRC, and adjusting the driving pressure (PIP − PEEP) affects the tidal volume.
The breaths are at a fixed rate and time-triggered—for example, at a rate of 30/min, a breath occurs every 2 seconds regardless of the infant’s respiratory effort.
The breaths are time-cycled so inspiration lasts a fixed period of time (T insp ) regardless of whether the infant is trying to inhale or exhale at the end of the ventilator breath.
Bias gas flow is fixed at a set flow rate (L/min), which means there is a set maximal inspiratory flow rate and a constant flow of bias gas in the expiratory limb of the ventilator circuit.
The advantage of TCPL IMV is mechanical and conceptual simplicity, and it works well for paralyzed or heavily sedated patients for whom the ventilator is doing all the work of breathing. However, it is a difficult mode to use effectively in a spontaneously breathing patient. If the patient is trying to breathe spontaneously, the ventilator onset of inspiration and duration of inspiration do not synchronize with the patient’s efforts. Not only are the baby’s inspiratory efforts not supported but, more importantly, the baby may be trying to exhale while the ventilator is delivering a breath. In addition, the fixed flow of the gas on the inspiratory side of the circuit can be a limitation if the patient (particularly a large, strong infant) has a higher inspiratory flow demand than the ventilator provides. For these reasons, TCPL IMV is rarely used except in anesthetized or paralyzed patients with no respiratory drive.
The development of accurate flow sensors led to neonatal ventilators which can deliver breaths in synchrony with the patient’s respiratory effort. The first of these modes to be developed was synchronized intermittent mandatory ventilation (SIMV). With SIMV, there is a fixed rate or number of breaths per minute (the “mandatory” part of the name) which last for a fixed duration (T insp ). However, the timing of these breaths coincides with at least some of the patient’s breaths. For example, at a set SIMV rate of 30, if the baby is breathing 50 times a minute, the ventilator will sense the baby’s respiratory rate and try to deliver 30 breaths that coincide with the onset of the baby’s breath, rather than delivering a breath exactly every 2 seconds. The baby’s other 20 breaths will be unsupported, with the baby inhaling from the bias gas flow which is maintaining PEEP. SIMV, which does not support all breaths, has been largely replaced by modes such as SIMV/PS or A/C which can support all breaths. Characteristics of SIMV include:
Size of the breath can be either volume-limited or pressure-limited.
The breaths are delivered at a set rate, but the ventilator tries to synchronize these breaths with the patient’s onset of inspiration. If the baby is apneic, the ventilator defaults to delivering time-triggered breaths at the set rate.
Depending on the ventilator and the mode, breaths can be either time-cycled (fixed T insp ) or flow-cycled (duration of the breath varies according to the patient’s inspiratory flow pattern). In general, if it is not specified as “SIMV with flow cycle,” SIMV refers to time-cycled breaths with a fixed T insp .
One of the advantages of synchronizing the ventilator breath with the patient’s breath is that a shorter T insp is usually effective in delivering an adequate tidal volume since the ventilator is not “fighting” the baby to deliver the breath. Lower PIP and slightly shorter T insp are often sufficient because the infant's inspiratory effort contributes to the generation of the V T .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here