Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Highlighting the importance of understanding physiology prior to prescribing medications will enable the provider to choose the preferred regimen for each particular patient.
Extrapolating data from older pediatric populations and adults must be done with caution, considering the unique physiology and differences in pharmacokinetics and pharmacodynamics in critically ill neonates and infants.
Understanding the mechanism of action and therapeutic effects (both desired and side effects) will enable the provider to better anticipate changes to be seen and monitor effects.
There are a number of cardiovascular medications that are used in neonatology on a regular basis. An understanding of physiology can guide the clinician toward the selection of a specific medication based on mechanism of action that is necessary to manage the patient-specific disease state. Each medication will have a range of side effects that should be considered when choosing a medication therapy regimen, and even more so when utilizing this class of medications in critically ill neonates. Extrapolating data from older pediatric or adult patients must be done with caution considering the unique physiology of critically ill neonates. For example, the premature neonatal heart contains only 30% contractile tissue, in contrast to 60% in the adult heart. Additionally, expression of sarcoplasmic reticulum and t-tubules is low, while mitochondria are abundant, resulting in disorganized myocyte activity. These maturational differences are tolerated in the intrauterine environment dominated by low placental resistance. However, with exposure to ex utero systemic vascular resistance, the stress of illness, and inotropes or vasopressors, cardiovascular function may be impaired.
The adrenergic system and myocardial innervation both mature throughout gestation. This maturation is driven to some extent by stimulation, and fetal adrenoreceptors have a low threshold for provocation. In preterm neonates this manifests as “denervation hypersensitivity,” in which myocardial adrenoreceptors demonstrate maximal response to even small concentrations of catecholamine. Importantly, maturation is not uniform, as active alpha-1-receptors outnumber beta-1-receptors in early gestation. Consequently, agents with non-specific activity like dopamine have a different dose-response profile in premature neonates, term neonates, and older patients. Critical illness further complicates dose-response. Renal maldevelopment or maladaptation has implications on drug elimination with lower albumin binding leading to increased drug available for metabolism in the setting of less efficient renal clearance of active drug and metabolites. Hypoxic-ischemic encephalopathy complicates the consequences of bradycardia (induced by therapeutic hypothermia or medications like dexmedetomidine in the setting of existing cardiac dysfunction induced by hypoxia-ischemia) and tachycardia (induced by exogenous catecholamines in the setting of metabolic insufficiency and/or hypocalcemia compromising right ventricular performance). Additionally, decreased renal clearance in this population may exacerbate the therapeutic or adverse effects of pharmacologic interventions. In confluence, this complex milieu highlights the vital nature of optimizing diagnostic techniques and understanding the specific impacts of available pharmacotherapies.
This chapter will discuss the mechanism of action and therapeutic effects of cardiovascular medications used most commonly in critically ill neonates and infants based on predominant pathology grouping ( Tables 5.1 and 5.2 ). We will not be discussing medication regimens for treatment of a hemodynamically significant patent ductus arteriosus (PDA), as these are discussed in detail in Chapter 17 . Critical evaluation of available pharmacologic studies and randomized controlled trials was vital to generation of the content of this chapter. However, this evaluation highlighted the profound limitations of the available data. Therefore the information in this chapter should be interpreted in the context of emerging evidence.
