Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neonatal jaundice is the most common physiologic variant encountered in the newborn. More than 60% of healthy term neonates, and even a greater percentage of breastfed infants, display some degree of visible jaundice during the first week of life. Usually the body’s regulatory mechanisms succeed in keeping the serum total bilirubin (STB) level within physiologic levels, and therefore at a concentration that is nontoxic. Indeed, STB concentrations within this range may even have beneficial antioxidant properties.
On occasion, STB levels may increase and significant hyperbilirubinemia may develop. Not all degrees of hyperbilirubinemia are necessarily dangerous, but because of the potential for the STB to continue to rise, phototherapy may be indicated. By facilitating bilirubin elimination, further rise of STB may be limited, thereby preventing the potential for bilirubin neurotoxicity. Rarely, the STB may increase to extreme levels at which bilirubin neurotoxicity may occur. In these cases, bilirubin—especially the unbound fraction—may enter vulnerable brain cells, especially the basal ganglia and auditory nerve tissue, causing acute bilirubin encephalopathy with the potential for progressing to the chronic form of bilirubin neurotoxicity, choreoathetoid cerebral palsy (kernicterus).
It is not our intention in this chapter to provide yet another all-inclusive treatise on neonatal hyperbilirubinemia. Rather, following some background information regarding neonatal hyperbilirubinemia, the reader will be presented with some actual clinical cases drawn from the authors’ experience. The reader is encouraged to put himself or herself in the “driver’s seat” and actually manage the patients, making clinical decisions from the options provided. The cases will provide the opportunity for in-depth discussions of the issues at hand and focus on practical issues that the practitioner may encounter on a daily basis.
A 36 weeks’ gestation, otherwise healthy infant aged 24 hours was being discussed on rounds in the regular newborn nursery. The STB was 15.0 mg/dL. The professor asked the residents what this value actually meant. The following possibilities were suggested.
Which answer do you think is correct?
One resident plotted the result on the hour-specific bilirubin nomogram. Because the value was greater than the 95th percentile, this resident concluded that increased hemolysis was present.
The second resident related to the late prematurity of this infant. The bilirubin conjugating system is immature, he claimed, resulting in the increased STB.
The third resident claimed that the pathogenesis of the high STB value was multifactorial and that both increased bilirubin production and hemolysis contributed to its development.
The third resident (C) supplied the correct answer. He correctly argued that several physiologic or pathophysiologic processes contributed to the STB. He claimed that no single process is responsible for an STB value at any point in time but that the STB value represents a combination of processes acting in tandem. The first resident’s answer (A) was incorrect because although increased hemolysis may have been present, he did not take bilirubin elimination into account. Similarly, the second resident (B) correctly identified late prematurity with diminished conjugation ability of the infant as a risk factor but neglected to take the potential for increased hemolysis into account.
The STB at any point in time, in any newborn, represents a combination of forces both affecting heme catabolism with subsequent bilirubin production, on the one hand, and bilirubin elimination—regulated by the processes of bilirubin conjugation and excretion—on the other. In the newborn, reabsorption of bilirubin from the bowel, as part of the enterohepatic circulation, adds to the bilirubin pool to be subsequently eliminated. As long as these processes remain in equilibrium, the STB may rise to physiologic levels but should not pose a threat to an otherwise healthy term newborn without hemolysis.
Should this delicate balance become compromised and bilirubin production exceed bilirubin elimination, the equilibrium will fail and hyperbilirubinemia may result. Severe hemolysis per se or immature bilirubin conjugation in and of itself may not necessarily result in hyperbilirubinemia. For example, an infant with blood type A, born to a woman with blood type O who has a positive direct antiglobulin test (DAT, also known as the Coombs test), can be expected to be a strong bilirubin producer but may not necessarily develop hyperbilirubinemia, should the bilirubin conjugation and elimination processes be well functioning. On the other hand, moderate hemolysis coupled with immaturity of UDP-glucuronosyltransferase 1A1 (UGT1A1, the bilirubin conjugating enzyme) as might occur in a late preterm infant, may result in lack of equilibrium between the aforementioned processes with resultant hyperbilirubinemia. A third cause of lack of equilibrium may result from nonfunction of the conjugation system in the absence of any hemolysis, as in Crigler-Najjar syndrome.
