Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hypotension of the premature newborn is a condition commonly encountered in the neonatal intensive care setting. Although no definite cut-off values exist in considering a given newborn to be hypotensive, certain blood pressure ranges will cause significant anxiety among caregivers. The goal of this chapter is to illustrate the complexity involved in the diagnosis and management of neonatal hypotension and guide the clinician in decision making using available evidence and a physiology-based individualized approach when caring for patients with neonatal circulatory compromise. We present a real case with a type of cardiovascular compromise frequently seen in the neonatal intensive care unit (NICU) and ask the reader to make clinical decisions. Along with the description of the course of the patient, we also present current evidence for and the pitfalls of the definition, diagnosis, and management of neonatal hypotension. Lastly, supplanting the case with additional information, we hope the reader arrives at a logical, reasonable, and pathophysiology-based treatment plan.
A 618-gram male preterm twin infant was born at 245⁄7 weeks’ gestation via cesarean section for fetal heart rate deceleration. The pregnancy was complicated by preterm labor and maternal fever treated with antibiotics. A full course of antenatal steroid was given before delivery.
At birth, the infant emerged with weak cry and a heart rate of more than 60 beats per minute. He was intubated in the delivery room and subsequently given one dose of surfactant. Apgar scores were 3, 7, and 9 at 1, 5, and 10 minutes respectively, and the patient was transferred to the NICU of the delivery hospital. On the third postnatal day, a course of indomethacin was started for a “large” patent ductus arteriosus. On this day, a right-sided grade III peri/intraventricular hemorrhage (P/IVH) was also discovered on cranial ultrasound. Repeat cardiac ultrasound on the sixth postnatal day revealed the persistence of the patent ductus arteriosus. However, his blood pressure was reported within acceptable range and thus the patient did not receive supportive cardiovascular treatment. After completion of the indomethacin course, patient was also started on trophic feedings with breastmilk by nasogastric tube receiving 2 mL every 3 hours.
On the seventh postnatal day, the infant had multiple bradycardic events accompanied by arterial oxygen desaturations. Abdominal radiograph revealed abdominal free air, and the arterial blood gas showed a pH of 6.9, Pa co 2 of 75 mm Hg, PaO 2 of 50 mm Hg, and a base deficit of 10 mEq/L. The infant was started on broad-spectrum antibiotic treatment and dopamine infusion at 2.5 mcg/kg/min and was transferred to a tertiary care center for provision of multidisciplinary pediatric medical and surgical subspecialty care. During transport, the infant developed progressively worsening systemic hypotension, and the dose of dopamine infusion was gradually increased to 10 mcg/kg/min. He subsequently also received 20 mL/kg isotonic saline bolus.
On arrival at the tertiary care center, he presented with a heart rate of 198 beats per minute, a respiratory rate of 40 per minute, and arterial systolic, diastolic, and mean blood pressures of 37, 12, and 19 mm Hg, respectively. On physical examination, the infant did open his eyes when stimulated but had minimal spontaneous movement. He appeared pale and mottled with a sunken fontanelle, breath sounds were equal bilaterally, and there was a loud holosystolic murmur over the heart. Abdomen was distended and appeared to have a bluish hue. He had had no urine output over the last 8 hours before admission. He remained appropriately ventilated on conventional ventilation and had a capillary blood pH, P co 2 , PaO 2 , bicarbonate and base deficit of 7.02, 52 mm Hg, 36 mm Hg, 13 mEq/L, and 16.6 mEq/L, respectively. His blood lactate level was 14.3 mmol/L (normal value ≤2.5 mmol/L). The complete blood count revealed a WBC of 8.11 × 10 3 /μL, Hgb and Hct of 9 g/dL and 27%, respectively, and a platelet count of 202×10 3 /μL. Patient’s serum cortisol concentration was appropriately elevated at 62 mcg/dL. Coagulation profiles were abnormal with a prothrombin time (PT) and partial thromboplastin time (PTT) of 20.3 and 103 seconds respectively, with a normal fibrinogen level at 176 mg/dL. Blood products were transfused to address the patient’s coagulation abnormality. Following the initial emergent medical management, pediatric surgeons placed a peritoneal Penrose drain at the bedside, producing 15 mL of meconium-like fluid. Blood culture at the referring hospital reported the growth of gram-negative bacilli. At this time, he was already on 20 mcg/kg/min of dopamine infusion with epinephrine added at 0.01 mcg/kg/min. However, patient’s blood pressure continued to decline with systolic, diastolic, mean blood pressure values being very low at 22, 10, 15 mm Hg, respectively. This finding triggered the administration of two more 10 mL/kg boluses of isotonic saline.
