Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
You are taking care of a 4-day-old male infant, born at 32 weeks’ gestation and weighing 1750 g. He was recently transitioned from mechanical ventilation to nasal cannula. He was doing well until this morning, when you noticed a few drops in his heart rate that were accompanied by desaturations. When you spoke to the nurse, she said he had three episodes like these the day before that self-resolved, but he had already had two this morning that required some tactile stimulation.
What physiologic mechanisms put this infant at risk for apnea of prematurity?
What mode of noninvasive respiratory support could be beneficial for this patient?
One of the definitions of apnea of prematurity is a cessation of breathing that lasts more than 20 seconds or a short pause in breathing that is accompanied by desaturation (Sp o 2 <80%) and/or bradycardia (HR ≤80 bpm) in infants that are less than 37 weeks’ gestation ( ). This is different from periodic breathing, which is also commonly seen in premature infants, and is characterized by brief and repetitive pauses lasting 5 to 10 seconds that may be accompanied by mild desaturations and bradycardia but do not require intervention.
Apnea of prematurity is a developmental disorder caused by physiologic immaturity of respiratory control and is inversely proportional to gestational age, being present in almost 100% of infants less than 29 weeks’ gestational age ( ). There are two important physiologic mechanisms implicated in apnea of prematurity: immature respiratory control and failure to maintain upper airway patency.
The immaturity of respiratory control is manifested by impaired ventilatory responses to hypoxia and hypercapnia and an exaggerated inhibitory response to stimulation of the upper airway receptors. Hypercapnia is the major chemical stimulant of breathing and is sensed primarily centrally in the brainstem. Preterm infants have decreased response to carbon dioxide (CO 2 ), hence increased ventilation is not triggered until higher levels of CO 2 are achieved. Also, in preterm infants hypoxia appears to depress the response to CO 2 ( ). Another physiologic mechanism related to apnea of prematurity is the CO 2 apneic threshold, which is the minimal P co 2 required to trigger respiration. In preterm infants the apneic threshold seems to be very close to the eupneic threshold, approximately 1.5 mm Hg ( ).
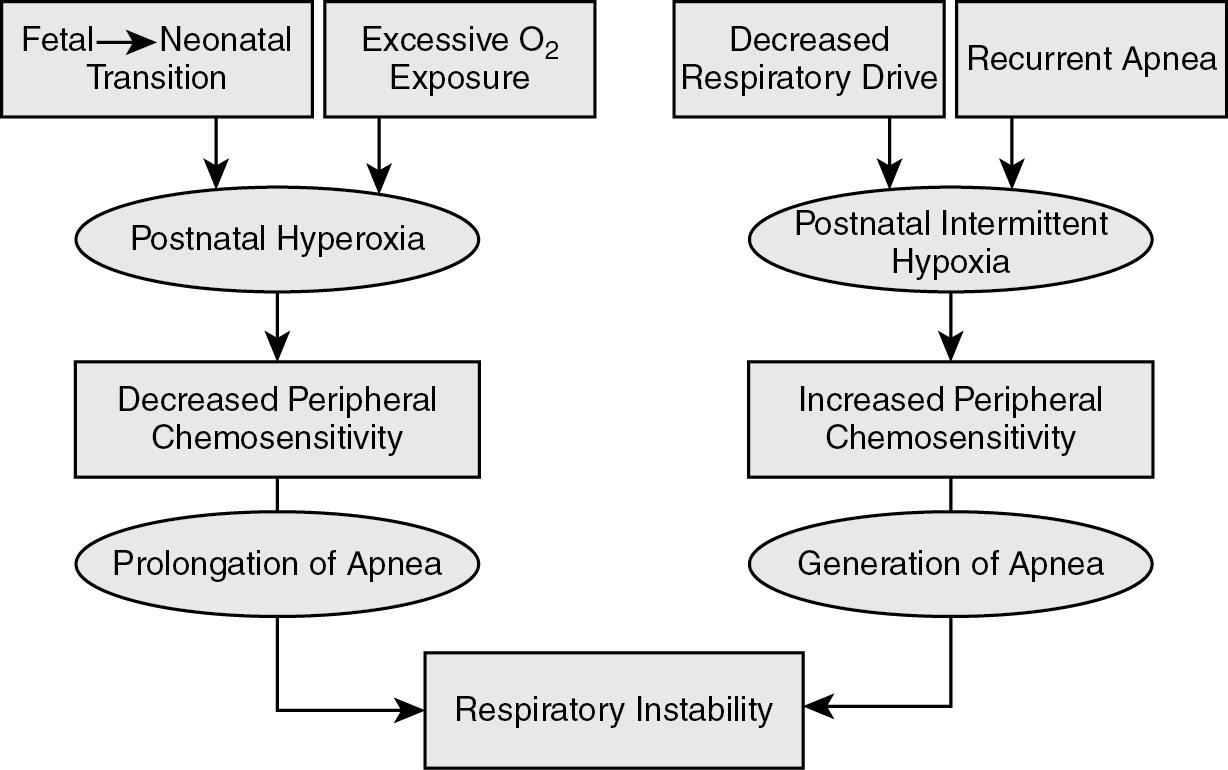
Chemosensitivity to hypoxia is sensed peripherally, and both enhanced and reduced peripheral chemoreceptor function of the carotid bodies may predispose to apnea, bradycardia, and desaturations in the preterm infants. The oxygen (O 2 ) sensitivity of the carotid body chemoreceptors in utero is adapted to a low Pa o 2 of approximately 25 mm Hg; after birth there is a fourfold increase in Pa o 2 that silences these peripheral chemoreceptors. This is followed by a gradual increase in hypoxic chemosensitivity. Exaggerated peripheral chemoreceptor stimulation may be caused by repeated hypoxic episodes and has been associated with periodic breathing. Such enhanced peripheral chemosensitivity may destabilize breathing and result in respiratory pauses secondary to hypocapnia seen after hyperventilation. Thus diminished peripheral chemosensitivity may prolong apnea while increased peripheral sensitivity may precipitate apnea ( Fig. 12.1 ).
