Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
On completion of this chapter, you should be able to:
Recognize normal neuroanatomy as it pertains to the ultrasound examination of the preterm and term neonate
Describe the coronal, sagittal, and mastoid view studies
Discuss the sonographic findings in neonatal brain pathology
Neurosonography is the primary imaging modality for high-risk and unstable premature infants because it is portable, nonionizing, noninvasive, and can be tolerated by the sickest infants, even immediately after birth. Furthermore, it is safe when adhering to the ALARA (as low as reasonably achievable) principle and is without contraindications, thus allowing for serial imaging of brain maturation and lesion evolution. In the hands of a skilled sonographer or physician, it is a reliable tool for the detection of most hemorrhage cystic and ischemic brain lesions, structural brain anomalies, calcifications, and cerebral infections. Although some conditions are not treatable, neurosonography allows for the assessment of neurologic prognosis, which aids parental counseling, as well as decisions on the continuation of neonatal intensive care. Furthermore, it helps to optimize treatment of the infant and provides support to the family, both during and after the neonatal period.
Neurologic impairment is one of the primary health concerns for premature infants. Prematurity is defined as a birth occurring before term, or 37 weeks of gestation. Most infants in the late-preterm category (34 to 36 weeks’ gestation) are often spared neurologic impairment, but the risk increases the more premature an infant is at birth ( Table 27.1 for prematurity categories). Intraventricular and subependymal hemorrhages (SEHs) occur in 40% to 70% of premature neonates under 34 weeks of gestation. Multifocal necrosis of the white matter, referred to as periventricular leukomalacia (PVL), may develop in 12% to 20% of infants weighing less than 2000 g (5 lb, 5 oz). Additionally, any neonate who suffered a difficult delivery associated with hypoxia or asphyxia may be examined for PVL. These lesions are associated with increased mortality and abnormal neurologic outcomes.
| Preterm Definition | Gestational Age at Birth | Birth Weight Categories and Corresponding Median Gestational Ages |
|---|---|---|
| Late preterm (or near-term) | 34 to <37 weeks |
|
| Moderately preterm | 32 to <34 weeks |
|
| Very preterm | 28 to <32 weeks |
|
| Extremely preterm | <28 weeks |
|
This chapter provides an introduction to the neonatal head examination, and therefore the focus is on normal cranial anatomy, along with sonographic findings and protocols. Pathology in this chapter includes hydrocephalus, intracranial hemorrhage (ICH), hypoxic-ischemic lesions, congenital malformations, and infection in the neonate.
Knowledge of the normal cranial anatomy is essential to perform the neonatal head examination confidently. The cranial cavity contains the brain and its surrounding meninges and portions of the cranial nerves, arteries, veins, and venous sinuses. The following neonatal head structures and sonographic findings are provided to aid in performing neurosonography.
The fontanels are unossified spaces between the bones of the infant skull, allowing for compression at birth and rapid brain growth thereafter. Colloquially termed “soft spots,” they provide the sonographer with acoustic windows, where the transducer is carefully placed to visualize and record brain structures ( Fig. 27.1 ). It is important to note their closure, which heavily hampers or completely impairs sonographic imaging. Generally, an acoustic window becomes limited at the beginning of the respective fontanel closure timeframe. Furthermore, these timeframes are relative to a term neonate, and preterm infants will be adjusted according to their gestational age at birth ( Box 27.1 ).
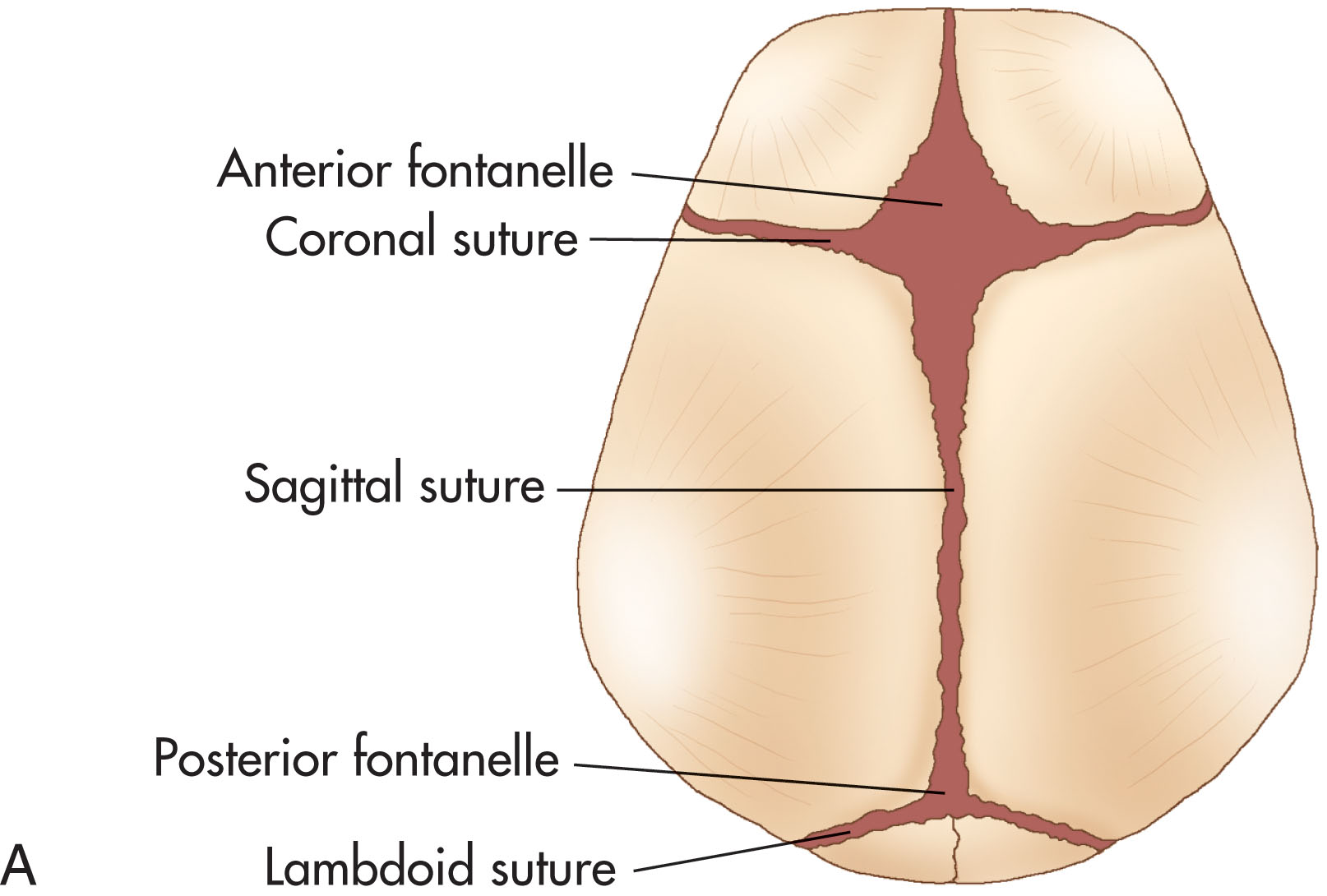
Anterior: 9–15 months (median, 13.8 months)
Mastoid: 6 and 18 months
Sphenoidal (temporal window): 6 months
Posterior: 8–12 weeks; some may be closed at birth
Neurosonography primarily utilizes the anterior and mastoid fontanels. The anterior fontanel is the largest at birth and provides an optimal sonographic view of the brain until 9 to 12 months of age, when it begins to close. This fontanel may remain open longer than the normal range in cases of prematurity, hydrocephalus, hypothyroidism, and some bone disorders and chromosomal abnormalities, such as trisomy 13, 18, and 21. If hydrocephalus is present, it is felt to be bulging. Compressed or overlapping fontanelles, on the other hand, due to oligohydramnios or a difficult delivery, may be difficult to palpate and provide a limited acoustic window to adequately image the structures of the brain.