| CARDIOVASCULAR SUPPORT OF HEART FUNCTION | |||||
|---|---|---|---|---|---|
| Receptors/Action | Predominant Effects | Dosing Range | Common Clinical Use | Comments | |
| Predominant Inotropes | |||||
|
|
|
2–20 mcg/kg/min | RV dysfunction in PH or HIE, cardiac dysfunction in premature infants | Improvement seen in premature infants at doses limited to 5–10 mcg/kg/min |
|
|
|
0.01–0.5 mcg/kg/min | Septic shock with cardiac dysfunction, severe cardiac dysfunction | Avoid in patients with hypertrophic cardiomyopathy |
| Inotropes With Vasodilator Effects | |||||
|
PDE-3 inhibitor | ↓ SVR, PVR, inotropy, and lusitropy | 0.25–1 mcg/kg/min | Low cardiac output syndrome after cardiac surgery, PLCS, PH, CDH | Caution in poor renal function or decreased renal clearance |
|
PDE-3 inhibitor | ↓ SVR, PVR, inotropy | 0.05–0.2 mcg/kg/min | Patients requiring afterload reduction and inotropy | Not available in United States or Canada |
| Primary Vasodilator | |||||
|
Arterial and venous vasodilator |
|
0.5–2 mcg/kg/min | Hypertensive crisis | Close monitoring needed to avoid severe hypotension and hypoperfusion |
| CARDIOVASCULAR SUPPORT FOR ACUTE AND/OR CHRONIC PULMONARY HYPERTENSION | |||||
| Pulmonary Vasodilator | |||||
|
Selective pulmonary vasodilator | ↓ PVR | 1–20 ppm | Acute and chronic PH | Should be avoided in left-sided outflow tract obstruction and increased PCWP |
| Phosphodiesterase Inhibitor | |||||
|
PDE-5 inhibitor |
|
|
Chronic and occasionally acute PH | Could contribute to worsened V:Q mismatch |
| Endothelin Receptor Antagonist | |||||
|
ET A and ET B blocker | ↓ PVR | 1–2 mg/kg BID | Chronic PH | Liver enzymes need to be followed closely |
| Prostacyclin Analogues | |||||
|
Pulmonary and systemic vasodilator | ↓ PVR, SVR |
|
Chronic PH and elevated PVR | Abrupt discontinuation results in rebound PH |
|
Analogue of epoprostenol | ↓ PVR, SVR | IV or subcutaneous infusion 2–20 ng/kg/min | Chronic PH | Optimal for prolonged subcutaneous infusion due to stability in solution and relatively long half-life |
|
Analogue of epoprostenol | ↓ PVR, SVR |
|
Chronic and occasionally acute PH | Less basic pH compared to epoprostenol facilitates nebulized delivery; however, data limited in neonates |
| Prostaglandin E1 | |||||
|
Vasodilation of vascular and PDA smooth muscle |
|
0.01–0.1 mcg/kg/min | CHD, PH with failing RV, acute PH | Apnea seen more often in <2 kg BW, higher starting doses, and during the first hour of infusion |
| Soluble Guanylate Cyclase Stimulators | |||||
|
sGC stimulation | ↓ PVR | 0.5–2 mg up to TID | Chronic PH | Should not be used within 24 hours of sildenafil |
