Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The nature of obstetrical practice requires consideration of two patients: mother and fetus. Their intrinsic biological interdependence creates unique challenges not typically encountered in other realms of medical practice. Often there is a paucity of objective data to support the evaluation of risks and benefits associated with a given clinical situation, forcing obstetricians to rely on their clinical acumen and experience. Family perspectives must also be integrated in clinical decision-making, along with the advice and counsel of other clinical providers. In this chapter, we review how to best utilize neonatology expertise in the obstetrical decision-making process.
Optimal perinatal care derives from collaboration between the obstetrician and neonatologist during pregnancy, and especially around the time of labor, to eliminate ambiguity and confusion in the delivery room and to ensure that patients and families understand the rationale for obstetrical and postnatal management decisions. The neonatologist can provide information regarding risks to the fetus associated with delaying or initiating preterm birth and can identify the optimal location for delivery to ensure that skilled personnel are present to support the newborn infant.
In addition to contributing information about gestational-age-specific outcomes, the neonatologist can also anticipate neonatal complications related to maternal disorders such as diabetes mellitus, hypertension, and multiple gestation and can define treatment pathways for fetal conditions such as congenital infections, alloimmunization, or congenital anomalies. When a lethal condition or high risk of death in the delivery room is anticipated, the neonatologist can assist with the formulation of a birth plan, develop parameters for delivery room intervention, and make plans for palliative care.
Preparing parents by describing delivery room management and resuscitation of a high-risk infant can demystify the process and reduce some of the fear anticipated by the expectant family. Making parents aware that premature infants are susceptible to thermal instability will reduce their anxiety when the newborn is rapidly moved after birth to a warming bed. The need for resuscitation is determined by careful evaluation of cardiorespiratory parameters and appropriate response according to published Neonatal Resuscitation Program guidelines.
Complications of pregnancy that affect infant well-being may be immediately evident after birth, such as hypotension related to maternal hemorrhage, or may present hours later, such as hypoglycemia related to maternal diabetes or thrombocytopenia related to maternal preeclampsia. Anemia and thyroid disorders related to transplacental passage of maternal immunoglobulin (Ig) G antibodies to platelets or thyroid, respectively, may present several days after delivery.
Diabetes during pregnancy is a prototypic example. Infants born to women with diabetes are often macrosomic, increasing the risk of shoulder dystocia and birth injury. After delivery, these infants may have significant hypoglycemia, polycythemia, and electrolyte disturbances, which require close surveillance and treatment. Lung maturation is delayed in the infant of a diabetic mother (IDM), increasing the incidence of respiratory distress syndrome (RDS) at a given gestational age. The IDM also has delayed neurologic maturation, with decreased tone typically leading to delayed onset of feeding competence. Less common complications include an increased incidence of congenital heart disease and skeletal malformations. Most neonatal complications of maternal diabetes are managed without long-term sequelae but may prolong length of hospital stay. Anticipatory guidance for parents can decrease anxiety and improve readiness for hospital discharge. Neonatal complications for the IDM are a function of maternal glycemic control. Thus careful screening of pregnant women by physicians and adherence to treatment regimens by patients will reduce neonatal morbidity due to maternal diabetes. In Table 73.1 we summarize other morbidities of pregnancy and their effects upon neonatal outcome. The list is not exhaustive and does not incorporate how multiple morbidities may interact to create additional complications. Any of these problems may contribute to increased length of hospital stay after delivery as well as long-term morbidity.
| Pregnancy Morbidity | Neonatal Outcome |
|---|---|
| Preeclampsia/chronic hypertension | SGA, thrombocytopenia, polycythemia |
| Congenital viral infection | Thrombocytopenia, SGA, intracranial calcifications, developmental delay |
| Smoking | SGA, polycythemia |
| Opioid use disorder | Feeding difficulties, hyperirritability, hypertonia, seizures |
| Obesity | LGA, hypoglycemia, feeding difficulties |
| Underweight | Preterm birth |
| pPROM with oligohydramnios | Pulmonary hypoplasia |
Chorioamnionitis has diverse effects upon the fetus and neonatal outcome. It is associated with preterm prelabor rupture of membranes and, therefore, preterm birth. Elevated levels of proinflammatory cytokines may predispose neonates to cerebral injury. Though suspected or proven neonatal sepsis is more common in the setting of chorioamnionitis, many neonates born to mothers with histologically proven chorioamnionitis are asymptomatic and appear unaffected, with normal pregnancy outcomes. Animal models and associated epidemiologic data suggest that chorioamnionitis can accelerate fetal lung maturation as measured by surfactant production and function. However, preterm infants born to mothers with chorioamnionitis are more likely to develop bronchopulmonary dysplasia (BPD). The neonatal consequences of chorioamnionitis are likely related to the timing, severity, and extent of the infection and the associated inflammatory response.
The effects of preeclampsia on the neonate are well known and include fetal growth restriction, hypoglycemia, neutropenia, thrombocytopenia, polycythemia, and electrolyte abnormalities such as hypocalcemia. Most of these problems relate to placental insufficiency with diminished oxygen and nutrient delivery to the fetus. With delivery and supportive care, most of these will resolve with time, although some patients will require treatment with intravenous calcium and/or glucose in the early neonatal period. Similarly, severe thrombocytopenia may require platelet transfusion therapy. Some studies suggest that preeclampsia may protect against intraventricular hemorrhage (IVH) in preterm infants, perhaps because of maternal treatment or other unknown factors. In contrast to intrauterine inflammation, preeclampsia does not appear to accelerate lung maturation. Predicting the consequences of maternal preeclampsia on neonatal outcome remains difficult.
Maternal autoimmune disease may affect the neonate through transplacental transfer of autoantibodies. The extent of antibody transfer drives severity of symptoms. Treatment is supportive and based on the affected neonatal organ system. For example, maternal Graves disease may cause neonatal thyrotoxicosis requiring treatment with propylthiouracil or beta-blockers. Maternal lupus or connective tissue disease is linked to congenital heart block that may require long-term atrial pacing after delivery. Myasthenia gravis during pregnancy can promote a transient form of the disease in the neonate. Supportive therapy during the early neonatal period will address most issues associated with maternal autoimmune disorders. Passively transferred autoantibodies gradually clear from the neonatal circulation with a half-life of 2–3 weeks. Preterm birth and small for gestational age are more likely among neonates born to mothers with autoimmune disorders.
Neonatal outcome associated with maternal nutritional status during pregnancy is of growing interest. The Dutch famine of 1944–45 created a unique circumstance for studying the consequences of severe undernutrition during pregnancy (caloric intake less than 1000 kcal/day). Mothers experienced significant third-trimester weight loss and offspring were underweight. Low maternal body mass index (BMI) is associated with increased risk of preterm birth. There is now growing evidence that infants undernourished during fetal life have higher risk for development of “adult” diseases such as atherosclerosis and hypertension. Poor maternal nutrition during intrauterine life may cause the fetus to modify metabolic pathways and blood pressure regulatory systems, with long-term health consequences lasting into late childhood and beyond.
Conversely, maternal overnutrition as defined by excessive caloric intake predisposes mothers to insulin resistance and large-for-gestational-age infants. In general, maternal BMI and birth weight have increased over time, even though mean gestational age at delivery has declined. Elevated maternal BMI is associated with excess stillbirth and neonatal mortality and may increase the risk for preterm birth. Maternal obesity (BMI >30) is a risk factor for cerebral palsy among a term population. The effects of maternal BMI on outcomes among preterm neonates are less clear. Some studies report an elevated risk of adverse neurodevelopmental outcomes, whereas others do not detect a link with maternal BMI. ,
Neonates from a multifetal gestation are, on average, smaller at a given gestational age than their singleton counterparts. They are also more likely to deliver before term and therefore are more likely to experience complications associated with low birth weight and prematurity described elsewhere in this chapter. Identical twins may also experience conditions unique to multiple gestation such as twin-twin transfusion syndrome. The associated discordant growth and additional problems of anemia, polycythemia, congestive heart failure, and hydrops may further complicate the clinical course following delivery, even after amnioreduction or selective fetoscopic laser photocoagulation. Of additional concern are cerebral lesions such as periventricular white matter injury and ventricular enlargement that may occur more frequently in the setting of twin-twin transfusion syndrome. Additional epidemiologic studies and long-term follow-up are needed to further our understanding.