There are three membranes called meninges that surround and form a protective covering for the brain: the dura mater, arachnoid mater, and pia mater. The pia mater, or “soft mother,” lies against the delicate brain parenchyma; the arachnoid membrane is in the middle; and the dura mater, or “tough mother,” is a double-layered outer membrane that forms the strongest barrier ( Fig. 27.2 ).
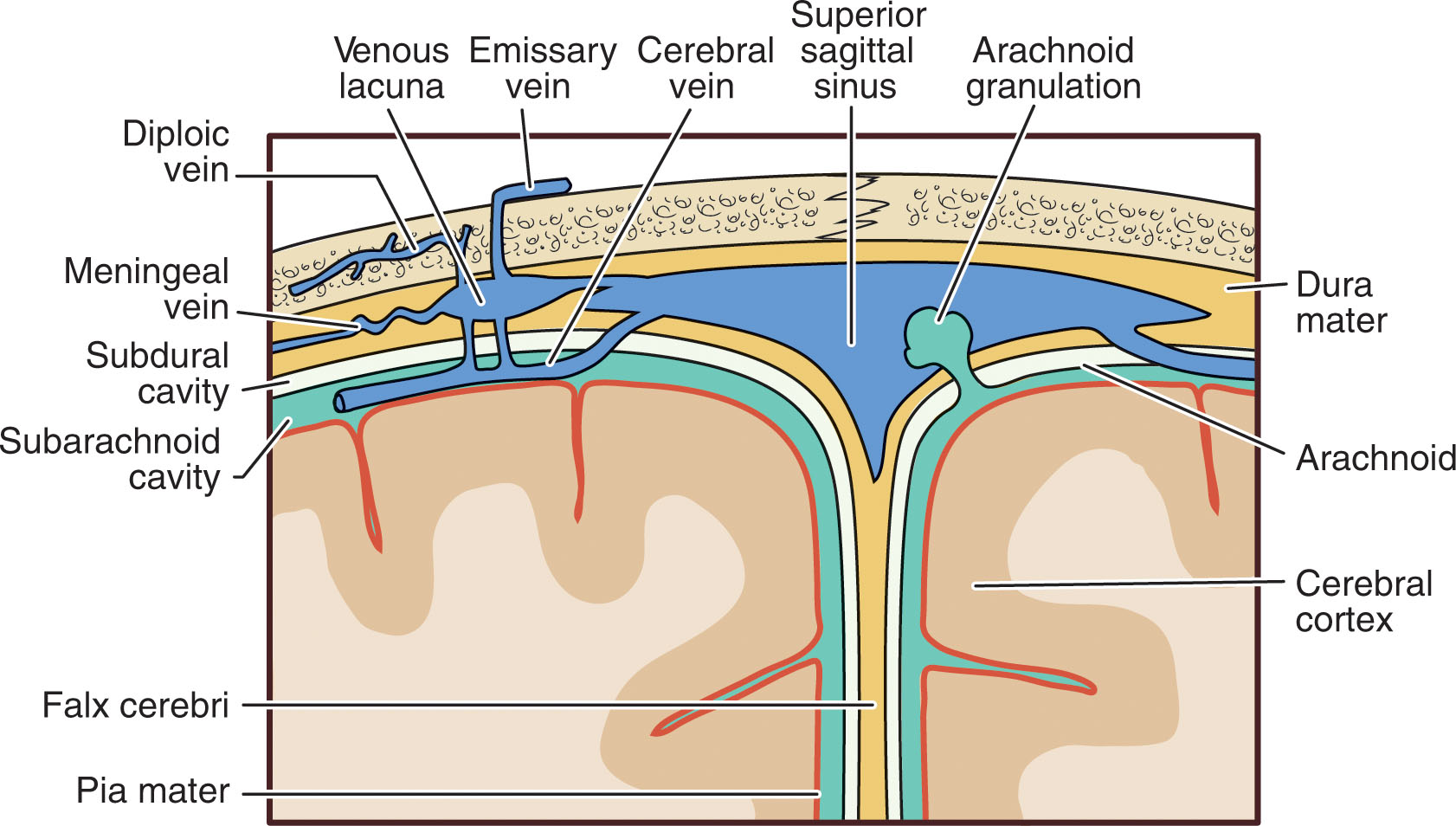
Lying between the delicate pia mater and arachnoid mater, the subarachnoid space contains cerebrospinal fluid (CSF) and branches of the arteries and veins of the brain. The intercommunication between these vascular channels and the CSF plays an important role in the blood-brain barrier. It connects to the pia matter via the arachnoid trabeculae, and to the dura matter, via arachnoid granulations.
The falx cerebri is a fibrous structure composed of a double fold of dura mater, separating the right and left cerebral hemispheres. The tentorium cerebelli is an extension of the falx cerebri and separates the cerebrum from the cerebellum. Structures superior to this inverted echogenic V-shaped structure are supratentorial, and structures below it, infratentorial. The infratentorial structures of the brain are collectively referred to as the posterior fossa .
The ventricular system is filled with CSF, which surrounds and protects the brain and spinal cord from physical impact. It also acts as a communication route for hormones and transmitters between areas of the central nervous system (CNS). The CSF-filled ventricular system includes the ventricles, their connecting foramina, and subarachnoid space, which are all contiguous with the central spinal column through the foramen magnum.
The lateral ventricles, located on either side of the brain, are the largest of the CSF-filled cavities located within the cerebral hemispheres and appear anechoic sonographically. The lateral ventricles are divided into four segments and are generally named after the lobe of the brain into which they project: the frontal horns, central body, temporal horns, and occipital horns. The body is the central section, just posterior to the frontal horn. The atrium (or trigone) of the lateral ventricle is the site where the body, occipital, and temporal horns join ( Fig. 27.3 ).