This concept has been likened to the filling of a kitchen sink with water. Provided the drainage is functional, an influx of water may not result in the water level increasing. Partial blockage of the drain may lead to a high water level even with a partly opened tap. Kaplan et al demonstrated this concept mathematically by using a production–conjugation index, which illustrates the contribution of the combined forces of bilirubin production and conjugation to the STB at any point in time. The blood carboxyhemoglobin concentration (corrected for inspired CO), an index of heme catabolism, and the serum total conjugated bilirubin (a reflection of intrahepatocytic conjugated bilirubin) have been used as components of this index. A rising index suggests an increasing lack of equilibrium between production and excretion.
It should be obvious that when evaluating a hyperbilirubinemic infant, both etiologic factors contributing to increased bilirubin production and diminished bilirubin conjugation should be taken into consideration. Given the unreliability of hematological indices to reflect hemolysis in the newborn, it may be difficult to distinguish disorders associated with increased production or increased excretion. These processes may include exaggerated heme catabolism (hemolysis), immaturity of UGT1A1, and reabsorption of bilirubin from the bowel to reenter the bloodstream. Immaturity in the enzyme UGT1A1 may be compounded by presence of the (TA) n polymorphism in the promoter of the UGT1A1 gene ( UGT1A1*28 ), resulting in diminished gene expression with decreased enzyme activity (Gilbert syndrome). Poor feeding may result in sluggish peristalsis and bowel stasis with increased reabsorption of bilirubin via the enterohepatic circulation. Factors affecting lack of equilibrium between the processes contributing to the serum total bilirubin are summarized in Table 5.1 .
| Increased hemolysis |
| Immaturity of the bilirubin conjugating enzyme, UDP-glucuronosyltransferase 1A1 (UGT1A1) |
| (TA)n promoter polymorphism of the encoding gene UGT1A1 with resultant diminished gene expression and enzyme activity (associated with Gilbert syndrome in adults) |
| Enterohepatic circulation |
Although, for practical purposes, the STB is used as the tool for the management of neonatal hyperbilirubinemia, including the indications for phototherapy and exchange transfusion, this test is actually not a good predictor of bilirubin-related neurologic outcome. Although it is unlikely that an otherwise healthy term infant with no obvious hemolytic condition will develop bilirubin neurotoxicity at STB levels under 25 mg/dL, there is actually no specific cutoff point at which an STB level will or will not be predictive of neurotoxicity. Certainly not all newborns with extreme hyperbilirubinemia go on to develop choreoathetoid cerebral palsy. For example, in one study of 140 newborns with STB values above 25 mg/dL who were treated with phototherapy or exchange transfusion, overall, 5-year outcomes were not significantly different from those of randomly selected controls. In a reanalysis of data from the Collaborative Perinatal Project, there was no relationship, overall, between maximum STB levels and subsequent IQ scores. However, in both these studies, the presence of a positive DAT resulted in a poorer prognosis. (See section on hemolysis.) Similarly, of 249 newborns admitted to a children’s hospital in Cairo, Egypt, all of whom had STB values 25 mg/dL and above, there was little correlation between admission STB and acute bilirubin encephalopathy. However, in babies with hemolytic risk factors including Rh incompatibility, ABO incompatibility, and sepsis, the threshold STB for identifying babies with bilirubin encephalopathy was lower relative to those without these factors.
Several studies have suggested that the unbound bilirubin fraction may be a more accurate predictor of bilirubin toxicity—including choreoathetoid cerebral palsy and sensorineural hearing loss—than STB, both in term and preterm infants. Use of the unbound fraction as an indication for institution of phototherapy or for performing exchange transfusion would take much of the guesswork out of the decision-making process and permit better identification of the infant at risk for brain damage. Currently, however, unbound bilirubin determinations are in the main unavailable for routine clinical use, and STB remains the cardinal laboratory indication used for clinical decision making in hyperbilirubinemic newborns.