Congenital malformations present significant challenges for caregivers and families. Prenatal diagnosis offers opportunity to plan for delivery room management and provide anticipatory guidance. Choosing the site of delivery should be based on optimizing availability of appropriate expertise. The neonatologist can facilitate appropriate delivery coverage and ensure availability of appropriate equipment, medications, and personnel. Table 73.2 summarizes some of the important considerations associated with neonatal management of congenital malformations. Management considerations include availability of expertise and equipment needed for optimal management. This list reflects the importance of multidisciplinary input for optimal management of patients with congenital malformations. Typically, such patients are best delivered in a setting with experienced delivery room attendants. If needed consultative services or equipment are not readily available, arrangement should be made for prompt transfer to a tertiary center. Successful transports depend upon clear communication between centers. For example, prompt notification of the delivery of an infant with gastroschisis ensures that the delivering hospital will provide adequate IV hydration and protection of exposed viscera, while alerting the referral center to make pediatric surgery expertise immediately available upon arrival.
| Malformation | Management Considerations |
|---|---|
| Clefts | Alternative feeding devices (e.g., Haberman feeder), genetics evaluation, occupation/physical therapy |
| Congenital diaphragmatic hernia | Skilled airway management, pediatric surgery, immediate availability of mechanical ventilation, nitric oxide, ECMO |
| Upper airway obstruction/micrognathia | Skilled airway management, otolaryngology, genetics evaluation/management, immediate availability of mechanical ventilation, tracheostomy tube placement |
| Hydrops/hydrothorax/peritoneal effusion | Skilled airway management, nitric oxide, ECMO, chest tube placement, paracentesis, immediate availability of mechanical ventilation |
| Ambiguous genitalia | Endocrinology, urology, genetics available for immediate evaluation |
| Neural tube defects | Sterile moist dressing to cover defect and prevent desiccation, IV fluids, neurosurgery, urology, orthopedics evaluation/management |
| Abdominal wall defects | Saline-filled sterile bag to contain exposed abdominal contents and prevent desiccation, IV fluids, pediatric surgery, genetics evaluation/management |
| Cyanotic congenital heart disease | IV access, prostaglandin E1, immediate availability of mechanical ventilation |
In the setting of premature labor, mothers are frequently treated with antibiotics and tocolytic agents. Maternal medications administered during pregnancy for nonobstetric diseases can have a significant impact on the neonate. A common challenge in many centers evolved from the treatment of opiate-addicted mothers with methadone or buprenorphine. The symptoms of neonatal abstinence syndrome (NAS, also called neonatal opioid withdrawal syndrome [NOWS]) vary as a function of the degree of prenatal opiate exposure and age after delivery. Many infants will appear neurologically normal at delivery, only to exhibit symptoms later, on the first, second, or even third day of postnatal life. Infants with NAS/NOWS typically demonstrate irritability, poor feeding, loose, frequent stools, and, in severe cases, seizures. Treatment options include nonpharmacologic intervention (swaddling, minimal stimulation), methadone, morphine, buprenorphine, or nonnarcotic drugs such as phenobarbital or clonidine, the latter as adjunct therapy with opioids. Often these infants require hospitalization for many days or weeks until their irritability is under sufficient control to allow for care in a home setting. Diagnostic criteria, management strategies, and treatment of NAS/NOWS continue to evolve.
There is clinical evidence that neonates may also exhibit similar symptoms following withdrawal from antenatal nicotine exposure. Maternal smoking during pregnancy is also strongly associated with low birth weight and sudden, unexpected infant death.
The consequences of other illicit drug use during pregnancy have been widely studied but are more difficult to assess due to diagnostic challenges and confounding variables. Maternal cocaine abuse has been associated with obstetrical complications such as placental abruption. In the neonate, vascular compromise is suspected to predispose these patients to cerebral infarcts and bowel injury. Developmental delay and behavioral problems are also noted, although associated factors such as poverty, lack of prenatal care, and low socioeconomic status clearly contribute as well. Methamphetamine use during pregnancy is associated with low birth weight, diminished linear growth, small head circumference, and structural alterations of the central nervous system.
Neonatal anemia may be a consequence of perinatal events such as placental abruption, ruptured vasa previa, or fetal-maternal transfusion. At delivery, the neonate may be asymptomatic or display profound effects of blood loss including high-output heart failure or hypovolemic shock. The duration and extent of blood loss along with any fetal compensation determines neonatal clinical status at delivery and subsequent management. In the delivery room, prompt recognition of acute neonatal blood loss and transfusion with O-negative blood can be a lifesaving intervention.
Alloimmune hemolytic disorders such as Rh hemolytic disease and ABO incompatibility can cause neonatal morbidity ranging from uncomplicated hyperbilirubinemia to severe anemia, hydrops, and high-output congestive heart failure. Rh hemolytic disease is now uncommon, but it still must be considered as a cause of unexplained hydrops, anemia, or heart failure in infants born to Rh-negative mothers, especially if there is a possibility of maternal sensitization. ABO incompatibility is common, with up to 20% of all pregnancies potentially at risk for hyperbilirubinemia. The responsible isohemagglutinins have weak affinity for blood group antigens. Therefore the degree of hemolysis and subsequent jaundice varies from patient to patient. Indirect immunoglobulin (Coombs) testing has limited value in predicting clinically significant jaundice. The typical neonatal morbidity is hyperbilirubinemia requiring treatment with phototherapy.
The mean duration of a spontaneous singleton pregnancy is 282 days or 40 menstrual weeks (38 postconceptional weeks). An infant delivered prior to completion of the 37th week of gestation is considered preterm (World Health Organization definition). Infant morbidity and mortality increase with decreasing gestational age at birth. Preterm birth remains the primary driver of global neonatal intensive care admissions and infant mortality. The risk of poor outcome, defined as death or lifelong handicap, increases dramatically as gestational age decreases, especially for very-low-birth-weight infants. The interplay between birth weight and gestational age, first documented by Lemons and colleagues and updated by Fanaroff and colleagues, , remains valid today. ( Fig. 73.1 ). Those born significantly below or above a weight appropriate for gestational age experience additional mortality risk.