Stimulation of the laryngeal chemoreflexes (LCRs) by physical contact of the laryngeal mucosa can lead to apnea, bradycardia, desaturations, and hypotension, by stimulation of inhibitory airway receptors. This can be observed when deep suction is attempted during resuscitation, which can lead to bradycardia and consequently apnea. The LCR is a protective reflex inhibiting inspiration in order to avoid aspiration and will later develop into cough and swallow reflexes in more mature infants. Maintenance of functional residual capacity (FRC) is important for better oxygenation and in decreasing the degree of desaturations during brief episodes of apnea. Premature infants have difficulty maintaining their FRC because of a high compliant chest wall.
Apnea of prematurity is classified into three different types: central, obstructive, and mixed, the latter being the most common. Central apnea is the total cessation of respiratory effort with a patent airway. In obstructive apnea there is respiratory effort and chest wall motion, but no nasal airflow due to an obstructed pharyngeal airway. Mixed apnea consists of respiratory efforts against an obstructed upper airway preceded or followed by central apnea.
Failure to maintain airway patency is caused by poor hypopharyngeal muscle tone leading to collapse of the airway, contribution of the inhibitory airway reflexes (LCR, mentioned earlier), and nasal obstruction and pharyngeal edema. The hypopharynx is a common site of upper airway obstruction because of its poor muscle tone, especially if the infant’s neck is flexed. The larynx and trachea are more rigid structures and are less likely to contribute to the upper airway obstruction, although this can be observed when there is vocal cord dysfunction, laryngeal edema or stenosis, laryngomalacia, or tracheomalacia. The newborn trachea and larynx are relatively superiorly positioned, resulting in close proximity between the epiglottis and the soft palate, facilitating sucking. This anatomy also confers a strong preference toward nasal breathing in infants. Hence, nasal swelling can also cause obstruction of the upper airway. This can be a result of prolonged use of nasogastric tubes or nasal prongs for certain respiratory support devices, as well as constant nasal suctioning.
Continuous positive airway pressure (CPAP) may benefit this patient because most of the longer apneas observed at less than 28 weeks of age are mixed apneas, and CPAP helps by splinting the upper airway, therefore decreasing upper airway closure and/or obstruction by mechanically dilating the supraglottic airway and lowering resistance in both inspiration and expiration ( ). CPAP also increases FRC, improves oxygenation, decreases respiratory frequency, and reduces the work of breathing. These effects not only help prevent apnea of prematurity but may also decrease the need for intubation.
Studies have been performed comparing noninvasive nasal intermittent positive pressure ventilation (NIPPV) and nasal CPAP (NCPAP) for management of apnea of prematurity. Some of these studies showed a reduction of apneic episodes utilizing NIPPV ( ), whereas others showed CPAP superiority in preventing apnea ( ). At this time there is no consensus regarding the potential benefits of one mode over the other for the treatment of apnea of prematurity.