| SUPPORT FOR SYSTEMIC HYPOTENSION | |||||
|---|---|---|---|---|---|
| Receptors/Action | Predominant Effects | Dosing Range | Common Clinical Use | Comments | |
| Predominant Vasoconstrictors | |||||
|
Arginine vasopressin, oxytocin, and purinergic receptors |
|
0.1–1.2 mU/kg/min (0.006–0.072 U/kg/h) | Vasodilatory shock, PH with preserved LV function, hypotension, hypertrophic cardiomyopathy | Sodium levels must be monitored during use, prolonged therapy may result in polyuria and fluid imbalance upon discontinuation |
|
Alpha-1, alpha-2, beta-1> beta-2 |
|
0.02–1 mcg/kg/min | Sepsis and shock | In presence of high oxygen concentrations, can induce pulmonary vasoconstriction |
|
CNS and peripheral dopaminergic, adrenergic (alpha-1, beta-1, beta-2), and serotonergic receptors |
|
2–20 mcg/kg/min | Sepsis in setting of no PH | Should be used with caution in premature infants due to the unpredictable nature of its effects |
|
Alpha-agonist | ↑ SVR | 0.1–0.5 mcg/kg/min | “Tet spells” | May cause severe bradycardia |
| Glucocorticoids | |||||
|
Genomic and non-genomic effects | Variable |
|
Adjunctive therapy for relative or absolute adrenal insufficiency and pressor-resistant hypotension when increased cell surface receptors required (e.g., adrenergic receptors) | Concurrent use with indomethacin should be avoided |
| SUPPORT FOR SYSTEMIC HYPERTENSION | |||||
| Dihydropyridine | |||||
|
CCB | ↓ SVR | 0.05–0.15 mg/kg QID | Acute hypertension | Rapid onset of action |
|
CCB | ↓ SVR | 0.05–0.3 mg/kg 1–2 times daily | Chronic hypertension | Slower onset of action and longer duration of effect |
| Angiotensin-Converting Enzyme (ACE) Inhibitors | |||||
|
Competitive inhibitor of ACE | ↓ SVR |
|
Hypertension, LV diastolic dysfunction, congestive heart failure | Use with caution in patients with impaired renal function or hypovolemia |
When attempting to support heart function, a combination of inotropes and vasodilators may be utilized. Inotropes are medications that act primarily on the cardiac myocyte ( Figure 5.1 ) to increase contractility, whereas vasodilators decrease wall tension leading to an improvement in stroke volume and cardiac output. In this section we will discuss two predominant inotropes, dobutamine and epinephrine; two inotropes with vasodilator effects, milrinone and levosimendan; and sodium nitroprusside, a primary vasodilator.
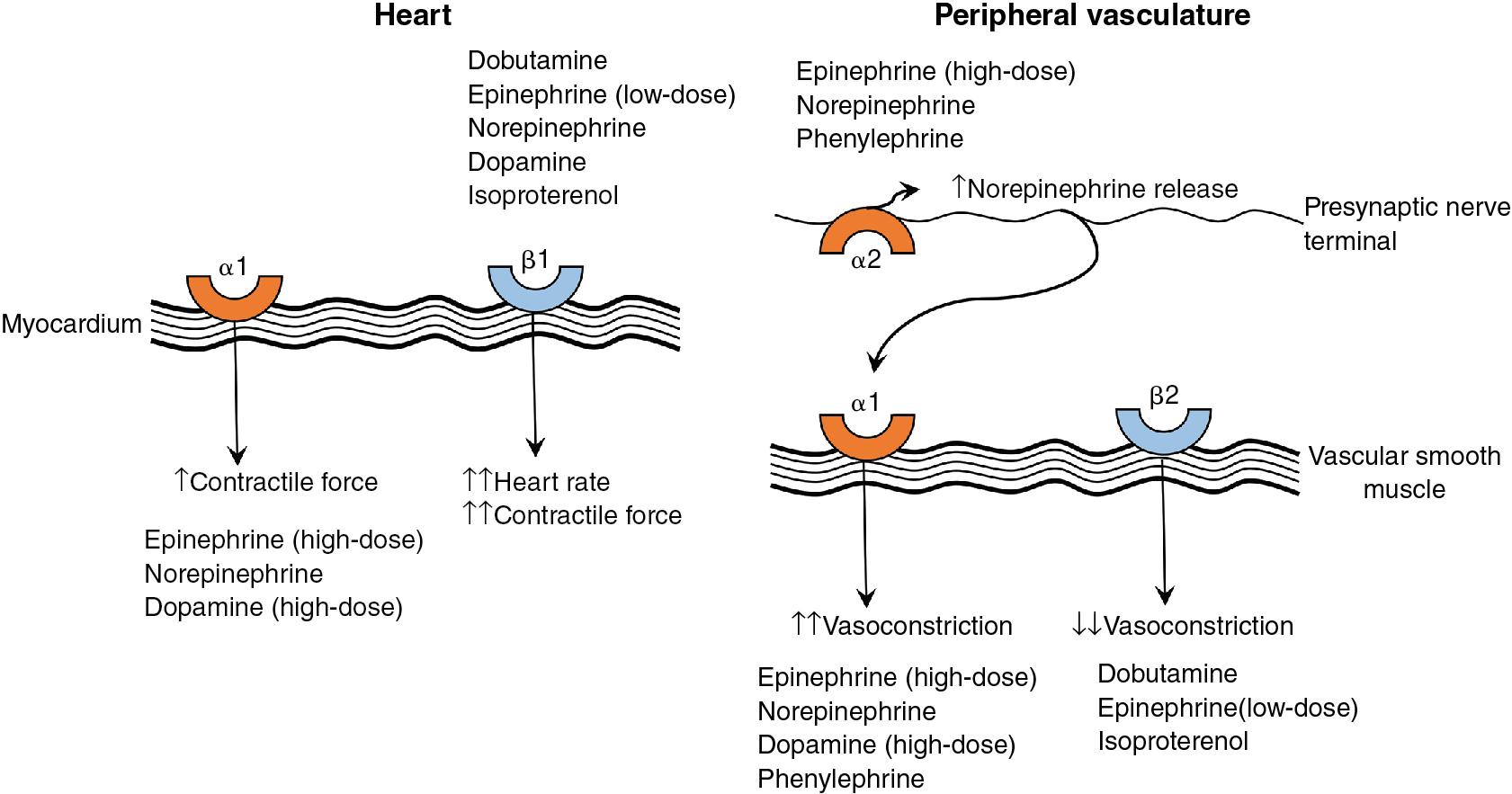