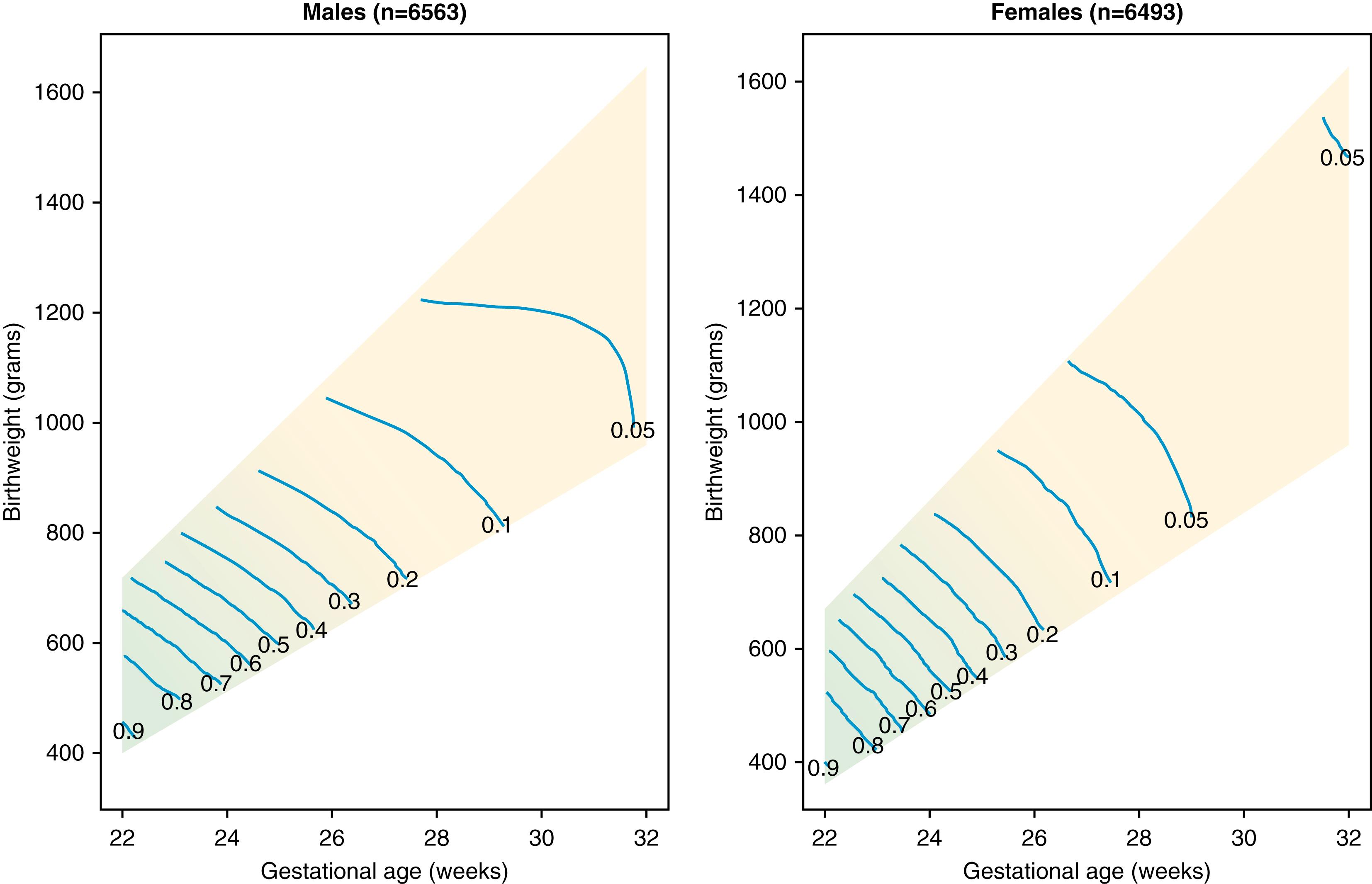
Beyond increased mortality risk, prematurity is also associated with increased risk of morbidity in nearly every major organ system. Bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis, and intraventricular hemorrhage are almost exclusively linked to the preterm state. Intrauterine growth restriction and increased susceptibility to infection are not restricted to the preterm infant but promote additional morbidity and mortality risk for the immature infant. Table 73.3 summarizes common complications of prematurity by organ system.
| Organ System | Morbidity |
|---|---|
| Pulmonary | Respiratory distress syndrome (RDS) Bronchopulmonary dysplasia (BPD) Pulmonary hypoplasia Apnea of prematurity |
| Cardiovascular | Patent ductus arteriosus (PDA) Apnea and bradycardia Hypotension |
| Gastrointestinal/liver | Necrotizing enterocolitis (NEC) Dysmotility/reflux Feeding difficulties Hypoglycemia |
| Central nervous system | Intraventricular hemorrhage (IVH) Periventricular leukomalacia Cerebral palsy Attention deficit disorders |
| Visual | Retinopathy of prematurity |
| Skin | Excess insensible water loss Hypothermia |
| Immune/hematologic | Increased incidence of sepsis/meningitis Anemia of prematurity |
Although certain changes in vital statistics methodology make direct decade by decade comparisons cumbersome, there is no doubt that the US preterm birth rate remains persistently elevated when compared with other developed countries. Explanations for our high preterm birth rates are complex and deserve close scrutiny. Among those closely associated with obstetric clinical practice include improvements in dating associated with use of early gestation ultrasound, an increase in multifetal gestation associated with rising maternal age, use of assisted reproductive technology, and an increase in indicated preterm births. This last category has major import because decisions affecting the timing of delivery can have a profound impact on neonatal outcome. Depending on other factors such as fetal weight and antenatal steroid exposure, the risk of death prior to hospital discharge roughly doubles when gestational age decreases from 25 weeks to 24 weeks. Therefore delaying delivery even for a few days may substantially improve neonatal outcome, especially prior to 32 weeks, with the caveat that the intrauterine environment remains safe to support fetal physiologic integrity. In certain clinical circumstances with high potential for preterm delivery, the quality of the intrauterine environment may be difficult to assess. Three common examples are preterm/prelabor rupture of membranes ( Chapter 39 ), placental abruption ( Chapter 43 ), and preeclampsia ( Chapter 45 ). In each situation, allowing gestation to continue is accompanied by an uncertain risk of rapid change in maternal status with a corresponding increasing risk of fetal compromise. Tests of fetal well-being are discussed in Chapter 32, Chapter 33 and patient safety in Chapter 46 . We must emphasize that the neonatologist can be a valuable partner as these clinical challenges evolve.
During the first decade of the 21st century, disturbing epidemiologic data emerged in the context of rising preterm birth rates in the United States. Among all preterm births (defined as delivery at less than 37 weeks’ gestational age), the fastest growing segment was between 34 and 36 weeks’ gestation. Notably, the peak gestational age at delivery in the United States had shifted from 40 weeks in 1991 to 39 weeks in 2002. This was despite no concordant increase in preeclampsia or chorioamnionitis over that same period. Further investigation determined that this population of late preterm infants experienced significantly higher morbidity and mortality risk, although these risks were often perceived to be of limited clinical significance at the level of an individual patient. Note that extremely preterm infants, typically defined as those born prior to 32 weeks’ gestation and/or weighing less than 1500 g, comprise 1%–2% of all deliveries, and the late preterm population accounts for 8%–9% of all births. Thus even uncommon morbidity in the late preterm population can generate a significant impact on the health care system.
Subsequent epidemiologic investigation has verified the vulnerability of the late preterm population relative to term infants and led to the implementation of obstetric practice guidelines meant to eliminate elective deliveries prior to 39 weeks’ gestation. , Though progress has ensued, elective late preterm birth remains a contributor to the US preterm birth rate. Some have advocated for and even implemented a hard-stop approach in the labor and delivery setting empowering any involved provider to notify maternity center leadership to initiate a standardized, real-time peer review.
It is also important to recognize that delivery prior to 39 weeks must be balanced against the risk of stillbirth during the remaining pregnancy. A recent historical cohort study evaluated the interplay between risk of stillbirth and late preterm/early term birth. In a 2-year period following implementation of rules designed to reduce elective delivery prior to 39 weeks, the investigators found a stable overall perinatal mortality rate with a very small but significant increase in stillbirths accompanied by a comparably significant decrease in infant deaths.