Which of the following is the mostly likely primary cause of hypotension at this time?
Decreased systemic vascular resistance (vasodilatory shock)
Decreased cardiac output (cardiogenic shock)
Ductal steal-associated decreased postductal systemic blood flow due to the presence of a hemodynamically significant patent ductus arteriosus with left-to-right shunting
Adrenal insufficiency
Decreased intravascular volume (hypovolemic shock)
By themselves or in combination, all of these conditions can cause systemic hypotension. However, before addressing the question, it would be useful to review the determinants of blood pressure and the definition of the “normal” blood pressure range in neonates.
In fundamental fluid mechanics, pressure within a tube is described by Ohm’s law:
Applying this equation to the cardiovascular system, the equation becomes:
Thus blood pressure (the mathematically dependent variable) is the product of two independent variables: cardiac output and systemic vascular resistance (SVR) . The heart pumps a certain volume of blood per minute (cardiac output = stroke volume × heart rate) into the arterial bed, where it meets a given resistance. Stroke volume is determined by preload, myocardial contractility, and afterload whereas the heart rate is affected by a number of factors, including autonomic sympathetic–parasympathetic balance, catecholamine production and release, and temperature. Adequate circulating blood volume and ventricular compliance are necessary for appropriate filling of the heart chambers during diastole, resulting in the generation of necessary sarcomere stretch before ventricular contraction.
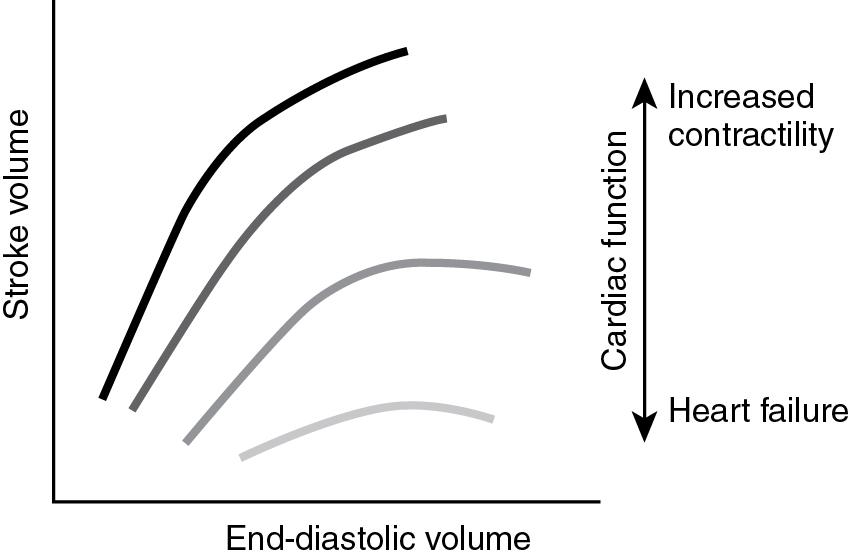
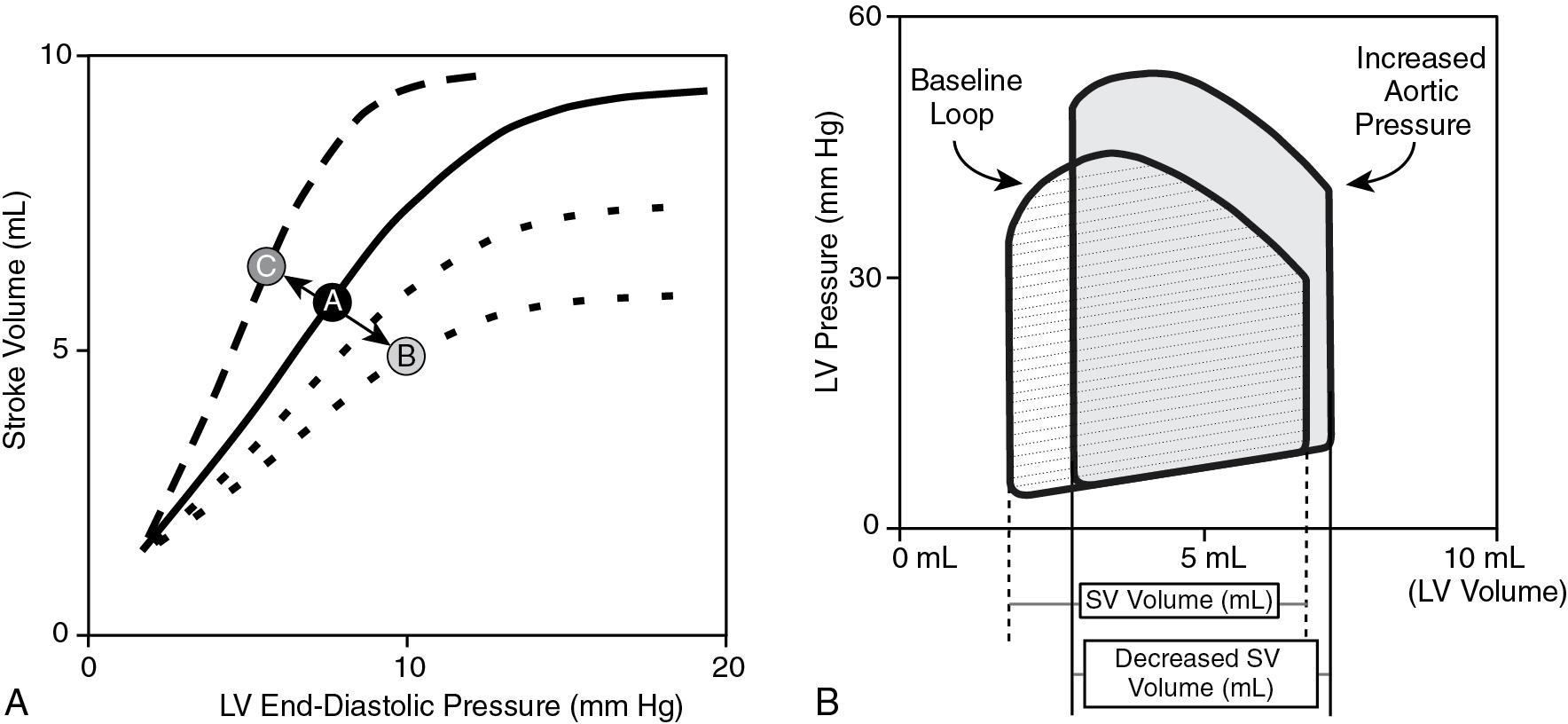