The terms jaundice and hyperbilirubinemia are sometimes, incorrectly, used interchangeably.
Jaundice refers to a yellow coloring of the sclera, skin, and mucous membranes caused by infiltration from the serum of the yellow pigment bilirubin. Hyperbilirubinemia , on the other hand, relates to a measurement of serum or transcutaneous bilirubin, the result of which is greater than an accepted norm.
In infants 35 weeks’ gestation or greater, a useful definition of hyperbilirubinemia is a STB value greater than the 95th percentile for age in hours on the Bhutani et al hour-specific bilirubin nomogram ( Fig. 5.1 ). Use of the nomogram adjusts for the dynamic changes in STB during the first postnatal week and obviates the concept whereby a single STB value is regarded as representative of hyperbilirubinemia. Thus an infant with an STB value of 10.0 mg/dL at 12 hours will be regarded as hyperbilirubinemic, whereas the same concentration 48 hours later will have little significance.
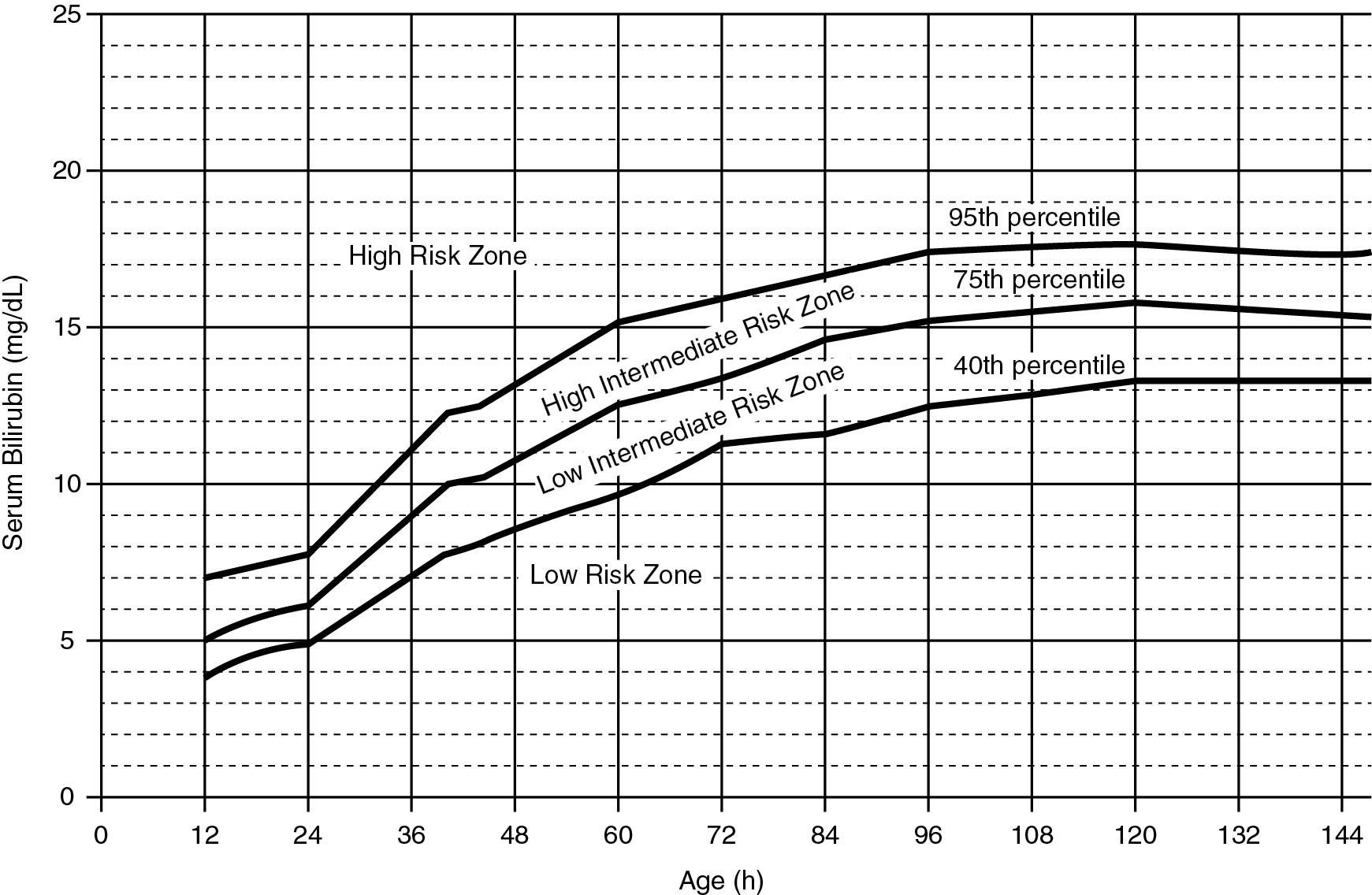
In newborns with lower gestational ages or with risk factors for hyperbilirubinemia, according to the 2004 AAP guidelines, phototherapy may be indicated at levels of STB below the 95th percentile. Thus many newborns receiving treatment may not actually meet these criteria for hyperbilirubinemia. Variations on this definition, to accommodate intervention with phototherapy, include use of an STB value within 1 mg/dL of the indications for phototherapy or an STB value exceeding the 75th percentile on the bilirubin nomogram.
The terms acute bilirubin encephalopathy and kernicterus are often used interchangeably, although the AAP recommends differentiating these two conditions ( ). Acute bilirubin encephalopathy relates to the acute manifestations of bilirubin neurotoxicity seen during or immediately following an episode of extreme hyperbilirubinemia. Permanent features of choreoathetoid cerebral palsy may ensue, but reversal, when appropriately treated, has been reported.