High-flow nasal cannula (HFNC), defined as flow greater than 2 l/min, has also been used as an alternative to CPAP to treat apnea of prematurity. Its benefits are ease of administration, increased ability for parents to hold and bond with their infants, and reduction in nasal damage as shown by previous studies ( ). These benefits must be weighed against the uncertainty of how much pressure is being delivered by the cannula ( ).
Recent publication of a small study seems to indicate that flow-synchronized NIPPV (SNIPPV) reduces the incidence of apneic episodes in preterm infants, <28 weeks compared with NCPAP and NIPPV ( ). The synchronization with patients’ breaths allows mechanical breaths to be delivered when the glottis is open, perhaps providing improved transmission of these breaths, ventilation, and maintenance of FRC.
Noninvasive neutrally adjusted ventilator assist (NIV-NAVA), another synchronized modality of assisted ventilation that has been used for ventilatory support of preterm infants, has shown reduction in the frequency and severity of desaturation episodes ( ). Perhaps this can be extrapolated to more oxygenation stability and fewer apneic episodes, but more studies are needed in this field.
The neonatal intensive care unit at Rainbow Babies & Children’s Hospital uses NCPAP with pressures between 3 and 6 mm Hg for prevention of apnea of prematurity and low-flow nasal cannula (<2 l/min) with great success. NIPPV and HFNC (heated and humidified) are used mostly for respiratory support. The suggested utilization of HFNC in our unit is for infants older than 2 weeks of age, greater than 2 kg, and older than 34 weeks’ postmenstrual age.
You recently admitted a female infant born at 28 weeks’ gestation. She required intubation for surfactant but was immediately extubated to CPAP and did well. At 48 hours of life, she started having apneic episodes, and although she self-recovered, they increased in frequency. Upon review of her medications, you noticed she is on antibiotics for 48 hours to rule out sepsis, but you realized that she was not started on caffeine.
Should this infant have been started on caffeine on day of life 1?
What are the caffeine dosing recommendations?
When would be the optimal time to discontinue the caffeine?
Methylxanthine therapy has been used for treatment of apnea since the mid- to late 1970s. Methylxanthines competitively inhibit adenosine receptors, resulting in stimulation of respiratory neural output. Although the basis of its beneficial effects is not completely understood, methylxanthine therapy reverses central hypoxic depression of breathing, increases minute ventilation, improves CO 2 sensitivity, enhances diaphragmatic activity, improves pharyngeal tone, and decreases periodic breathing. There may also be a dose-dependent antiinflammatory effect of caffeine.
Schmidt and colleagues published the largest randomized controlled trial of caffeine therapy for apnea of prematurity (CAP) demonstrating both respiratory and neurodevelopmental benefits for caffeine- versus placebo-treated infants ( ; ). The risk of BPD was significantly reduced in the caffeine group (OR 0.63, 95% CI 0.52–0.76). At 18 to 21 months follow-up, caffeine-treated infants had less cognitive impairment (0.81, 95% CI 0.66–0.99) and decreased incidence of cerebral palsy (0.58, 95% CI 0.39–0.87). Although the 5-year follow-up suggested attenuation of these cognitive and motor benefits, follow-up at school age has revealed benefit of caffeine for developmental coordination disorder and decrease in motor impairment ( ; ).
Traditional indications for methylxanthine therapy initiation were treatment of apnea and facilitation of extubation. However, prophylactic use for infants at risk for apnea is now widespread. Increasing efforts to avoid intubation and mechanical ventilation have contributed to the overall shift from selective therapeutic toward widespread prophylactic use of methylxanthine therapy for apnea of prematurity. The infant in this case would be a candidate for this therapy, but data to support the practice of prophylactic methylxanthine use in preterm infants is limited ( ).
See Table 12.1 .
| Pro | Con | |
|---|---|---|
| Early onset |
|
|
|
|
|
| Higher doses |
|
|
Data supporting the early use of caffeine are based on retrospective analyses, such as a large Canadian cohort that showed an association between caffeine given in the first 2 days (vs later dosing) and decreased duration of invasive and noninvasive ventilatory support ( ). Unfortunately, early caffeine prophylaxis may not significantly decrease the risk of CPAP failure and the need for invasive ventilation ( ). Most recently, early caffeine also has not decreased the age of first successful extubation in ventilated preterm infants ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here