The Frank-Starling law describes the sarcomere length-dependent changes of cardiac contractility, where increased preload, up to a certain point, improves myocardial contractility ( Fig. 15.1 ). During systole, SVR contributes to afterload as the heart has to generate a pressure to overcome the resistance and produce forward flow. In addition to the blood pressure generated, afterload is directly determined by the diameter and is inversely related to the thickness of the left ventricle. An increase in afterload shifts the Frank-Starling curve and decreases stroke volume ( Fig. 15.2 ). The overal resistance that is overcome to create blood flow within the vascular network is the SVR. This value is not directly measured but derived from the measurement of blood pressure and cardiac output based on Ohm’s law. SVR is the mean value of the resistance to blood flow within a vascular network irrespective of the pulsatile changes in resistance throughout the cardiac cycle. Regional differences in vascular resistance exist to allow for autoregulation of end-organ blood flow within a given range of perfusion pressure (autoregulatory blood pressure range). Based on fluid dynamics and the Poiseuille’s equation:
where L = vessel length, μ = blood viscosity, r = vessel radius. From this equation, we can appreciate that vessel diameter (inversely to the 4th power) is the most potent regulator of vascular resistance, because vessel length and blood viscosity usually do not change acutely. Regulation of the smooth muscle tone of the blood vessels is complex and is influenced by local, endothelial-derived substances such as nitric oxide, endothelin, and prostacyclin as well as neural and humoral factors (antidiuretic hormone, angiotensin II, catecholamines, etc.). In sepsis, inflammatory cytokines such as tumor necrosis factor, the interleukin-1 family, and prostaglandins are the most potent mediators of vasodilatory shock.