Most neonatal complications of late preterm birth are easily treated, but their economic and social consequences are substantial, and long-term sequelae are incompletely understood. For example, brain growth and development proceed rapidly during the third trimester and continue for the first several years of life. An infant born at 35 weeks’ gestation has approximately one-half the brain volume compared to a term infant. Although IVH is unusual after 32 weeks’ gestation, the periventricular white matter and other regions of the central nervous system continue to undergo rapid myelination during the third trimester of pregnancy. Studies demonstrate a clear association between late preterm birth and long-term neurodevelopmental concerns such as learning disabilities and attention deficit disorders. Additional neurologic and epidemiologic study is needed to establish mechanistic connections between late preterm delivery and these long-term outcomes. Table 73.4 provides a summary of complications of prematurity, comparing early preterm versus late preterm populations. Later preterm infants also experience excess infant mortality compared to their full-term counterparts: the infant mortality rate for infants born at 34–36 weeks’ gestation is approximately threefold higher than those delivered at 40 weeks. Clearly, ongoing scrutiny of immediate and long-term birth outcomes related to recently established practice guidelines is still essential.
| Complication of Prematurity | Incidence in Early Preterm a | Incidence in Late Preterm b |
|---|---|---|
| Respiratory distress syndrome | 10%–80% depending on gestational age, antenatal corticosteroid treatment | <5% |
| Bronchopulmonary dysplasia | 22% <1500 g , | Uncommon |
| Retinopathy of prematurity | ∼20% <1500 g , , | |
| Intraventricular hemorrhage with ventricular dilation or parenchymal involvement | 12% <1500 g , | Rare |
| Necrotizing enterocolitis | 5%–7% <1500 g , | Uncommon |
| Patent ductus arteriosus | 30% <1500 g , | Uncommon |
| Feeding difficulty | >90% | 10%–15% |
| Hypoglycemia | NA | 10%–15% |
In addition to the clinical management issues associated with preterm birth noted above, the role of key social determinants of health must be noted as a central driver of excessive preterm birth rates in the United States. The racial disparity of preterm birth (and infant mortality) is particularly disturbing. In the United States, Black infants are more than twice as likely to deliver early compared to their White counterparts, a disparity that is not explained away by demographic factors such as insurance status, income, or educational attainment. Racism in many manifestations, including systemic versions, is now recognized as a driver of the racial disparities of birth outcomes. Strategies to remediate these challenges are urgently needed and will require the same rigorous thinking and investment traditionally applied to other major health care challenges such as cancer and heart disease.
Decisions regarding treatment of infants at the “limit of viability” are often the most difficult for families and health care professionals. The challenge stems from the lack of clarity in defining that limit, which has decreased by approximately 1 week every decade over the past 40 years in the United States. Among developed countries, most identify the limit of extrauterine viability at 22 to 25 weeks’ gestation. ,
Clinical decision making at these gestational ages typically centers around a dichotomy of immediate medical intervention at the time of birth versus palliative or so-called comfort care. Taking time to gather relevant objective information and share that information among the obstetric and neonatal providers is crucial and should be a priority. The objective basis of the presumed gestational age should be verified and discussed. Ideally, counseling should be based upon local outcome data and up-to-date experience. If known, estimated fetal weight and gender are important variables that impact predicted survival and major morbidity risk. Other variables to consider are noted in Table 73.5 . At present, there is clear consensus that infants born prior to 22 weeks’ gestation do not survive, regardless of resuscitative efforts. Beginning at 22 0/7 weeks, survival is documented, although data are difficult to interpret due to variation in resuscitation practices. Evidence-based calculators designed to estimate survival and long-term outcomes are useful tools but should not be the sole resource used to counsel families (see https://www.nichd.nih.gov/research/supported/EPBO/use ).
| Variable | Comment |
|---|---|
| Gestational age | Small variations impact survival and morbidity |
| Fetal/neonatal weight | Small variations impact survival and morbidity |
| Antenatal steroid treatment | Growing recognition of importance to survival |
| Delivery site | Survival advantage with delivery at a specialty perinatal center |
| Transport | Higher morbidity and mortality risk with intercenter transport |
| Comorbidities | For example, congenital malformations |
Obviously, at extremely early gestational ages, a decision not to attempt resuscitation always results in mortality for the infant. Long-term outcome data are also dependent on follow-up rates, the duration of follow-up, and the quality of developmental testing. Finally, outcome is clearly driven by the site of delivery. Although under ideal circumstances, all periviable deliveries should take place at a specialty perinatal center, some do not. Availability of state-of-the-art resuscitation equipment and skilled, experienced delivery room personnel clearly impacts outcomes, although quantitative data are very limited. The published recommendations of a joint workshop convened by the Eunice Kennedy Shriver National Institute of Child Health and Human Development remain relevant at the time of this writing and, along with other published guidelines, , can be construed as foundational documents for caregivers working with families under these extremely challenging circumstances.
Ideally, discussion between physicians and parents should begin prior to birth in a nonemergency situation and should include obstetric and neonatal providers. If simultaneous presence is not possible, it is imperative that obstetric and neonatal providers communicate directly prior to delivery to ensure clarity and consistency of intent. Even during active labor, communication with the family should be initiated as a prelude to postdelivery discussions. The family should understand that plans for delivery management are based upon maternal and fetal considerations. Outcome data are typically very limited and are based upon populations of similar individuals (e.g., cohorts of 23-week gestation infants, rather than an individual). Finally, it should be emphasized that additional information available only after delivery, such as actual birth weight and neonatal physical findings, may modify a previously predicted predelivery prognosis.
As emphasized earlier, a birth plan developed by the parents in collaboration with obstetrical and pediatric/neonatology providers should be established. The neonatologist will provide information about planned interventions in the delivery room, transfer to the neonatal intensive care unit, anticipated initial hospital course, and the significance of potential complications in the context of prior experience with neonates of similar gestational age, weight, and clinical circumstances. Even when time does not permit such discussions, the neonatologist should carefully assess gestational age at delivery and the response to initial resuscitation. This information can be instrumental in assisting families regarding viability or nonviability of an extremely premature infant. Even though resuscitation measures for extremely preterm neonates follow standard Neonatal Resuscitation Program (NRP) protocols, every effort should be made to engage the participation of the most clinically experienced neonatal provider to evaluate gestational age, estimate weight, assess response to resuscitative efforts, and provide medical leadership for the delivery room care team to support decision making. In cases when a precipitous delivery occurs and no family communication is possible, the physician/provider should employ their best judgment to initiate resuscitation until a parent can be brought into the discussion, erring on the side of resuscitative measures if the appropriate course is initially uncertain.
It is generally recognized that parents are the surrogate decision makers who should determine the goals of care for their newborn child. Decisions regarding lifesaving treatment should emphasize what is believed to be best for the newborn and are greatly impacted by the provided expertise of knowledgeable health care providers. Some recent legislative dictums have emphasized an imperative toward resuscitation for any liveborn infant. Such imperatives are likely to evolve over time. Providers should be proactive in knowledge of current institutional, local, and national regulatory norms. Every effort should be made to utilize up-to-date locally relevant outcome data. It is important to note that health care providers tend to be pessimistic about outcomes based solely on recollections of their experience and subjective reasoning.
Clinicians can assist parents by providing (1) technical information (evidence-based outcome data, prognostic information), (2) individual clinical information (specific data regarding their newborn), and (3) theoretical background (ethical guidelines, local experience). It is crucially important to remember that parents bring profound emotional and psychological investment to their comprehension and decision-making processes. They also often lack technical expertise needed to evaluate outcome data and convert that into a risk/benefit framework that is consistent with their values. Finally, parents typically bring powerful instincts to advocate for treatment, no matter how futile. The phenomenon of selective listening is common. If possible, written documentation of key concepts for future reference and discussion may be helpful. In rare circumstances when clinical providers and parents come into conflict about resuscitation, involvement of an institutional ethics committee may be helpful. Legal remedies should be a last resort.
Though decisions about resuscitation should ideally be individualized to each circumstance and family, ethical frameworks can provide an important foundation. The Nuffield Council on Bioethics report “Critical Care Decisions in Fetal and Neonatal Medicine: Ethical Issues” published in 2006 remains one of the most comprehensive, although some evolution of thought regarding the precise threshold of viability has ensued since the time of publication. The cornerstone ethical principles of medical care, autonomy, beneficence, nonmaleficence, and justice remain relevant and should be a starting point.