Kernicterus, on the other hand, refers to chronic and permanent sequelae attributable to bilirubin neurotoxicity, the result of bilirubin deposition in the target nuclei of the brain.
An understanding of the basic concepts of bilirubin physiology is necessary for perceptive management of the hyperbilirubinemic newborn. As detailed reviews of this subject are available in standard texts, only an outline will be provided here as a basis for comprehension of the subsequent portions of the chapter. Variations in bilirubin physiology peculiar to the newborn, contributing to the development of hyperbilirubinemia, are interspersed among the descriptions of basic bilirubin physiology.
Most heme is produced by the destruction of red blood cells (RBC) in the reticuloendothelial system, although some is produced from turnover of hemoproteins such as myoglobin. Heme itself is catabolized to biliverdin by the enzyme heme oxygenase 1 and thence to bilirubin. This bilirubin component is termed unconjugated or indirect bilirubin. In newborn infants, the RBC mass is larger, the turnover of the RBC is more rapid, and the cell lifespan is shorter than in adults. There is thus a relatively large heme load that contributes to the bilirubin pool.
To facilitate transportation to the liver, indirect bilirubin is bound to serum albumin. This step is very important in our current understanding of the pathophysiology of bilirubin neurotoxicity. As long as the bilirubin molecule is bound to albumin, it is not expected to cross the blood–brain barrier and to cause bilirubin neurotoxicity. Should the albumin-binding sites be saturated and the bilirubin unable to bind, unbound, or free, bilirubin will result. The unbound bilirubin fraction is thought to be that capable of entering bilirubin-sensitive brain cells and causing neurotoxic damage. Potential causes of unbound bilirubin formation, raising the risk for neurotoxicity, should always be kept in mind when evaluating an infant for hyperbilirubinemia. Some causes potentiating unbound bilirubin formation are listed in Table 5.2 .