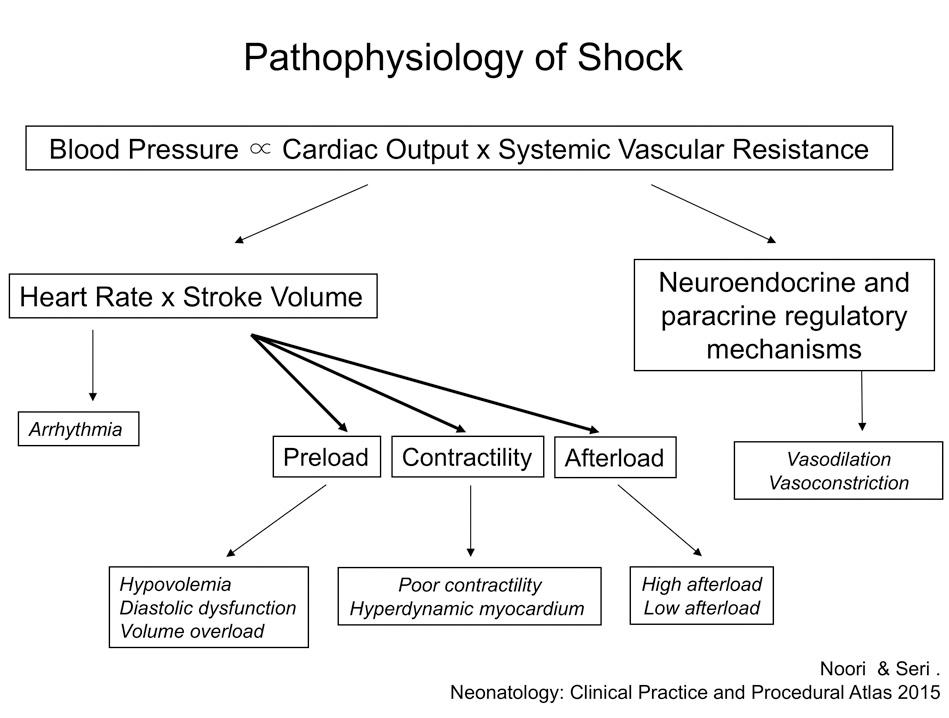
Upon understanding these determinants of blood pressure, one can realize that the different etiologies in neonatal shock correspond to a compromise in a particular determinant or a combination of determinants (cardiac output and/or SVR) without adequate compensation. The ability to identify the specific hemodynamic components and their interplay that determine blood pressure will aid the clinician in making more appropriate management decisions, such as the choice of vasopressor-inotrope, inotrope, or lusitrope, and in deciding if volume administration is needed. The clinical components that affect blood pressure are shown in Fig. 15.3 . As—in addition to heart rate—blood pressure is the most readily and accurately monitored clinical hemodynamic variable, the bedside clinician is often dependent on its measurement in the management of shock. However, the particular blood pressure value at which it is considered “too low” in a neonate is unclear.
A number of investigators have reported population-based normal ranges of blood pressure values based on gestational age, postnatal age, or birthweight. The majority of the data support the notion that larger and more mature infants have higher blood pressures. In addition, blood pressure also increases with postnatal age ( Table 15.1 ) ( ). One of the common clinical practices is to consider the minimum acceptable mean blood pressure value to numerically equal the gestational age of the infant. This rather simplistic but practical approach is based on the 1992 recommendations from the Joint Working Group of the British Association of Perinatal Medicine ( ). Of note is that a recent study of extremely preterm infants reported a higher survival rate without major morbidity and a lower rate of severe cerebral abnormalities when isolated, clinically not symptomatic hypotension (defined as the numeric value of blood pressure lower than that of the gestational age in weeks) was treated with volume, vasopressor-inotropes, and/or corticosteroids during the first 3 postnatal days ( ). These findings suggest that in the present clinical practice, undetectable but clinically relevant changes in organ (brain) blood flow and oxygen delivery might accompany decreases in blood pressure that are considered harmless by many (“permissive hypotension”).
| Birth Weight (g) | Time (h) Postnatal Age | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 | |
| 500 | 35/23 | 36/24 | 37/25 | 38/26 | 39/28 | 41/29 | 42/30 | 43/31 | 44/33 |
| 600 | 35/24 | 36/25 | 37/26 | 39/27 | 40/28 | 41/29 | 42/31 | 44/32 | 45/33 |
| 700 | 36/24 | 37/25 | 38/26 | 39/28 | 42/29 | 42/30 | 43/31 | 44/32 | 45/34 |
| 800 | 36/25 | 37/26 | 39/27 | 40/28 | 41/29 | 42/31 | 44/32 | 45/33 | 46/34 |
| 900 | 37/25 | 38/26 | 39/27 | 40/29 | 42/30 | 43/31 | 44/32 | 45/34 | 47/35 |
| 1000 | 38/26 | 39/27 | 40/28 | 41/29 | 42/31 | 43/32 | 45/33 | 46/34 | 47/35 |
| 1100 | 38/27 | 39/37 | 40/29 | 42/30 | 43/31 | 44/32 | 45/34 | 46/35 | 48/36 |
| 1200 | 39/27 | 40/28 | 41/29 | 42/30 | 43/32 | 45/33 | 46/34 | 47/35 | 48/37 |
| – | – | – | – | – | – | – | – | – | – |
| 1400 | 40/28 | 42/29 | 42/30 | 43/32 | 44/33 | 46/34 | 47/35 | 48/36 | 49/38 |
| 1500 | 40/29 | 42/30 | 43/31 | 44/32 | 45/33 | 46/35 | 48/36 | 49/37 | 50/38 |
Another approach is to use population-based norms as the accepted blood pressure range ( Fig. 15.4 ) ( ).