Mechanism of action: Dobutamine is a synthetic catecholamine that is a racemic mixture of two parts (negative and positive isomers) and promotes direct stimulation of adrenergic receptors in the myocardium. The negative isomer is an alpha-1-receptor agonist and increases systemic vascular resistance (SVR) and myocardial contractility. The positive isomer is a beta-1- and beta-2-receptor agonist, which increases myocardial contractility and heart rate while decreasing SVR. It is also a potent alpha-1-receptor antagonist that blocks the effects of the negative isomer. The resulting effects of dobutamine are increased inotropy and chronotropy, with no effect on, or a decrease in, SVR. ,
Dosing is via continuous infusion; the usual dosing range is 2–20 mcg/kg/min. Improved left ventricular (LV) performance in preterm infants is achieved at doses of 5–10 mcg/kg/min with changes in cardiac output within 20 minutes and improvement in systemic markers after 8–10 hours.
Pharmacokinetics: Dobutamine is metabolized by the liver and excreted in the urine. The half-life is 3–36 minutes in preterm neonates.
Examples of clinical use: Right ventricular (RV) dysfunction in the setting of pulmonary hypertension (PH) or hypoxic-ischemic encephalopathy, LV dysfunction following interventional closure of PDA, myocarditis, or asphyxia.
Adverse effects include tachycardia, arrhythmias, hypertension, and hypotension. ,
Special considerations: Expression of α-adrenergic receptors is upregulated in preterm infants as compared to β-adrenergic expression, which may result in attenuated decreases in SVR when compared to term infants. Dobutamine has been shown to improve superior vena cava flow in preterm neonates when compared to dopamine.
Mechanism of action: Epinephrine is an endogenous hormone secreted by the adrenal medulla. It is a potent stimulator of both α- and β-adrenergic receptors and its effects on body organ systems are expressed in a dose-dependent manner. The cardiac and vascular beta-1- and beta-2-adrenoreceptors are primarily stimulated at low doses (0.01–0.1 mcg/kg/min), which leads to increased inotropy, chronotropy, and conduction velocity and peripheral vasodilation (primarily in the muscles). Doses >0.1 mcg/kg/min stimulate vascular and cardiac alpha-1-receptors leading to vasoconstriction and increased inotropy, while the alpha-2-receptor effects are less prominent. Epinephrine has been shown to increase coronary artery blood flow, , cerebral perfusion, and SVR and pulmonary vascular resistance (PVR) in a 1:1 ratio or less with some dose-dependent variability. However, it has also been shown in high doses to irreversibly disrupt the myocardial sarcolemma and swell the mitochondria with calcium deposition in a neonatal animal model.