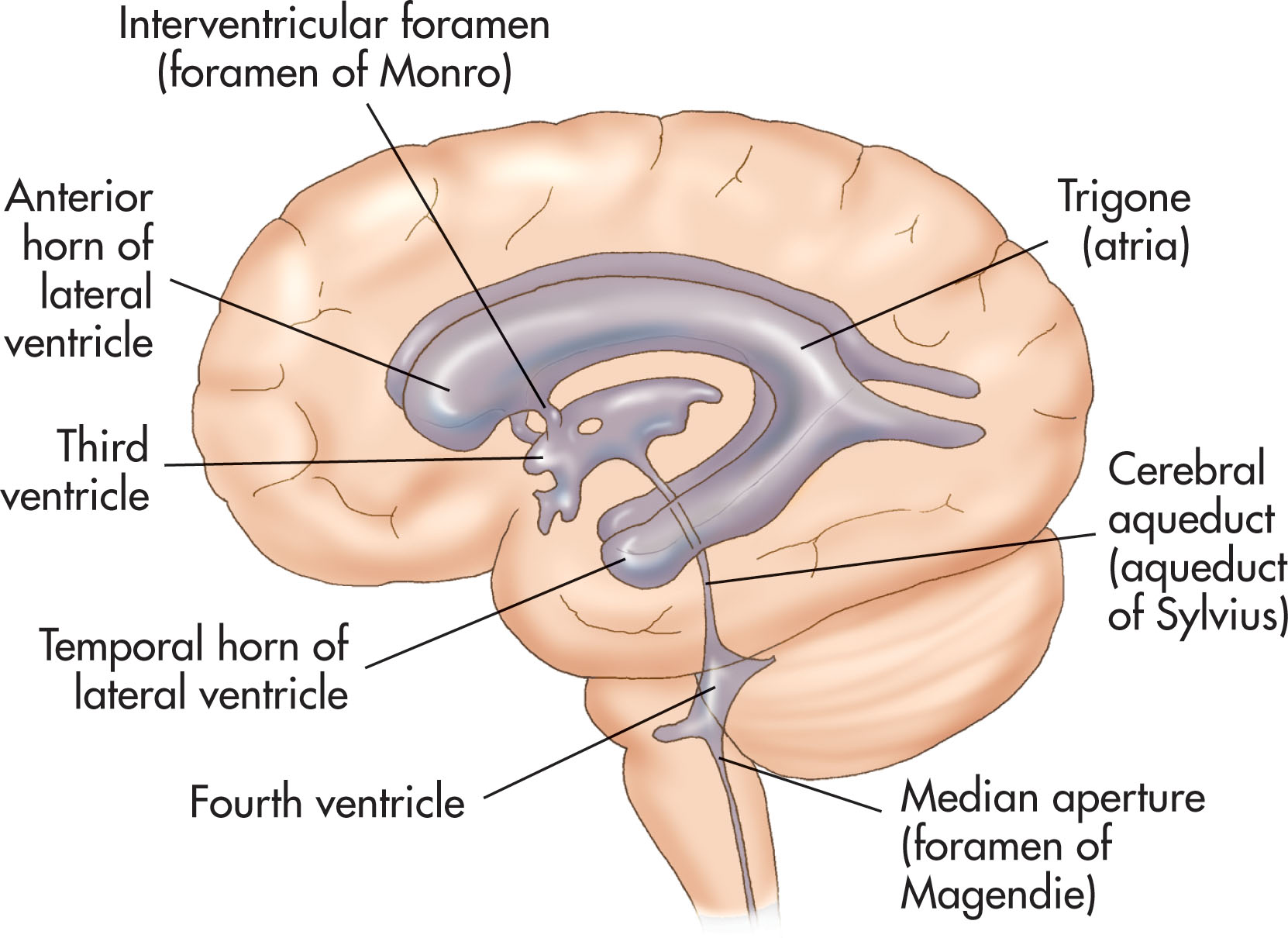
The lateral ventricles communicate with the third ventricle through paired foramina, individually referred to as the intraventricular foramen, or foramen of Monro. The third ventricle is a narrow, irregularly shaped opening, which sits inferior and midline between the lateral ventricles. The roof is formed by the corpus callosum. Often, the third ventricle is not visualized beyond 32 weeks of gestation. The cerebral aqueduct, or aqueduct of Sylvius, connects the third and fourth ventricles and is the narrowest passage. It is also the most common site for intraventricular blockage of CSF in the neonate.
The fourth ventricle is diamond-shaped and sits within the pons, or upper portion of the brain stem, between the cerebellar peduncles. Anteriorly, the lower floor surface is formed by the medulla oblongata. The roof is formed by the cerebellar vermis posteriorly. Three apertures allow the CSF to leave the fourth ventricle, one centrally and two laterally, into the subarachnoid cisterns. The lateral angles of the fourth ventricle form the foramina of Luschka, which drain into the lateral cerebellopontine cisterns. The inferior angle, the middle foramen of Magendie, drains into the cerebellomedullary cistern, which is continuous with the cisterna magna and central canal of the spinal cord.
Like the ventricles, the subarachnoid space and cisterns also play a role in the flow of CSF. The narrow subarachnoid space surrounding the brain and spinal cord contains a small amount of CSF. The subarachnoid cisterns are the spaces at the base of the brain where the arachnoid becomes widely separated from the pia, giving rise to large cavities. The cisterna magna is one of the largest of these subarachnoid cisterns and is consistently seen with ultrasound, appearing anechoic; it is in the posterior fossa between the medulla oblongata, cerebellar hemispheres, and occipital bone.
The sonographer should be aware that ventricular size varies with gestational age, and the premature infant will normally have larger-appearing ventricles than the term infant. Also, dilation of the lateral ventricles often begins in the occipital horns. Minor asymmetry of the lateral ventricles is not uncommon, occurring in 20% to 40% of infants. The left side is often larger than the right. Another rarer variant is coarctation of the ventricle ( Fig. 27.4 ), which will appear as a cyst in coronal view at the superior and lateral ventricle, often at the level of the intraventricular foramen. In both variant cases, care should be taken to make certain the cause is not a sequela, or sequential result, of an intraventricular hemorrhage (IVH) , such as ventricular dilation or a subependymal cyst.
CSF from the lateral ventricles passes through the foramen of Monro to the third ventricle. The CSF then passes through the aqueduct of Sylvius to the fourth ventricle. From that point, the CSF may leave through the central foramen of Magendie or the lateral foramen of Luschka into the basal subarachnoid cisterns and cisterna magna. The anterior flow continues upward through the chiasmatic cisterns, sylvian fissure, and the pericallosal cisterns up over the hemispheres, where it is reabsorbed by the arachnoid granulations in the sagittal sinus. Posteriorly the CSF flow moves around the cerebelli, through the tentorial incisure, the quadrigeminal cistern, the posterior callosal cistern, and up over the hemispheres. The remaining small amount flows down into the subarachnoid space of the spinal canal, bathing the spinal cord ( Fig. 27.5 ).