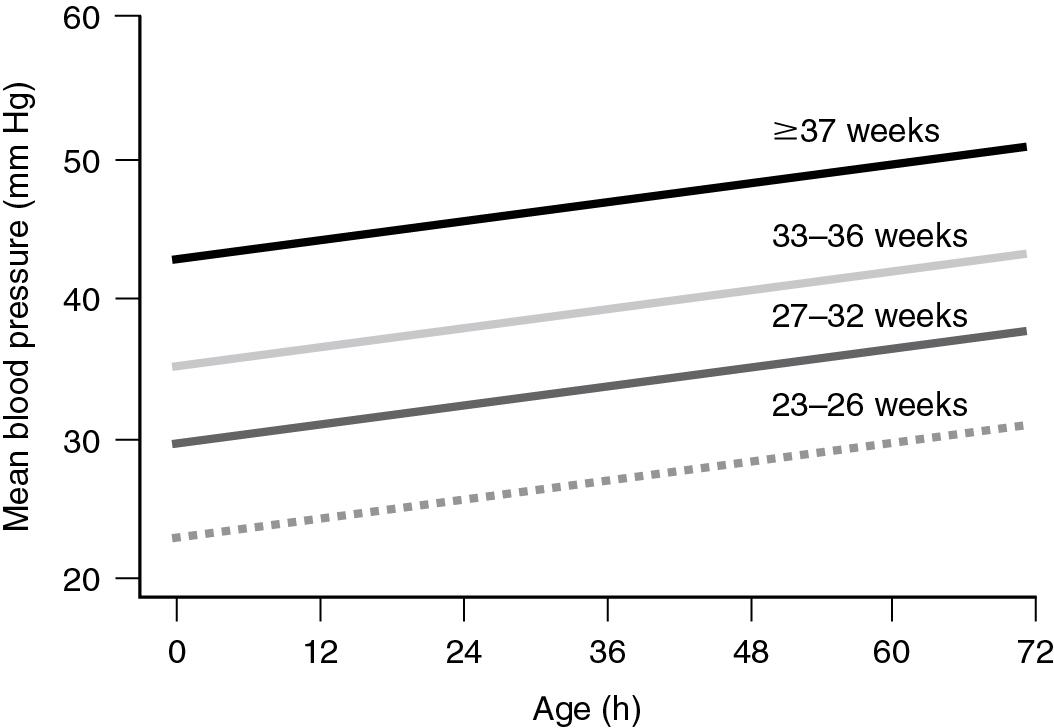
Finally, based on the presumptive lower elbow of the cerebral blood flow autoregulatory curve, others have proposed the use of 28 to 30 mm Hg as the critical lower limit of acceptable blood pressure in preterm infants during the immediate transitional period ( ; ). Furthermore, it has been suggested that at mean arterial pressure below 28 to 30 mm Hg, the compromised and pressure passive cerebral blood flow may contribute to the development of intraventricular hemorrhage and periventricular leukomalacia ( ; ). However, high level of evidence supporting this latter notion is lacking.