Dosing is via continuous infusion with a usual dosing range of 0.01–0.5 mcg/kg/min. ,
Pharmacokinetics: Epinephrine is metabolized in the liver and excreted in the urine as inactivated compound. Data characterizing the pharmacokinetics of epinephrine in neonates are limited; the half-life in adults is less than 5 minutes.
Examples of clinical use: Septic shock with cardiac dysfunction and diseases associated with severe cardiac dysfunction.
Adverse effects include tachycardia, lactic acidosis independent of improvement in hemodynamic status, and hyperglycemia requiring insulin seen in premature infants. ,
Special considerations: In patients with acute PH caution is advised due to the potential increase in PVR and pulmonary artery pressure seen in animal studies. Epinephrine, as is true with all drugs with β-adrenergic activity and chronotropic effects, should be avoided in patients with hypertrophic cardiomyopathy.
Mechanism of action: Milrinone is a selective phosphodiesterase-3 (PDE-3) inhibitor that inhibits the degradation of cyclic adenosine monophosphate (cAMP). The increased concentration of cAMP leads to the multiple cardiovascular effects of milrinone, including vasodilation in the systemic and pulmonary vascular beds, inotropy through activation of contractile proteins, and a lusitropic effect by prolonging the relaxation period of the cardiac cycle.
Dosing is via continuous infusion, with the usual dosing range being 0.25–1 mcg/kg/min. Loading doses ranging from 25 to 75 mcg/kg have been described in the literature but have been associated with hypotension, with some recommending avoidance. A normal saline bolus of 10–15 mL/kg administered during the first hour of infusion may assist in the prevention of low diastolic blood pressure (BP) due to vasodilation.
Pharmacokinetics: Most of the circulating milrinone is not metabolized and is excreted unchanged in the urine. The mean half-life is approximately 4 hours in term neonates and 10 hours in preterm neonates. ,
Examples of clinical use: Milrinone has been shown to improve hemodynamics in low cardiac output syndrome after cardiac surgery, post-ligation cardiac syndrome, PH, , and congenital diaphragmatic hernia.
Adverse effects include hypotension and arrhythmias; less commonly seen are thrombocytopenia, hypokalemia, and other laboratory value changes.
Special considerations: Caution is advised in therapeutic situations associated with poor renal function or decreased renal clearance of medications. Patients with hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia have been shown to exhibit profound hypotension with milrinone administration, likely due to vasodilation after accumulation from poor excretion. Clearance will vary based on gestational and chronological age. , Additionally, it has been postulated in an animal model that PDE-4 is the predominant enzyme in fetal life leading to a potential delay in the inotropic effect for the first several days. ,
Mechanism of action: While levosimendan has not been approved for use in the United States or Canada, it is used in over 60 countries, including many in the European Union and Latin America. It enhances myocardial contractility by binding to troponin C, a cytosolic calcium-dependent interaction, thereby also improving LV diastolic function without promoting arrhythmogenesis or altering myocardial oxygen demand. , Levosimendan also inhibits cardiac PDE-3 at higher doses , and promotes vasodilation by activation of adenosine triphosphate (ATP)–sensitive potassium channels. , Affected vasculatures include coronary, pulmonary, renal, splanchnic, cerebral, and systemic arteries as well as saphenous, portal, and systemic veins.
Dosing: More studies are required to establish a safe dosing range in neonates and premature infants. Reported dosing range for continuous infusion is 0.05–0.2 mcg/kg/min with variable use of loading doses.
Pharmacokinetics: The metabolism of levosimendan results in active metabolites that are likely responsible for prolonged hemodynamic effects after discontinuation of continuous infusion. These metabolites are excreted in the feces and urine. Pharmacokinetic data on its use in neonates are not available; in adults the half-life of the parent drug is approximately 1 hour, while the active metabolite has a prolonged elimination half-life of 70–80 hours.
Examples of clinical use: Since the hemodynamic profile is similar to milrinone, levosimendan may be beneficial in patients requiring afterload reduction and inotropy (e.g., post-ligation cardiac syndrome).
Adverse effects include, as with any medications that cause vasodilation, hypotension. Further studies in neonates and infants are needed to delineate other potential adverse effects.