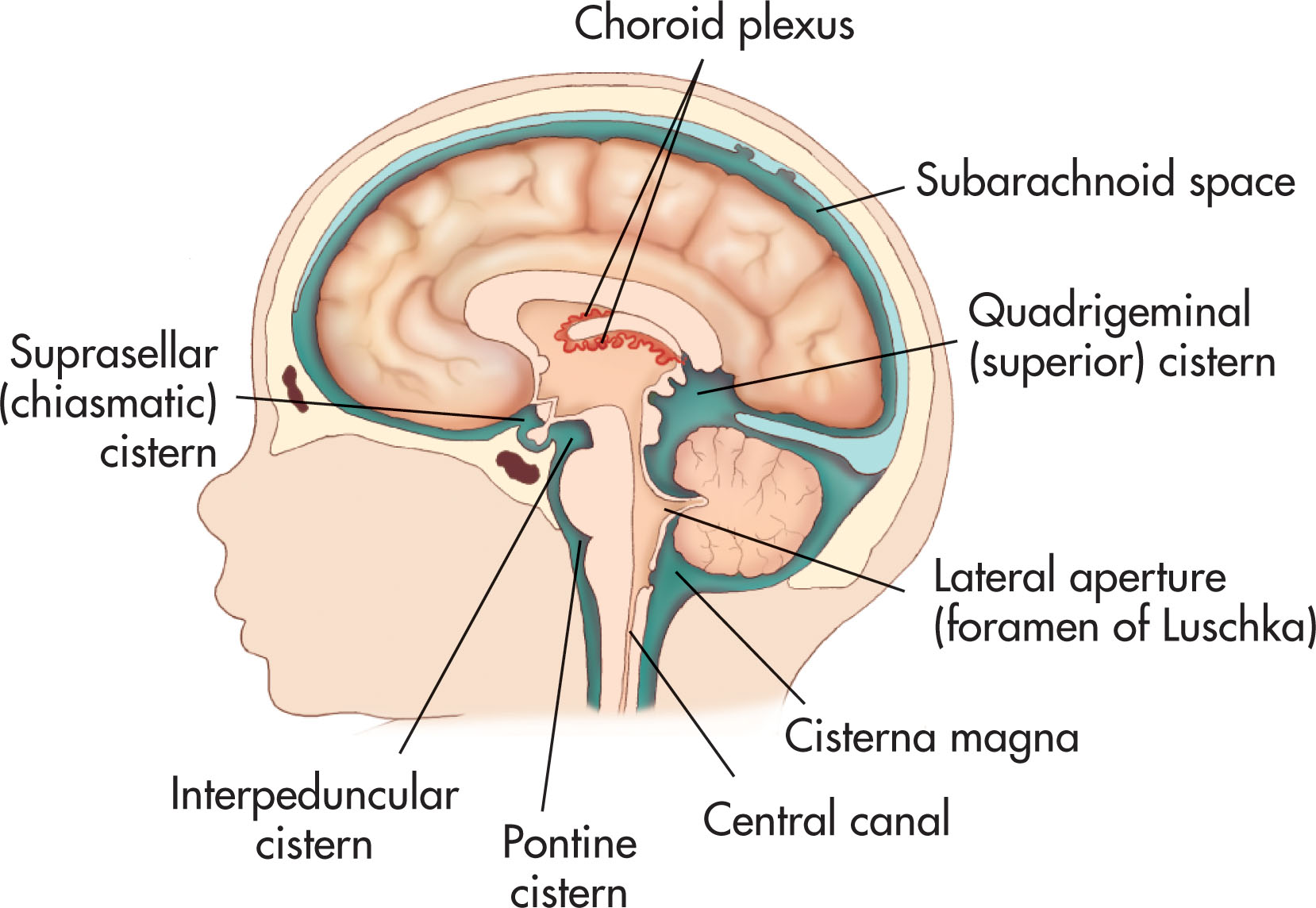
The choroid plexus is a mass of specialized cells located in the lateral, third, and fourth ventricles. The main and largest choroid is in the atria (atriums) of the lateral ventricles and is responsible for most of the CSF production. The choroid is prominent in preterm infants under 25 weeks of gestation and often develops a normal appearance by 30 weeks.
The atrial glomus is the largest part of the choroid plexus and tapers posteriorly. The glomus is a site for intraventricular bleeding in the term neonate compared with the much more common intraventricular hemorrhage in the caudothalamic groove in premature infants. The periventricular blush, or white matter, is the area of parenchyma surrounding the lateral ventricle containing the glomus. It is a major site for hypoxic-ischemic injury. The choroid plexus should always appear more echogenic than the surrounding brain tissue ( Fig. 27.6 ).
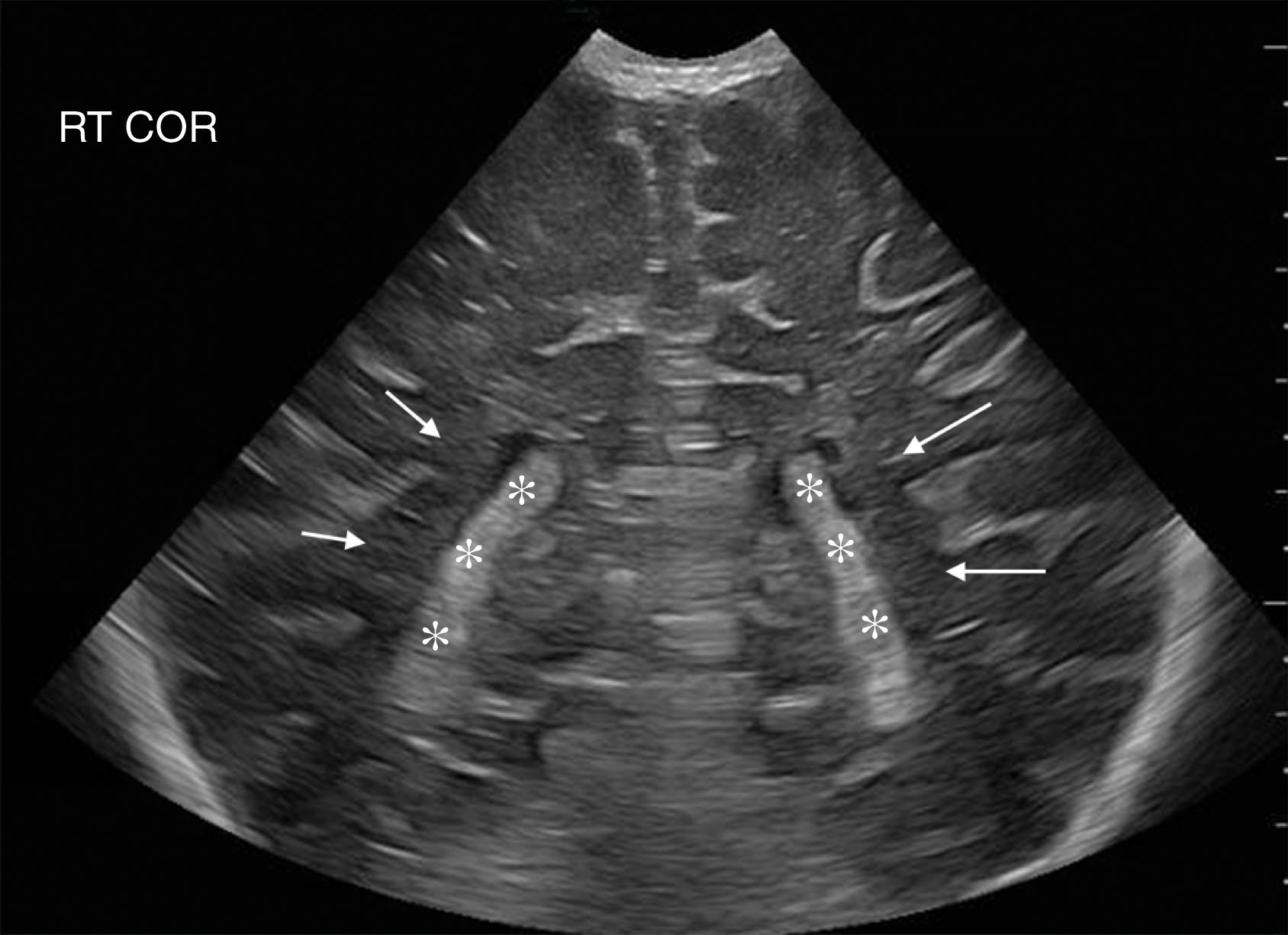
Other structures that produce CSF in the ventricular system include the intracranial and spinal subarachnoid lining. Additionally, the ventricles are lined with specialized ependyma that also produce CSF. These cells, along with the choroid plexus, regulate the normal intraventricular pressure by both secreting and absorbing CSF. However, in the setting of pathology, CSF production does not appreciably change, and absorption does not keep up, which may result in the ventricles enlarging, putting increased pressure on the brain parenchyma (intracranial pressure).