From these differing approaches, one can easily see why it has become extremely difficult to appropriately define neonatal hypotension ( ) and to reach a general consensus on blood pressure management in the preterm neonate ( ; ). The main reason for the confusion in defining neonatal hypotension is the simple fact that, aside from the heart rate, blood pressure is the only continuously and routinely monitored hemodynamic variable. As blood pressure is the mathematically dependent variable in the equation determining systemic blood flow (CO = BP × SVR), a clinically relevant definition of hypotension and its treatment cannot be solely based on the blood pressure value. Indeed, with the exception of a recent large trial ( ) evidence from randomized control studies is lacking on clinically relevant outcome measures of treatment of preterm infants with mean arterial pressures below gestational and postgestational age-dependent norms. However, the heterogeneity of clinical presentations and patient populations, the differences in the approach to treatment among the studies, and the differences in the patients’ ability to compensate for the decreased blood pressure–associated decline in systemic and organ blood flow might, at least in part, explain why conclusive evidence could not be found to identify the lowest acceptable blood pressure range in preterm neonates in the transitional period. Importantly, this “magic” number is likely different among patients and also changes with time in the same patient due to the patient’s ability to mount a successful compensatory response to maintain acceptable blood flow and oxygen delivery to the vital organs. Thus such a critical value cannot be determined for entire populations of preterm and term neonates. Rather, it will have to be defined in each patient at each point in time applying the concept of “precision medicine” ( ).
Interestingly, a number of studies have found an association between early hypotension and subsequent peri/intraventricular hemorrhage (P/IVH) and poor neurodevelopmental outcome in premature infants ( ; ; ; ; ; ; ; ; ). Of note, although the association between early postnatal hypotension and subsequent brain injury has been established, causation remains to be proven. On the other hand and as mentioned earlier, some studies have found blood pressure below gestational age alone did not affect neurodevelopmental outcome, possibly explained by the finding that treatment of hypotension by volume bolus or low to moderate dose dopamine did not change regional cerebral oxygen saturation significantly ( ; ).
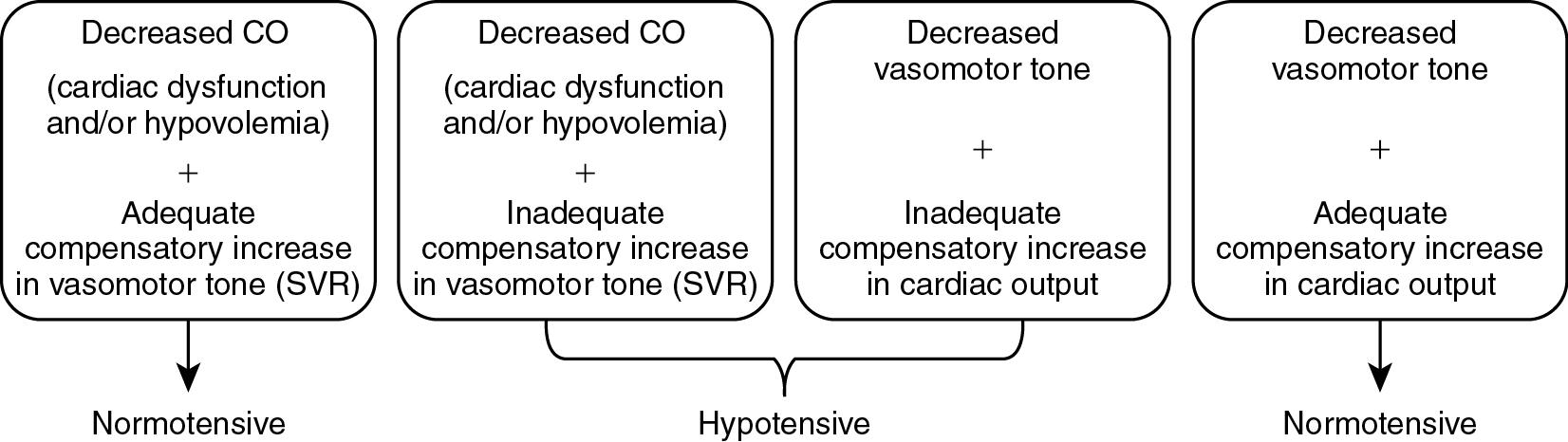
In summary, derangements in blood pressure are the result of uncompensated shock as also seen in our case. However, from the equation based on Ohm’s law, we can also appreciate that even in the setting of cardiac dysfunction or impaired vasomotor tone, compensatory capacity even of the immature cardiovascular system might maintain blood pressure in the “normal” range. Accordingly, we can classify the hemodynamic status of a neonate into four major categories:
Cardiac dysfunction with compensatory increase in vasomotor tone,
Cardiac dysfunction without adequate compensation in vasomotor tone,
Vasomotor dysregulation leading to vasodilation with compensation in cardiac output, and
Vasomotor dysregulation leading to vasodilation without adequate compensation in cardiac output ( Fig. 15.5 ).
Next, we turn our attention to direct and indirect hemodynamic assessments of systemic and end-organ perfusion along with the limitations of these approaches.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here