Special considerations: More data on its use in neonates and infants are required to evaluate potential effects and associations with clinical procedures (e.g., therapeutic hypothermia) and diagnoses (e.g., renal failure).
Mechanism of action: Sodium nitroprusside is a non-specific vasodilator (arterial and venous) with no direct effect on myocardial contractility or heart rate. It functions as a prodrug, reacting with sulfhydryl groups on erythrocytes, albumin, and other proteins to release nitric oxide (NO), which activates guanylate cyclase stimulating production of cyclic guanosine monophosphate (cGMP). Its main hemodynamic effect is reducing SVR, resulting in decreased afterload. The vasodilator properties lead to decreased ventricular filling pressures, reduced wall stress, and lower myocardial oxygen demand. Together, these result in increased cardiac output and systemic tissue oxygenation as long as coronary perfusion pressure is adequate.
Dosing is via continuous infusion, with a usual dosing range of 0.5–2 mcg/kg/min, though maximum dosing as high as 10 mcg/kg/min has been reported for control of hypertensive emergencies. Doses higher than 1.8 mcg/kg/min and/or prolonged therapy have been associated with elevated cyanide levels. ,
Pharmacokinetics: Sodium nitroprusside combines with hemoglobin, forming cyanide and cyanmethemoglobin. This process is rapid, with a half-life of approximately 2 minutes. Cyanide is then converted to thiocyanate in the liver and kidney, a process with limited capacity which can be overwhelmed in high doses, resulting in toxicity. Sodium nitroprusside is excreted in the urine as thiocyanate. The half-life of thiocyanate is approximately 3 days in adults with normal renal function.
Examples of clinical use: Hypertensive crisis (e.g., paradoxical hypertension following correction of coarctation of the aorta).
Adverse effects include cyanide toxicity (especially in those who have hypoalbuminemia and hepatic impairment and those undergoing cardiopulmonary bypass or therapeutic hypothermia), hypotension, increased intracranial pressure, and methemoglobinemia.
Special considerations: Upon initiation of sodium nitroprusside, it is important to maintain close monitoring to avoid life-threatening hypotension and hypoperfusion, especially in populations at increased risk, such as premature infants.