We have already emphasized the importance of collaboration between neonatal and obstetric providers. Nonetheless, moral distress, the ethical confrontation between a plan of care and one’s own ethical principles, can occur despite best efforts to achieve consensus. This is most likely to occur when providers on the same care team have differing personal values and moral principles. Education and communication in the form of debriefing sessions, workshops, and ethics training are important tools to ensure that the resilience and optimal functionality of the health care team. There is no formula or guideline that can be consistently applied to each clinical situation. Clinicians will benefit their patients and families by taking time to consider the large body of literature focused on care of the periviable infant, while listening carefully to each family. It is also important to emphasize that clinical decision making regarding care for the periviable infant does not end in the delivery room. Several complications of prematurity listed in Table 73.4 may present in devastating fashion during the hours and days following delivery, most notably severe IVH, bowel perforation, and respiratory failure with air leak. For an extreme preterm infant, transitioning from delivery room to NICU is the beginning of a long medical journey taking from months to years.
No aspect of the transition from fetal to neonatal life is more dramatic than the process of pulmonary adaptation. In a normal term infant, the lungs expand with air, pulmonary vascular resistance rapidly decreases, and vigorous, consistent respiratory effort ensues within a minute of separation from the placenta. The process is dependent upon crucial physiologic mechanisms including production of functional surfactant, dilatation of high-resistance pulmonary arterioles, bulk transfer of fluid from airspaces, and physiologic closure of the ductus arteriosus and foramen ovale. Complications such as prematurity, infection, neuromuscular disorders, developmental defects, or complications of labor may interfere with the transition to normal neonatal respiratory function. Below, common respiratory problems of neonates are reviewed.
Transient tachypnea of the newborn (TTN), commonly known as “wet lungs,” is a mild condition affecting term and late preterm infants. This is the most common “respiratory cause” of admission to the special care nursery. Transient tachypnea of the newborn is self-limiting, with no risk of recurrence or residual pulmonary dysfunction. It rarely causes hypoxic respiratory failure secondary to persistent pulmonary hypertension of the newborn.
During the last trimester, a series of physiological events lead to changes in the hormonal milieu of the fetus and its mother to facilitate neonatal transition. Rapid clearance of fetal lung fluid is essential for successful transition to air breathing. The bulk of this fluid clearance is mediated by transepithelial sodium reabsorption through amiloride-sensitive sodium channels in the respiratory epithelial cells. The mechanisms for such an effective “self-resuscitation” soon after birth are not completely understood. Traditional explanations based on Starling forces and vaginal squeeze for fluid clearance account only for a fraction of the fluid absorbed.
This condition is classically seen in infants delivered late preterm/early term, especially after cesarean birth before the onset of spontaneous labor. Absence of labor is accompanied by impaired surge of endogenous steroids and catecholamines necessary for a successful transition. Additional risk factors such as multiple gestations, excessive maternal sedation, prolonged labor, and complications resulting from excessive maternal fluid administration have been less consistently observed.
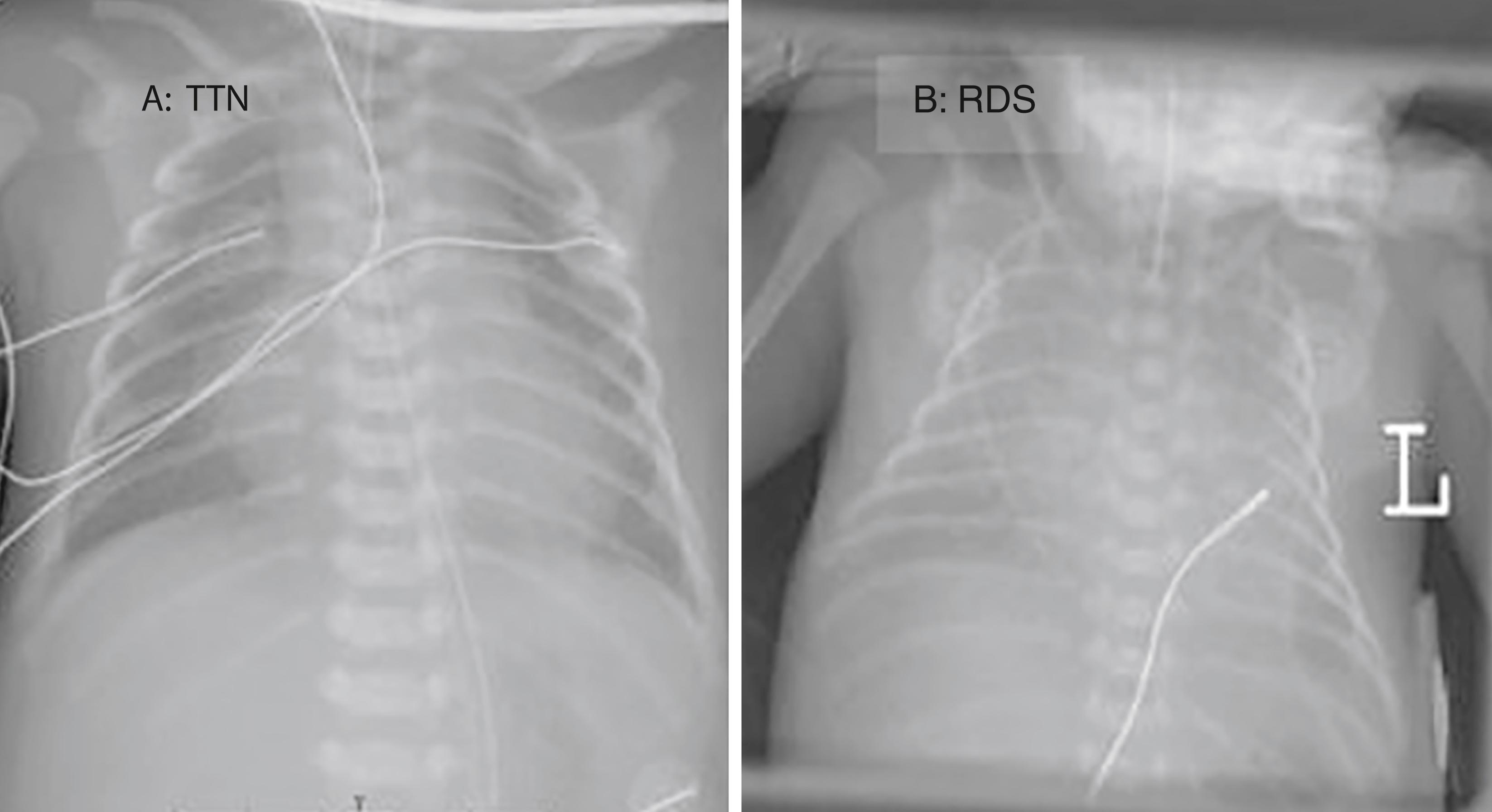
The clinical features of TTN include a combination of grunting, tachypnea, nasal flaring, and mild intercostal and subcostal retractions along with mild central cyanosis. The grunting can be prominent and sometimes misdiagnosed as respiratory distress syndrome secondary to surfactant deficiency. The chest x-ray usually shows prominent perihilar streaking that represents engorged pulmonary lymphatics and blood vessels. The presence of fluid in the fissures is a common nonspecific finding. Clinical symptoms rapidly improve in the first 24–48 hours after birth. TTN is a diagnosis of exclusion, and it is important that other potential causes of respiratory distress in the newborn be excluded. The differential diagnosis of TTN includes pneumonia/sepsis, air leaks, surfactant deficiency, and congenital heart disease. Other rare diagnoses are pulmonary hypertension, meconium aspiration, and polycythemia.
This is primarily a clinical diagnosis. Chest x-rays typically demonstrate mild pulmonary congestion, with hazy lung fields. The pulmonary vasculature may be prominent. Small accumulations of extrapleural fluid, especially in the minor fissure on the right side, may be seen. Ultrasound of the lung is increasingly used to diagnose TTN.