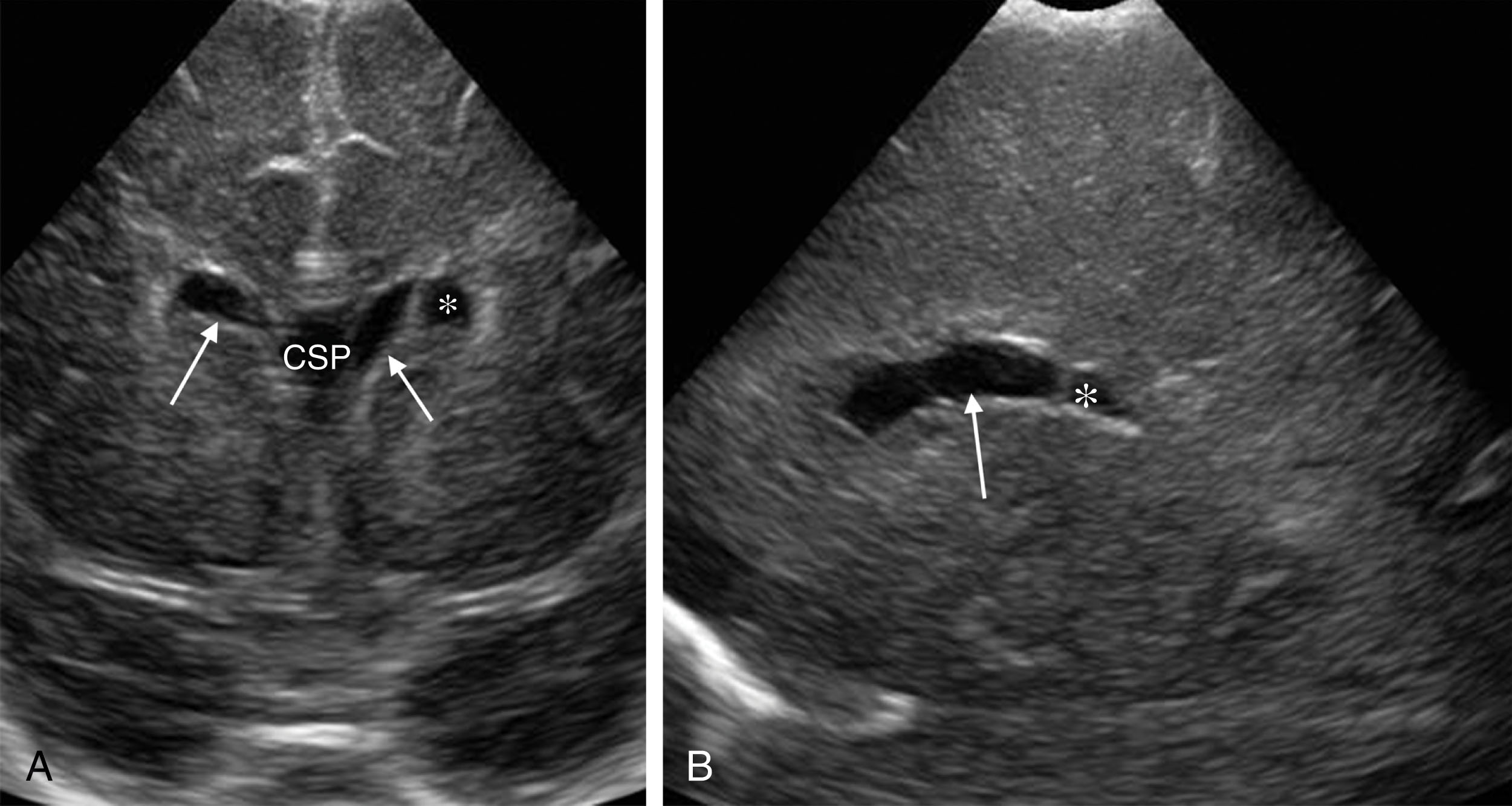
The cavum septum pellucidum (CSP) is a thin triangular space filled with CSF and lies between the anterior horn of the lateral ventricles and forms the floor of the corpus callosum. Thus, the CSP is inferior to the corpus callosum. The CSP is present at birth in 50% to 61% of normal neonates and often closes within the first 3 to 6 months of life. However, it may persist for life in some individuals. The cavum vergae is a posterior extension of the CSP and is often closed in term neonates. However, it may be seen in some preterm infants, as it normally closes around 6 months gestation.
There are two cerebral hemispheres connected by the corpus callosum. They extend from the frontal to the occipital bones above the anterior and middle cranial fossae. Posteriorly, they extend above the tentorium cerebelli. They are separated by a longitudinal fissure into which the falx cerebri projects. The cerebrum consists of the gray and white matter. The outermost portion of the cerebrum is the cerebral cortex (composed of gray matter). White matter is located at the innermost portion of the cerebrum. The largest and densest bundle of white matter is the corpus callosum.
The cortex is divided into four sections or lobes. These lobes correspond to the cerebral cortex located under the cranial bone of the same name and include the frontal, parietal, occipital, and temporal lobes ( Fig. 27.7 ).
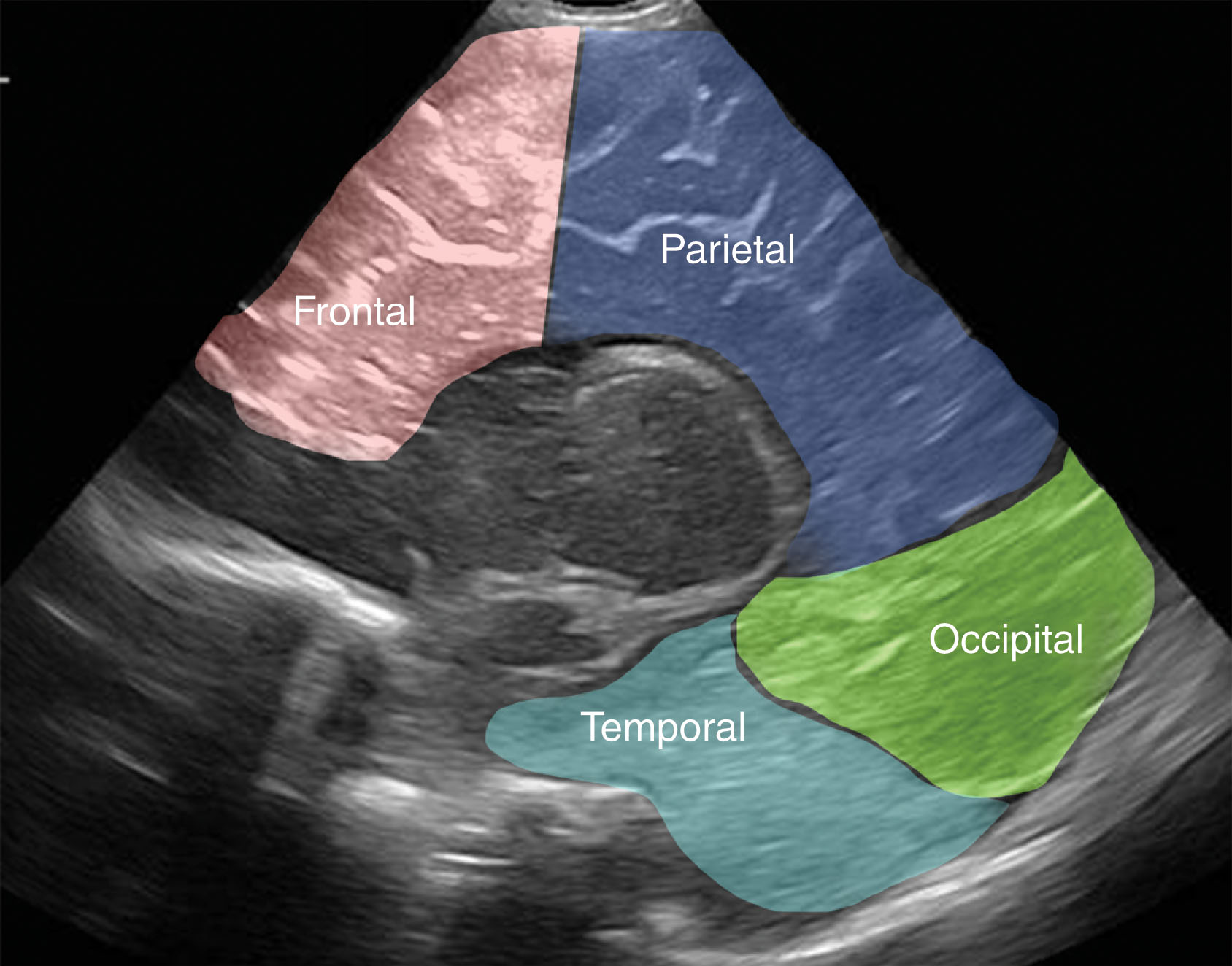
The gyri are convolutions on the surface of the brain caused by infolding of the cortex. The sulcus is a groove or depression on the surface of the brain separating the gyri. The sulci further divide the hemispheres into frontal, parietal, occipital, and temporal lobes. Sulci and gyri development is heavily dependent on the age of an infant. Sulci develop first around 22 weeks of gestation, with most formed by 28 weeks. However, sulci are not detected sonographically until around 26 weeks, and extremely premature brains have a smooth appearance. The midline cingulate sulcus forms fully between 28 and 31 weeks of gestation. Gyral development occurs after sulci development, and even at 32 weeks, this may be seen as asymmetric, with the right side more advanced.