The major goal of medication therapy in PH in the acute setting is to improve pulmonary blood flow, and, more often than not, fast-acting medications are utilized first-line (e.g., pulmonary vasodilator). In addition, there are likely to be secondary beneficial effects to RV function and cardiac output. In the chronic setting , while fast-acting agents may be utilized initially, more often than not, chronic medication therapy is required to affect pulmonary pressures. In both cases, evaluation, and support, when decreased, of both RV and LV heart function is still required.
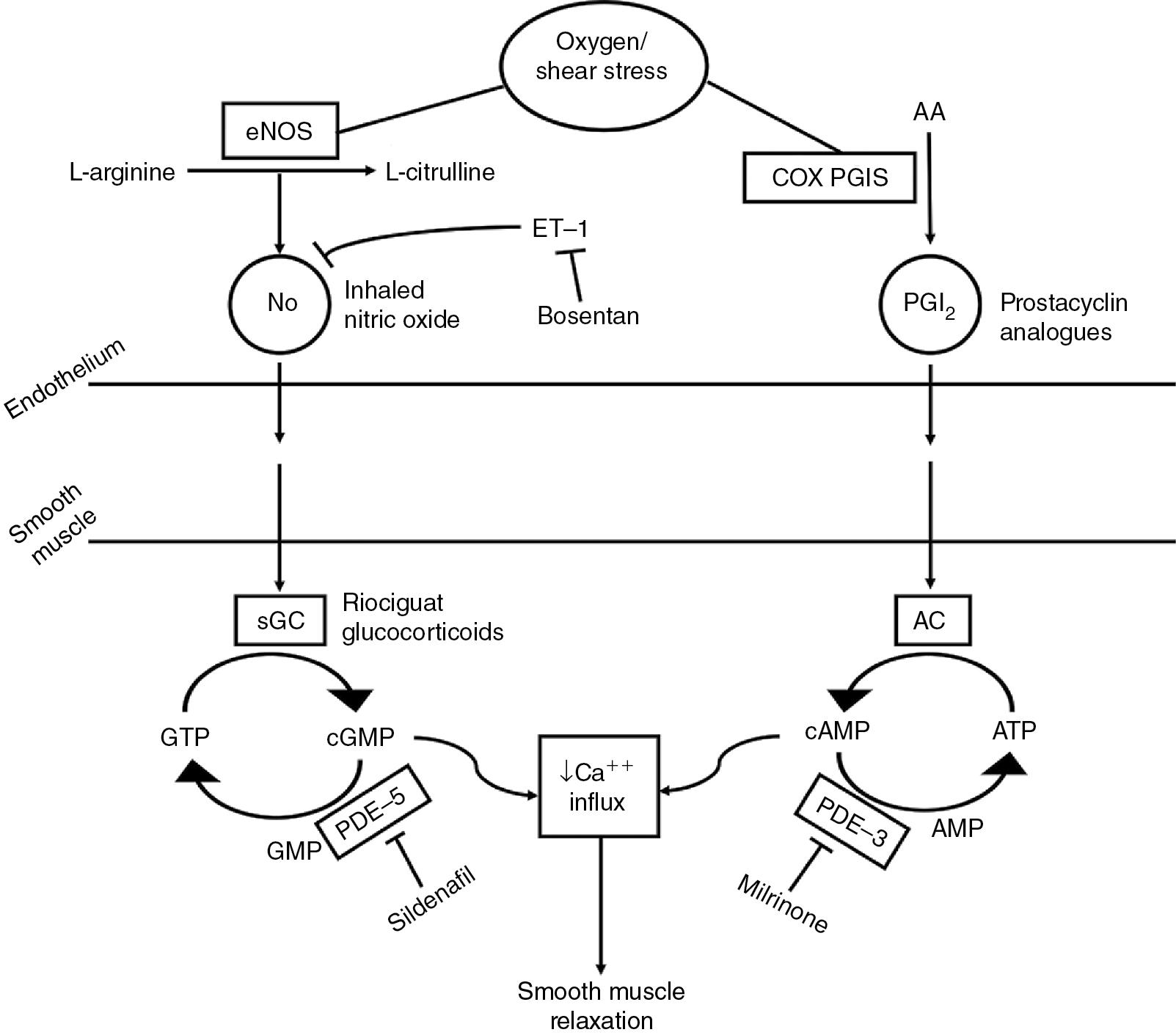
Mechanism of action: Inhaled nitric oxide (iNO) acts as a selective pulmonary vasodilator that relaxes vascular smooth muscle by binding to the heme moiety of guanylate cyclase, which leads to its activation and increased levels of cGMP. It improves oxygenation via reduction of PVR, improved ventilation/perfusion matching, and decreased right to left extrapulmonary shunting. These actions together improve pulmonary blood flow, which leads to improved pulmonary venous return and LV output.
Dosing is via inhalation, with a usual dosing range of 1–20 ppm, with starting doses ranging from 5 to 20 ppm. Doses >20 ppm are not recommended due to increased risk of methemoglobinemia and pulmonary injury from elevated nitrogen dioxide.
Pharmacokinetics: iNO combines with oxyhemoglobin to produce methemoglobin and nitrate. The half-life of NO is 2–6 seconds in neonates. Nitrate is excreted in the urine.
Examples of clinical use: Acute or chronic PH.
Adverse effects include methemoglobinemia, which should be measured within 4–8 hours of initiation and periodically throughout administration, inflammation and lung tissue injury caused by nitrogen dioxide, and possible decreased platelet aggregation without increased bleeding time. In the presence of high concentrations of oxygen, it is possible that iNO may react with reactive oxygen species to form reactive nitrogen species (e.g., peroxynitrite) that can lead to cytotoxicity. Use in patients with LV systolic or diastolic dysfunction may increase pulmonary capillary pressure resulting in pulmonary edema. iNO should be avoided in patients with left-sided outflow tract obstruction (e.g., hypoplastic left heart syndrome) or those with excessive pulmonary blood flow (e.g., anomalous pulmonary venous return or vein stenosis, PDA).