Prenatal prevention strategies include antenatal betamethasone prior to late preterm infant birth and elective cesarean birth between 37 and 39 weeks. This intervention decreases all common causes of neonatal respiratory morbidities; however, potential long-term adverse effects of this intervention need to be closely monitored. , Current American Academy of Pediatrics (AAP) guidelines recommending scheduling of elective cesarean births after 39 completed weeks of gestation should significantly reduce the incidence of TTN. Postnatal management is mainly supportive. Supplemental oxygen is provided to keep the O 2 saturations greater than 90%. Infants are usually given IV fluids and not fed orally until their tachypnea resolves. Rarely, infants may need continuous positive airway pressure to relieve symptoms. Diuretic therapy is not effective; fluid restriction and inhaled beta-agonists (salbutamol) have been tried with limited success. ,
TTN can contribute to significant morbidity related to delayed initiation of oral feeding, which may in turn interfere with parental bonding and establishment of successful breastfeeding. The hospital stay is prolonged for mother and infant. Recently, studies have found an association between TTN and wheezing/asthma in later childhood ( Fig. 73.2 ).
Lung development begins during the first trimester when the ventral foregut endoderm projects into adjacent splanchnic mesoderm (see Chapter 3 ). Branching morphogenesis, epithelial differentiation, and acquisition of a functional interface for gas exchange ensue through the remainder of gestation and are not completed until the second or third year of postnatal life. Clinical conditions associated with pulmonary hypoplasia and approaches to prevention and treatment are discussed here.
Perturbation of lung development at any time during gestation may lead to clinically significant pulmonary hypoplasia. Two general pathophysiologic mechanisms contribute to pulmonary hypoplasia: extrinsic compression (e.g., congenital diaphragmatic hernia) and neuromuscular dysfunction (e.g., myotonic dystrophy). There is no gold standard clinical diagnostic method to diagnose pulmonary hypoplasia. Computed tomography (CT) is commonly used to confirm the clinical suspicion. Postmortem pathological criteria such as lung weight to body weight and radial alveolar count have been used in clinical research.
Oligohydramnios, whether due to periviable preterm prelabor rupture of membranes (pPROM) or diminished fetal urine production (renal agenesis), can lead to severe pulmonary hypoplasia. pPROM in the periviable period occurs during the canalicular phase of fetal lung development and drives pulmonary hypoplasia. Prediction of clinical outcome is difficult in these infants. The reduction in branching morphogenesis and surface area for gas exchange may be lethal or clinically imperceptible. The degree of pulmonary hypoplasia and neonatal survival depend on gestational age at rupture and delivery and parental wishes for resuscitation. In a recent review, Gibson and colleagues reported significant mortality risk in infants born to women with periviable (20–25 weeks) PROM and oligohydramnios. Therapeutic interventions such as amnioinfusion and resealing technologies do not currently show benefit and are undergoing further investigation.
The degree of pulmonary hypoplasia in congenital diaphragmatic hernia (CDH) is directly related to the extent of herniation. Large hernias occur earlier in gestation. In most cases, the contralateral lung is also hypoplastic. Recent studies document some degree of catch-up lung growth following delivery. The interplay of the three salient pathophysiologic processes in CDH (pulmonary hypoplasia, pulmonary hypertension, and ventricular dysfunction) determines the baby’s survival. Prenatal diagnosis of pulmonary hypoplasia is discussed in Chapter 22 .
Postnatal treatment for pulmonary hypoplasia is largely supportive. A subset of infants with profound hypoplasia will have insufficient surface area for effective gas exchange. These patients typically display profound hypoxemia, respiratory acidosis, pneumothorax, and pulmonary interstitial emphysema shortly after delivery. At the other end of the spectrum, some infants will have no clinical evidence of pulmonary insufficiency at birth but have diminished reserves when stressed. In between is a cohort of patients with respiratory insufficiency responsive to mechanical ventilation and pharmacologic support. Typically, these patients have adequate oxygenation and ventilation, suggesting adequate gas exchange capacity. However, many develop pulmonary hypertension. The pathophysiologic sequence begins with limited cross-sectional area of resistance arterioles, followed by smooth muscle hyperplasia in these same vessels.
Early use of pulmonary vasodilators such as nitric oxide is the mainstay of management for increased pulmonary vasoreactivity. Optimizing pulmonary blood flow reduces the potential for hypoxemia thought to stimulate pathologic medial hyperplasia. If oxygenation, ventilation, and acid-base balance are maintained, nutritional support over time can allow sufficient lung growth to support the infant’s metabolic demands. In many cases the process is lengthy, requiring mechanical ventilation and treatment with pulmonary vasodilators such as sildenafil, bosentan, prostaglandin E 1 , epoprostenol, or treprostinil for weeks to months. Prenatal prognostic indicators for CDH such as percentage predicted lung volume and lung-head ratio measurements on fetal magnetic resonance imaging (MRI) are useful for guiding management and parental counseling.
Respiratory distress syndrome (RDS) is a significant cause of early neonatal mortality and long-term morbidity. However, in the last 3 decades, significant advances have been made in its management with a consequent decrease in associated morbidity and mortality.
The classic risk factors for RDS are prematurity and low birth weight. In 2017, according to Vermont Oxford Network the incidence of RDS was around 80% at 28 weeks increasing up to 90% at 24 weeks. Factors that negatively affect surfactant synthesis include maternal diabetes, perinatal asphyxia, cesarean delivery without labor, and genetic factors (Caucasian race, history of RDS in siblings, male sex, and surfactant protein B deficiency). Congenital malformations that lead to lung hypoplasia such as CDH or giant omphalocele are also associated with significant surfactant deficiency.
The need for assessing fetal lung maturity was considered critical in timing delivery of high-risk pregnancies to minimize respiratory morbidity associated with immature lungs. There were a number of biochemical, functional, and physical lung maturity tests performed on amniotic fluid obtained by amniocentesis or from pooled vaginal secretions after rupture of the membranes; however, none of them individually or collectively had a clinically significant sensitivity or specificity to detect lung maturity. The most widely used tests were lamellar body count (LBC), the L/S ratio, and PG test (phosphatidylglycerol). The less commonly used tests were surfactant/albumin ratio (TDx FLM), foam stability test, and lung profile. Studies found that neonates delivered late preterm despite mature fetal lung testing were at increased risk for RDS, hyperbilirubinemia, and hypoglycemia; hence, recent American College of Obstetricians and Gynecologists (ACOG) and Society for Maternal-Fetal Medicine (SMFM) guidelines cite the unreliability of these tests and advised clinicians to discontinue the practice of performing routine amniocentesis for fetal lung maturity testing in suboptimally dated and medically indicated or nonmedically indicated early deliveries. ,
Quantitative ultrasound (quantusFLM) is a new technique increasingly used to detect fetal lung texture to predict lung maturity. The fetal lung is visualized at the level of the four-chamber cardiac view and images uploaded to a cloud-based database where it is quickly analyzed, and results are transmitted back. This technique has been validated in a large prospective multicenter study to have reliable (reproducible) and accurate results comparable to prior invasive methods. Magnetic resonance imaging and MRI spectroscopy are being investigated to measure lipid content of amniotic fluid but not clinically used due to high cost and maternal discomfort while obtaining images.
Antenatal steroids (ANS) are the most effective prenatal intervention to mature fetal lungs and reduce associated neonatal morbidity and mortality. The most popular treatment is to use a 1:1 mixture of betamethasone phosphate and betamethasone acetate (Celestone) in pregnant women between 24 and 34 weeks’ gestation with a high risk for preterm delivery within 7 days. A single rescue course is recommended for threatened preterm birth if >7 days after a previous full course of steroids. The use of ANS has now expanded to threatened late preterm births (34–37 weeks) and to elective cesarean delivery without labor at term to reduce respiratory morbidity. Betamethasone and dexamethasone have been compared in well-designed large trials and found to be equally effective with no difference in neurosensory impairment at 2 years. ,
More recently, potential long-term adverse effects of ANS have been shown in both animals and human epidemiological studies. Large cohort studies from Finland and Canada have shown an increase in behavioral-emotional and psychological development effects, anxiety, and depression in long-term follow-up studies of infants who received ANS. To reduce ANS exposure, investigations are currently ongoing to examine a lower dose of betamethasone (full dose versus half dose) and to evaluate betamethasone acetate alone to reduce the peak concentrations obtained when combined with betamethasone phosphate.