The interhemispheric fissure is the area in which the falx cerebri sits and separates the two cerebral hemispheres. The sylvian fissure is located along the lateral-most aspect of the brain and is the area through which the middle cerebral artery courses ( Fig. 27.8 ). The quadrigeminal fissure is located posterior and inferior to the cavum vergae. The vein of Galen is also posterior, so the sonographer must be aware that Doppler should be utilized to make sure it is a fissure, when prominent, and not a malformation of the vein of Galen.
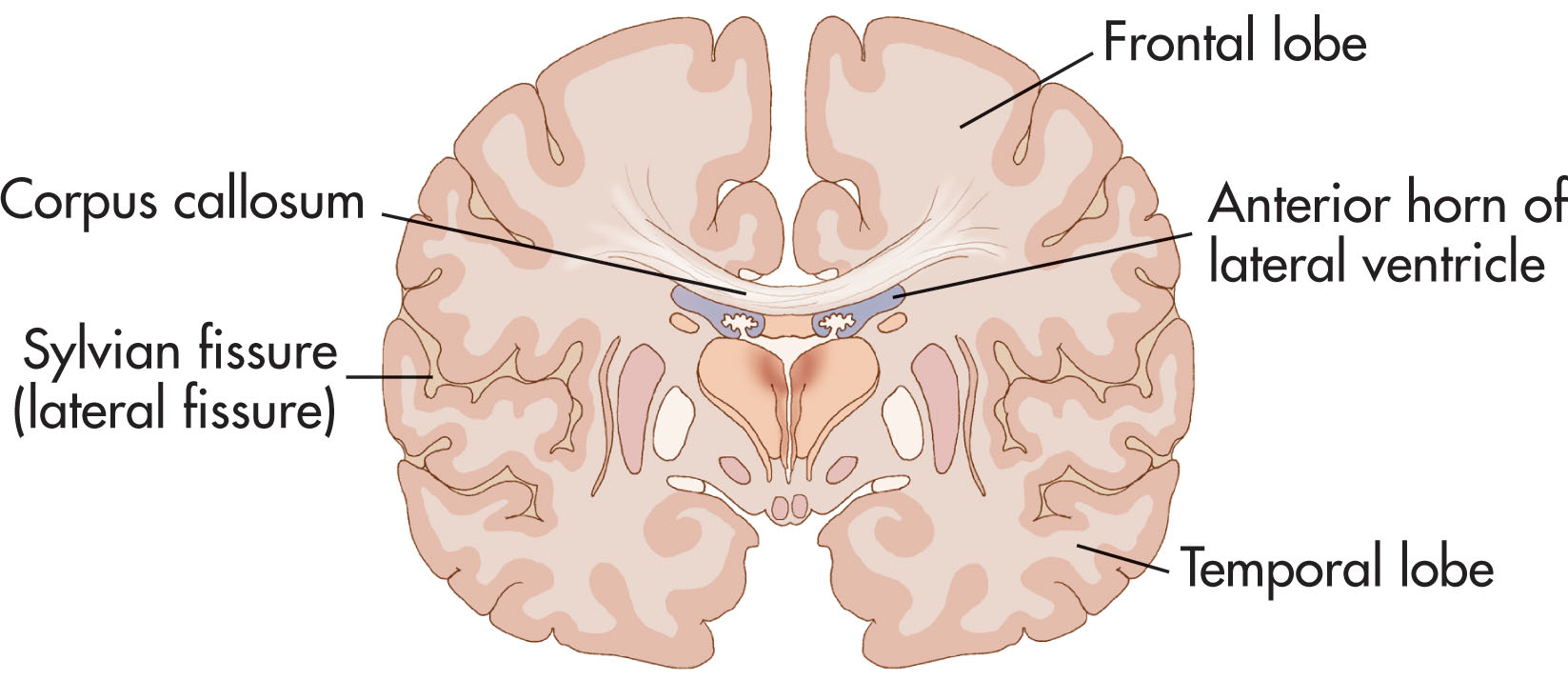
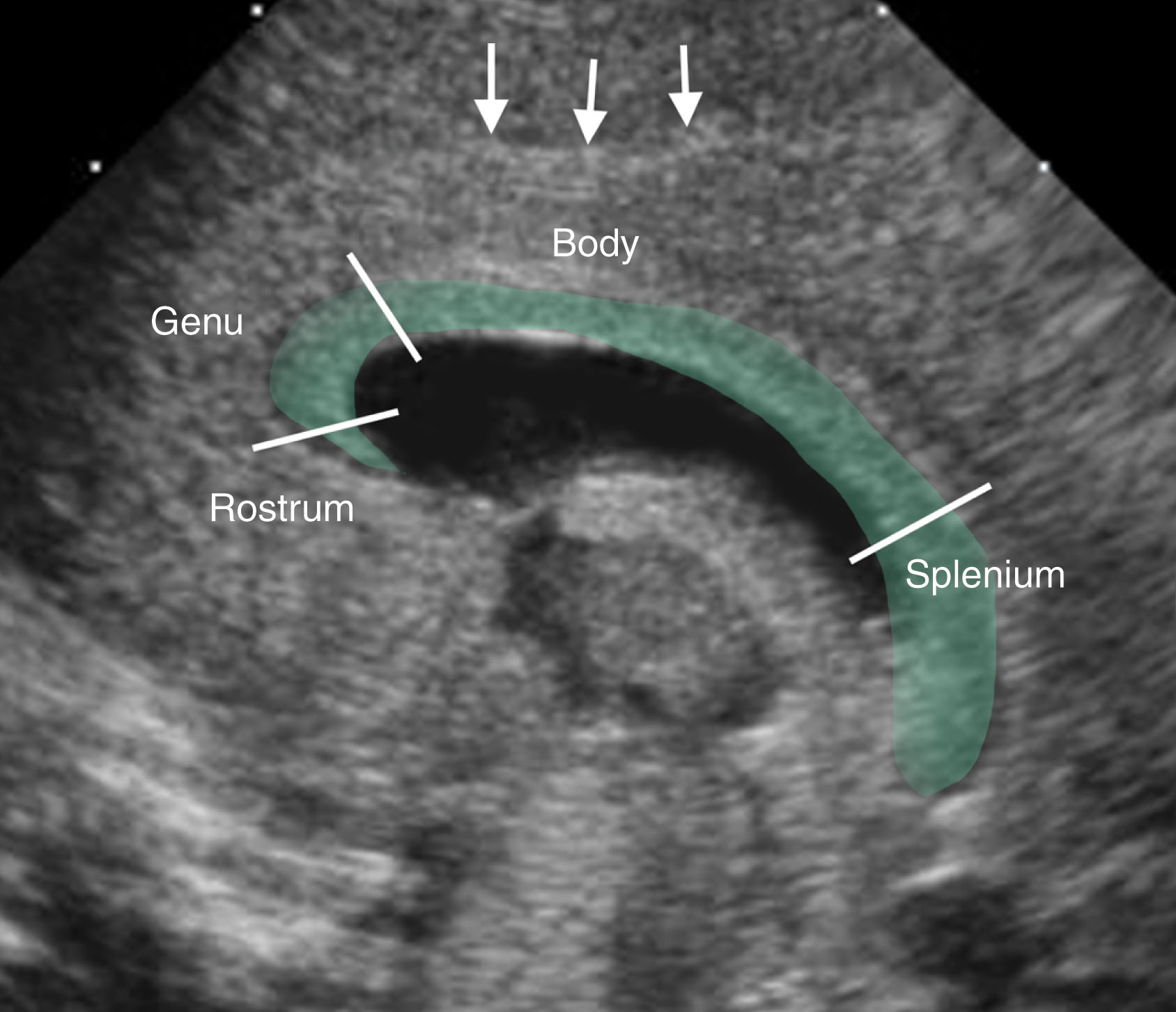
The corpus callosum forms broad bands of connecting fibers between the cerebral hemispheres. This structure forms the roof of the lateral ventricles. The corpus callosum sits superior to the CSP ( Fig. 27.9 ). The development of the corpus callosum occurs between the 8th and 18th week of gestation, beginning ventrally and extending dorsally. The genu of the corpus callosum develops first, followed by the body and splenium (the posterior element). The rostrum develops last. If a uterine insult occurs, development may be partially arrested, or complete agenesis may occur. If partial, the genu will be preserved, whereas those portions that develop later will be absent. This is known as agenesis or dysgenesis of the corpus callosum, respectively.