| Hypoalbuminemia |
| Excessive hemolysis even in the presence of normal serum albumin concentrations |
| Metabolic acidosis |
| Hypothermia |
| Sepsis |
| Drugs such as sulfa-containing antimicrobials |
| Prematurity (possible) |
Uptake of bilirubin into the liver is controlled by the solute carrier organic anion transporter protein 1B1, SLCO1B1, also known as OATP2. Varying expression of this sinusoidal transporter gene, the result of polymorphisms, may affect bilirubin kinetics and metabolism. For example, the SLCO1B1*1b variant is associated with neonatal hyperbilirubinemia in Taiwanese newborns, especially when coupled with UGT1A1 variants. Similarly, coexpression of SLCO1B1*1b with G6PD A− was associated with hyperbilirubinemia in a study from the United States.
Following uptake into the hepatocyte, indirect bilirubin is conjugated with glucuronic acid to form water soluble mono- and diglucuronides. These complexes are known as conjugated or direct bilirubin. The enzyme controlling the conjugation process is UGT1A1. Immaturity of UGT is an important contributor to hyperbilirubinemia in both term and preterm infants. In term infants, activity of UGT is only about 1% that of adults, and it is even less in preterm infants. Developmental immaturity with slowing of the conjugation process is actually the bottleneck of the neonatal bilirubin elimination process and the reason that the majority of newborns exhibit some degree of visible jaundice during the postnatal period.
There is increasing appreciation that the modulation of serum bilirubin levels and the development of hyperbilirubinemia may be under genetic control. A detailed account of all the genes contributing to bilirubin metabolism is beyond the scope of this text. Because of the practical nature of the enzyme UGT1A1, its genetic control is discussed in some detail.
The enzyme UGT1A1 is encoded by the gene UGT1A1, mapped to chromosome 2q37. This gene contains both a noncoding promoter region and a coding region. Polymorphisms of the promoter region, such as the (TA) n polymorphism, result in diminished expression of a normally formed enzyme and are associated with Gilbert syndrome. On the other hand, coding area mutations as seen in Crigler-Najjar syndrome result in an abnormally structured enzyme that has no or little conjugating ability. Coexpression of genes, presence of several mutations or polymorphisms, and interactions with environmental factors may potentiate the genetic contribution to the pathophysiology of neonatal hyperbilirubinemia. A paradigm of this concept may be found in the pathophysiology of neonatal hyperbilirubinemia in glucose-6-phosphate dehydrogenase (G6PD) deficient neonates, in which interaction between environmental factors (triggering hemolysis), the G6PD deficiency in and of itself, and (TA) n promoter polymorphisms of UGT1A1 (UGT1A1*28) may potentiate severe hyperbilirubinemia.
Direct bilirubin is secreted into the bile and then to the bowel from which it is excreted in the stool. The presence of the enzyme beta-glucuronidase in the colon deconjugates bilirubin-glucuronides and allows the reabsorption of bilirubin into the bloodstream, thereby adding to the bilirubin pool. A delay in enteral feeding or poor intake may diminish intestinal motility. The resultant increased bowel stasis with decreased elimination will allow for even greater reabsorption of bilirubin.
Given the universal immaturity of the enzyme UGT1A1, it is fair to suggest that almost all newborns have suboptimal bilirubin conjugation. Taking the bilirubin production–conjugation equilibrium into account, it stands to reason that hemolysis must therefore be a cardinal factor in the pathogenesis of hyperbilirubinemia in many newborns.
Baby AB was born at term gestation to a blood group O, Rh-negative mother. On admission to the nursery, the nurses thought that the baby’s skin had a yellow tinge. The physician believed this was only very mild jaundice and chose to ignore it. An astute nurse, however, took an STB at age 12 hours, the result of which was 9.2 mg/dL. “Not very high,” responded the physician. By the next day (28 hours) the STB value was 15 mg/dL.
What would you do?
Observe the baby and repeat the STB in another 24 hours
Place the infant under intense phototherapy and repeat the STB in 4 to 6 hours
Begin phototherapy and proceed to exchange transfusion
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here