Symptoms are typically evident in the delivery room, or shortly thereafter, including tachypnea, nasal flaring, subcostal and intercostal retractions, cyanosis, and expiratory grunting. The characteristic expiratory grunt is secondary to expiration through a partially closed glottis, providing continuous distending airway pressure to maintain functional residual capacity (thereby preventing alveolar collapse). These signs of respiratory difficulty are not specific to RDS and can occur from a variety of pulmonary and nonpulmonary causes such as transient tachypnea, air leaks, congenital malformations, hypothermia, hypoglycemia, anemia, polycythemia, or metabolic acidosis. Progressive worsening of symptoms in the first 2–3 days followed by recovery characterizes the typical clinical course. This timeline (curve) is modified by administration of exogenous surfactant with a more rapid recovery. Classic radiographic findings include low-volume lungs with a diffuse reticulo-granular pattern and air bronchograms. Point-of-care lung ultrasound is being increasingly used in Europe to diagnose and determine severity of RDS. , The diagnosis can be established chemically by measuring surfactant activity in tracheal or gastric aspirates, but this is not routinely done. ,
Infants are managed in an incubator or under a radiant warmer in a neutral thermal environment to minimize oxygen requirement and consumption. Arterial oxygen tension (PaO 2 ) is maintained between 50 and 80 mm of mercury with saturations between 90% and 95%. Hypercarbia and hyperoxia are avoided. Heart rate, blood pressure, respiratory rate, and peripheral perfusion are monitored closely. As sepsis cannot be excluded, screening blood culture and complete blood counts with differential counts are performed and infants are started on broad-spectrum antibiotics for 48 hours, until culture results are available.
Surfactant replacement is one of the safest and most effective interventions in neonatology. The first successful clinical trial of surfactant use was reported in 1980 using surfactant prepared from an organic solvent extract of bovine lung to treat 10 infants with RDS. By the early 1990s, widespread use of surfactant led to a progressive decrease in RDS-associated mortality. Three strategies for treatment are commonly used: (1) prophylactic surfactant, in which surfactant is administered via endotracheal tube before the first breath to all infants at risk of developing RDS; (2) rescue therapy, where surfactant is given via endotracheal tube after the onset of respiratory signs; and (3) minimally invasive methods of surfactant administration for infants on noninvasive ventilation. The advantages of prophylactic administration include a better distribution of surfactant when instilled into a partially fluid-filled lung along with the potential to decrease trauma related to resuscitation. Avoiding treatment of unaffected infants and related cost savings are the advantages of rescue therapy. Less-invasive methods of surfactant administration include nebulization, instillation into the pharynx before the first breath, and administration via laryngeal mask or thin catheter. The thin catheter method allows the instillation of surfactant into a spontaneously breathing infant (better surfactant distribution), without the disruption of nasal continuous positive pressure, and potentially avoids positive pressure–induced lung injury. Biologically active surfactant can be prepared from bovine, porcine, human, or synthetic sources. When administered to patients with surfactant deficiency and RDS, all of these preparations show improvement in oxygenation and a decreased need for ventilator support, along with decreased air leaks and death. The combined use of antenatal corticosteroids, noninvasive ventilation, and postnatal surfactant has significantly improved neonatal outcomes.
In infants with acute RDS, continuous positive airway pressure (CPAP) prevents atelectasis, minimizes lung injury, and preserves surfactant function, allowing infants to be managed without endotracheal intubation and mechanical ventilation. Early delivery room CPAP therapy decreases the need for mechanical ventilation and the incidence of long-term pulmonary morbidity. , Increasing use of CPAP has led to decreased use of surfactant and decreased BPD. Common complications of CPAP include pneumothorax and pneumomediastinum. Rarely, the increased transthoracic pressure leads to progressive decrease in venous return and decreased cardiac output. Brief intubation and administration of surfactant followed by transition to CPAP (InSurE technique) is an additional RDS treatment strategy increasingly used in Europe and Australia. Recently, the advantages of CPAP have been combined with less-invasive surfactant administration (LISA) and have led to reduced need for mechanical ventilation and BPD. Prospective, randomized trials in extremely-low-birth-weight infants comparing early delivery room CPAP versus early prophylactic surfactant therapy demonstrate equivalency as defined by death or bronchopulmonary dysphasia. Three meta-analyses of these trials support the superiority of delivery room CPAP in reducing BPD. These findings led to the recommendation by the European Association of Perinatal Medicine and AAP to endorse delivery room CPAP as the primary mode of respiratory support for treating RDS. Noninvasive respiratory support techniques such as synchronized nasal intermittent positive ventilation (SNIPPV) and heated humidified high-flow nasal cannula have been shown to be equally effective as nasal CPAP in primary support for RDS in recent noninferiority clinical trials.
The goal of mechanical ventilation is to limit lung injury (volutrauma and barotrauma) without causing progressive atelectasis while maintaining adequate oxygenation and gas exchange. Recent evidence suggests that volume-targeted intermittent mandatory ventilation and high-frequency oscillators cause the least amount of iatrogenic lung injury. Complications associated with mechanical ventilation include pulmonary air leaks, endotracheal tube displacement or dislodgement, obstruction, infection, and long-term complications such as BPD and subglottic stenosis.
Acute complications include pneumothorax, pneumomediastinum, pneumopericardium, and pulmonary interstitial emphysema. The incidence of these complications has decreased significantly with surfactant treatment. Infection, intracranial hemorrhage, and patent ductus arteriosus occur more frequently in very-low-birth-weight infants with RDS. Long-term complications and comorbidities include BPD, poor neurodevelopmental outcomes, and retinopathy of prematurity. Incidence of these complications is inversely related to decreasing birth weight and gestation. Studies of early inhaled nitric oxide and supplementary inositol for prevention of long-term BPD failed to demonstrate significant effectiveness. ,
The classic form of BPD was first described in a group of preterm infants who were mechanically ventilated at birth and who later developed chronic respiratory failure with characteristic radiographic findings on chest x-ray. These infants were larger, late-preterm infants with lung changes attributed to mechanical ventilation and oxygen toxicity. More recently, smaller, extremely preterm infants with lung immaturity and prenatal exposure to antenatal glucocorticoids have developed a more subtle form, labeled the “new BPD.” This disease now primarily occurs in infants less than 1000 g who have very mild or no initial respiratory distress. The clinical diagnosis is currently based on the need for supplemental oxygen at 36 weeks’ corrected gestational age. , Recently diagnostic criteria have been revised to improve prediction of childhood respiratory morbidity based on mode of respiratory support at 36 weeks’ gestational age, irrespective of the level or duration of oxygen therapy. Advances in pulmonary imaging are now guiding risk stratification to predict development of BPD and to formulate treatment strategies.
Clinically, the transition from RDS to BPD is subtle and gradual. Radiographic manifestations of classic BPD include areas of shifting focal atelectasis and hyperinflation with or without parenchymal cyst formation. Chest x-rays of infants with the new BPD show bilateral haziness reflecting diffuse microatelectasis without multiple cystic changes. These changes lead to ventilation-perfusion mismatching and increased work of breathing. Preterm infants with BPD either gradually wean off respiratory support and oxygen or continue to worsen with progressively severe respiratory failure, pulmonary hypertension, and a high mortality risk.
Risk factors predisposing preterm infants to BPD include extreme prematurity, oxygen toxicity, mechanical ventilation, and inflammation (prenatal and postnatal). The pathological findings characterized by severe airway injury and fibrosis in the old BPD have been replaced in the new BPD with large simplified alveolar structures, impaired capillary configuration, and variable degrees of interstitial cellularity and/or fibroproliferation. Airway and vascular abnormalities tend to be associated with more severe disease.