Special considerations: Echocardiography, prior to iNO use, should be considered to confirm features of PH (especially in premature infants), to determine RV and LV function, and to establish normal cardiac anatomy. Abrupt discontinuation of iNO may lead to worsening of oxygenation and/or rebound pulmonary hypertension; weaning is recommended. ,
Mechanism of action: Sildenafil is a selective PDE-5 inhibitor in the smooth muscle of the pulmonary vasculature, which promotes increased cGMP and pulmonary vasculature relaxation. While sildenafil is more selective for the pulmonary vasculature, both pulmonary and systemic vasodilation may occur.
Dosing may be via intravenous (IV less commonly) or oral route. Recommended dosing for continuous infusion is 0.4 mg/kg load over 3 hours followed by 1.6 mg/kg/day. , IV intermittent dosing is 0.4 mg/kg given over 1–3 hours every 6 hours. Oral dosing in neonatal case series of acute PH ranges from 0.5 to 3 mg/kg/dose q 6–8 hours. , , Chronic oral therapy is typically limited to a maximum dose of 2 mg/kg/dose, or 10 mg, orally every 8 hours.
Pharmacokinetics: Sildenafil is metabolized in the liver by CYP3A4 (major) and CYP2C9 (minor) forming a major metabolite (desmethylsildenafil), which retains 50% of the activity of sildenafil. Elimination increases with increasing postmenstrual age (half-life of the parent compound ∼56 hours in term newborns with maturation to ∼48 hours by week 1 of age). Excretion is mainly as metabolites via feces and some urine. Clearance may be decreased in patients with hepatic or renal impairment.
Examples of clinical use: Chronic and, less frequently, acute PH.
Adverse effects include hypotension and flushing. Due to risk of severe hypotension, concurrent use of riociguat (a soluble guanylate cyclase stimulator) is contraindicated and should not be given less than 24 hours apart. Caution is required with other vasodilators.
Special considerations: Pulmonary vasodilatory effects are not confined to well-ventilated portions of the lung, as is the case with iNO, which could contribute to worsened V:Q (ventilation:perfusion) mismatch.
Mechanism of action: Bosentan blocks endothelin (ET) receptors, which induce vasoconstriction, on the endothelium and in vascular smooth muscle. While it blocks both ET A and ET B receptors, it has a slightly higher affinity for the A subtype receptor.
Dosing is via the oral route with an initial dose of 1 mg/kg/dose twice daily, which may be increased to 2 mg/kg/dose twice daily if necessary. ,
Pharmacokinetics: Bosentan is metabolized in the liver via CYP2C9 and CYP3A4 to one active and two inactive metabolites. Bosentan also induces both enzymes. The maximum concentration occurs at a median of 12 hours after administration of initial doses but this time decreases to a median of 7.5 hours by day 5 of therapy. It is mostly excreted in the feces as metabolites, with a small amount excreted in the urine as unchanged drug.
Examples of clinical use: Chronic PH.
Adverse effects include hepatotoxicity and anemia requiring close monitoring of liver enzymes (baseline, then monthly) and hemoglobin (baseline, 1 and 3 months, then every 3 months).
Special considerations: Bosentan is only available in the United States through a Risk Evaluation and Mitigation Strategy (REMS) program. It has been classified as a hazardous substance due to its associated risk of birth defects. Single gloving is recommended for administration of intact tablets or capsules. Double gloving, a protective gown, and eye/face protection are recommended for manipulating tablets and administration of oral suspension to neonates and infants. A suspension recipe has been shown to be stable for up to 1 month. Bosentan was shown to rapidly improve oxygenation index in the absence of iNO but was shown to have limited acute efficacy as an adjunct to iNO.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here