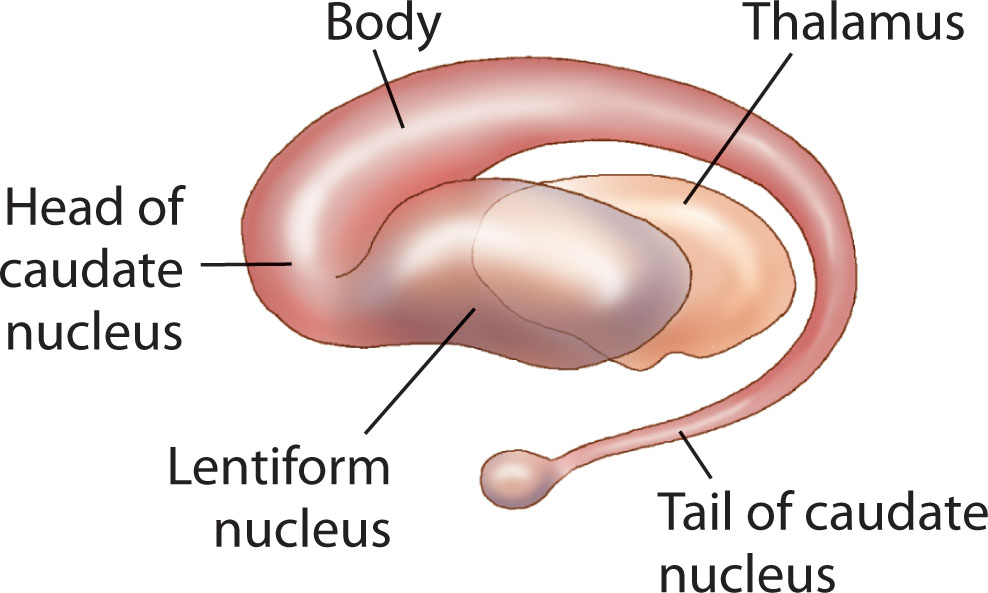
The basal ganglia are a collection of gray matter that includes the caudate nucleus, lentiform nucleus, claustrum, and thalamus. The caudate nucleus is the portion of the brain that forms the lateral borders of the frontal horns of the lateral ventricles and lies anterior to the thalamus ( Fig. 27.10 ). It is further divided into the head, body, and tail.
The head of the caudate nucleus, at the caudothalamic groove or notch ( Fig. 27.11 ), is the most common site for hemorrhage. The caudate nucleus and lentiform nucleus are the largest basal ganglia. They serve as relay stations between the thalamus and the cerebral cortex.
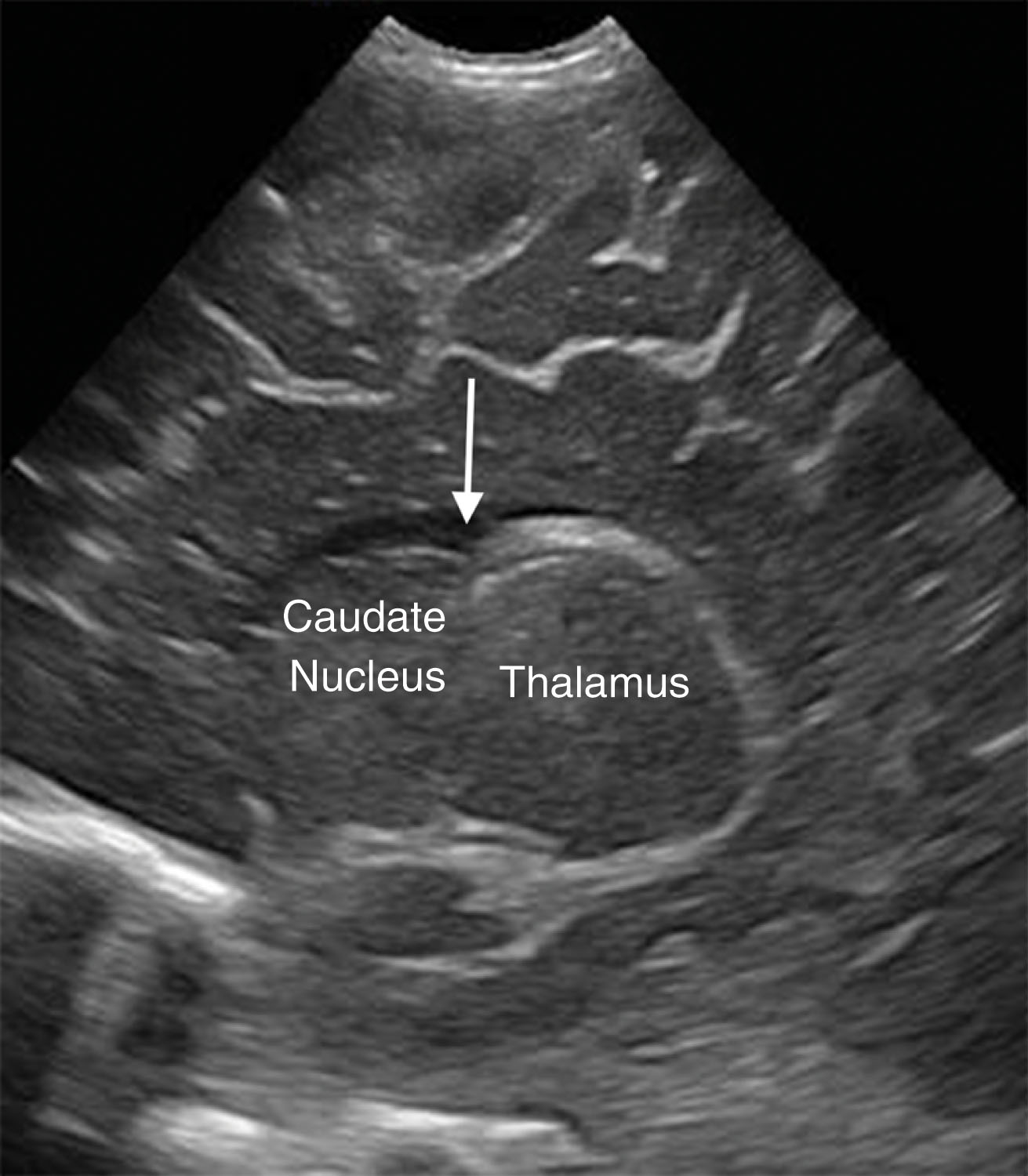
The thalamus consists of two ovoid, egg-shaped brain structures situated on either side of the third ventricle, superior to the brainstem. The thalamus borders the third ventricle and connects through the middle of the third ventricle by the massa intermedia, which is composed of gray matter and exists in most neonatal brains. The hypothalamus forms the floor of the third ventricle. The pituitary gland is connected to the hypothalamus by the infundibulum.