Oxygen-induced lung injury is an important contributing factor. Exposure to oxygen in the first two weeks of life and as chronic therapy has been associated in clinical studies with the severity of BPD. , Similarly, in animal models, hyperoxia has been shown to mimic many of the pathological findings of BPD. As there are potential benefits and harm of hyperoxemia and hypoxemia, effects of targeting two different saturation ranges from birth have been studied in multiple clinical trials. Meta-analysis revealed no difference in BPD rates; however, assignment to a higher target range reduced the risk of death and severe necrotizing enterocolitis but increased the risk of treated retinopathy.
Concerns regarding oxygen toxicity are reflected in the most recent update of the neonatal resuscitation guidelines. Blended oxygen (30%) or, if not available, room air is now recommended for initial resuscitation of preterm infants in the delivery room, along with continuous monitoring via pulse oximetry.
Barotrauma and volutrauma associated with mechanical ventilation have been identified as major factors causing lung injury in preterm infants. Surfactant replacement therapy is beneficial in decreasing symptoms of RDS and improving survival. The efficacy of surfactant to decrease the incidence of subsequent BPD is less well established. Chronic inflammation and edema associated with positive-pressure ventilation cause surfactant protein inactivation leading to decreased lung compliance and need for higher positive pressures. Newer ventilation strategies have been developed to reduce lung injury and optimize repair and lung growth. As intrauterine inflammation is increasingly recognized as a cause of preterm parturition, antenatal inflammation is gaining more attention in the pathogenesis of BPD and other morbidities of prematurity. Chorioamnionitis has been shown to be strongly associated with impaired pulmonary and vascular growth, a typical finding in the new BPD.
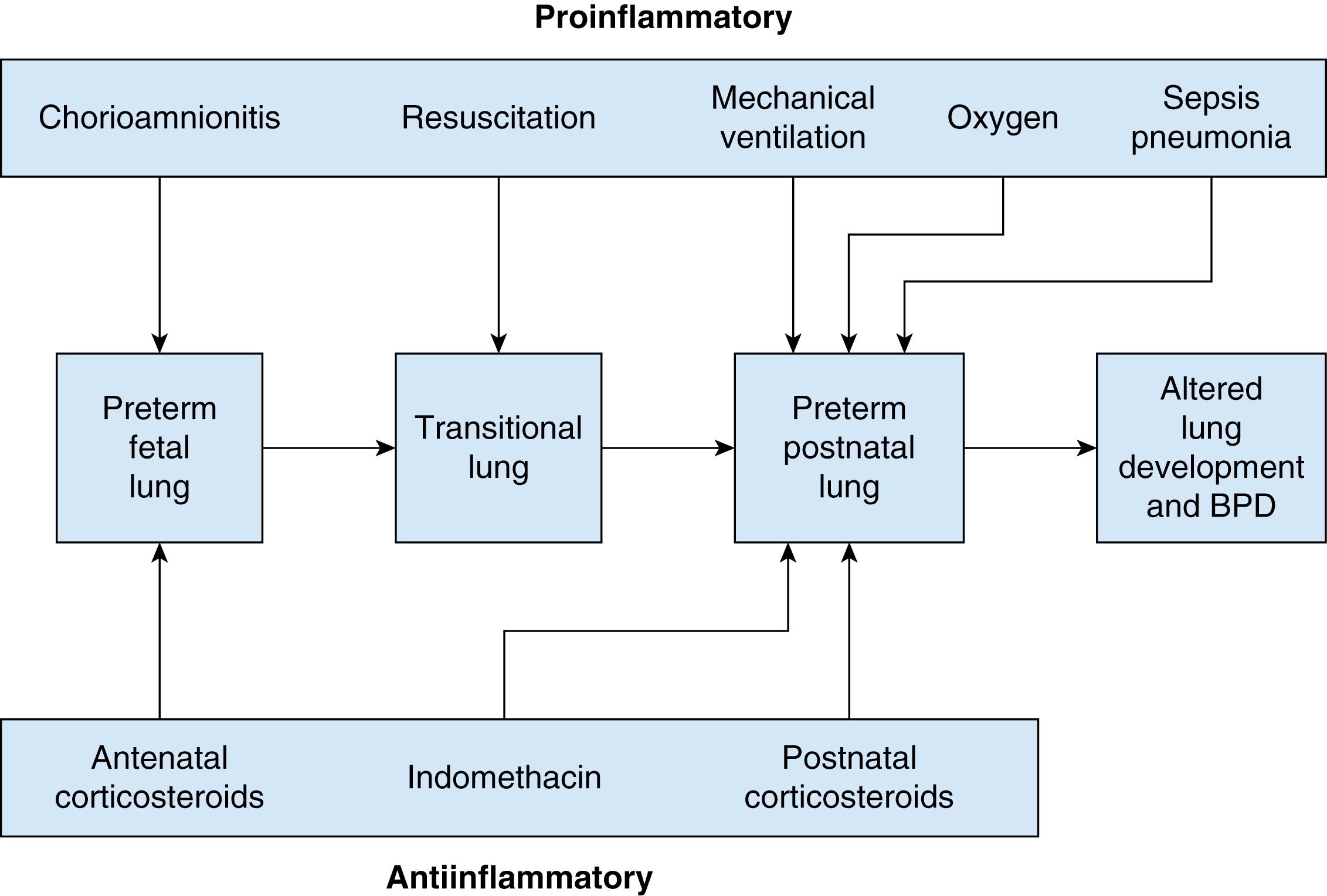
Most deliveries before 30 weeks’ gestation are associated with histological chorioamnionitis, which except for preterm initiation of labor is otherwise clinically silent. The more preterm the delivery, the more often histological chorioamnionitis is detected. Increased levels of proinflammatory mediators in amniotic fluid, placental tissues, tracheal aspirates, lung, and serum of extremely-low-birth-weight preterm infants support an important role for both intrauterine and extrauterine inflammation in the development and severity of BPD. The proposed interaction between the proinflammatory and antiinflammatory influences on the developing fetal and preterm lung is detailed in Fig. 73.3 . Several animal models and preterm studies demonstrate that mediators of inflammation including endotoxins, tumor necrosis factor, interleukin (IL)-1, IL-6, IL-8, and transforming growth factor alpha can enhance lung maturation but concurrently impede alveolar septation and vasculogenesis, contributing to the development of BPD. Chorioamnionitis alone is associated with BPD, but the probability is increased when these babies receive a second insult such as mechanical ventilation or postnatal infection. , , ,
Maternal genital mycoplasma infection, particularly with M ycoplasma hominus and U reaplasma urealyticum , is associated with preterm delivery and BPD. The pulmonary microbiome is emerging as an important factor in the pathophysiology of BPD as studies reveal differences in airway microbiome in preterm infants with and without BPD. Numerous studies have isolated these organisms from amniotic fluid and placentas in women with spontaneous preterm birth (preterm birth due to preterm labor or pPROM). Following birth, these organisms are known to colonize and elicit a proinflammatory response in the respiratory tract leading to BPD. Preliminary evidence suggests azithromycin therapy in preterm infants colonized with ureaplasma reduces a combined outcome of BPD and death. Additional, more rigorous study is needed before broadly recommending this as an established practice.
The unpredictable variation in the incidence of BPD, despite adjusting for low birth weight and prematurity, suggests a genetic predisposition to the occurrence and the severity of BPD. Expression of genes critical to surfactant synthesis, vascular development, and inflammatory regulation are likely to play a role in the pathogenesis of BPD. Twin studies have recently shown that the BPD status of one twin, even after correcting for contributing factors, is a highly significant predictor of BPD in the second twin. In this cohort, after controlling for covariates, genetic factors accounted for 53% of the variance in the liability for BPD. Genetic polymorphisms in the inflammatory response are increasingly recognized as important in the pathogenesis of preterm parturition (see Chapter 7 ) and may be similarly important in the genesis of inflammatory morbidities in the preterm neonate as well.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here