The germinal matrix includes periventricular tissue and the caudate nucleus. It is located 1 cm above the caudate nucleus in the floor of the lateral ventricle, at the caudothalamic groove. It sweeps from the frontal horn posteriorly into the temporal horn. It is indicated in 90% of premature brain bleeds and is made up of a highly vascular bed of delicate blood vessels, especially in infants under 34 weeks of gestation.
The brainstem is the part of the brain connecting the forebrain (cerebral hemispheres, thalamus, and hypothalamus) and the spinal cord. It consists of the midbrain, pons, and medulla oblongata.
The midbrain portion of the brain is narrow and connects the forebrain to the hindbrain. It consists of two halves called the cerebral peduncles, the cerebral aqueduct, the tectum, and the tegmentum.
The pons and medulla oblongata are part of the brainstem and hindbrain. The pons is found on the anterior surface of the cerebellum below the midbrain and above the medulla oblongata.
The medulla oblongata extends from the pons to the foramen magnum, where it continues as the spinal cord. This structure contains the fiber tracts between the brain and the spinal cord and the vital centers that regulate important internal activities of the body (heart rate, respiration, and blood pressure).
The cerebellum , part of the hindbrain, is composed of two hemispheres that have a cauliflower-like appearance. The central cerebellar white matter is referred to as the arbor vitae (“tree of life”) due to its appearance and function of bringing motor and sensory information to and from the cerebellum. The cerebellum lies in the posterior cranial fossa under the tentorium cerebelli. The two hemispheres are connected by the vermis.
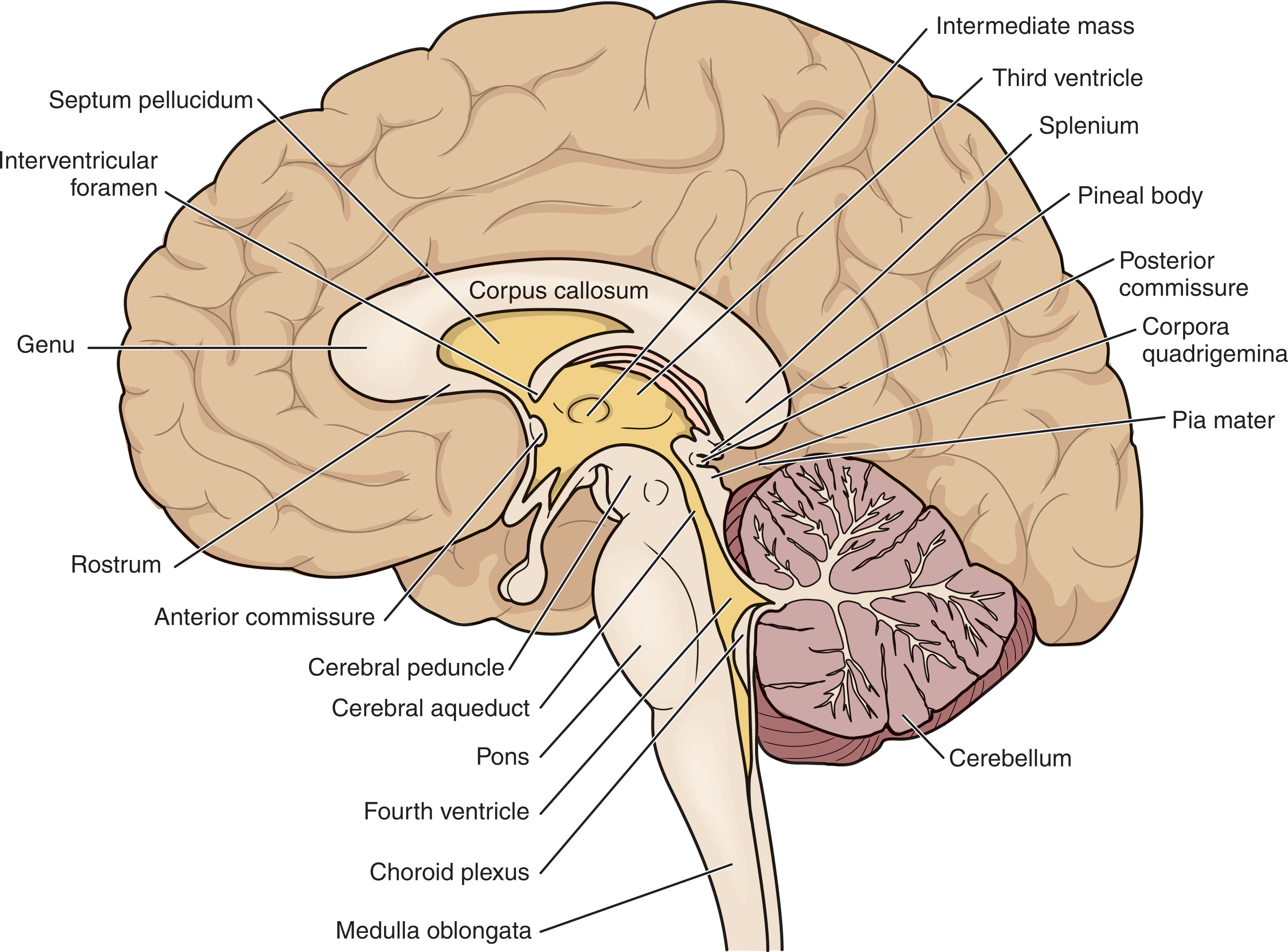
Fig. 27.12 summarizes the major anatomy of the midline sagittal plane. Fig. 27.13 summarizes the major sonographic anatomy of the midsagittal view for both a term infant and an extremely premature infant. (Notice the development of the gyri and cingulate sulcus and lack of a cavum vergae pellucidum in the term neonate. Also, the structures are better visualized in the premature infant due to a wider anterior fontanel and more prominent ventricular system.)
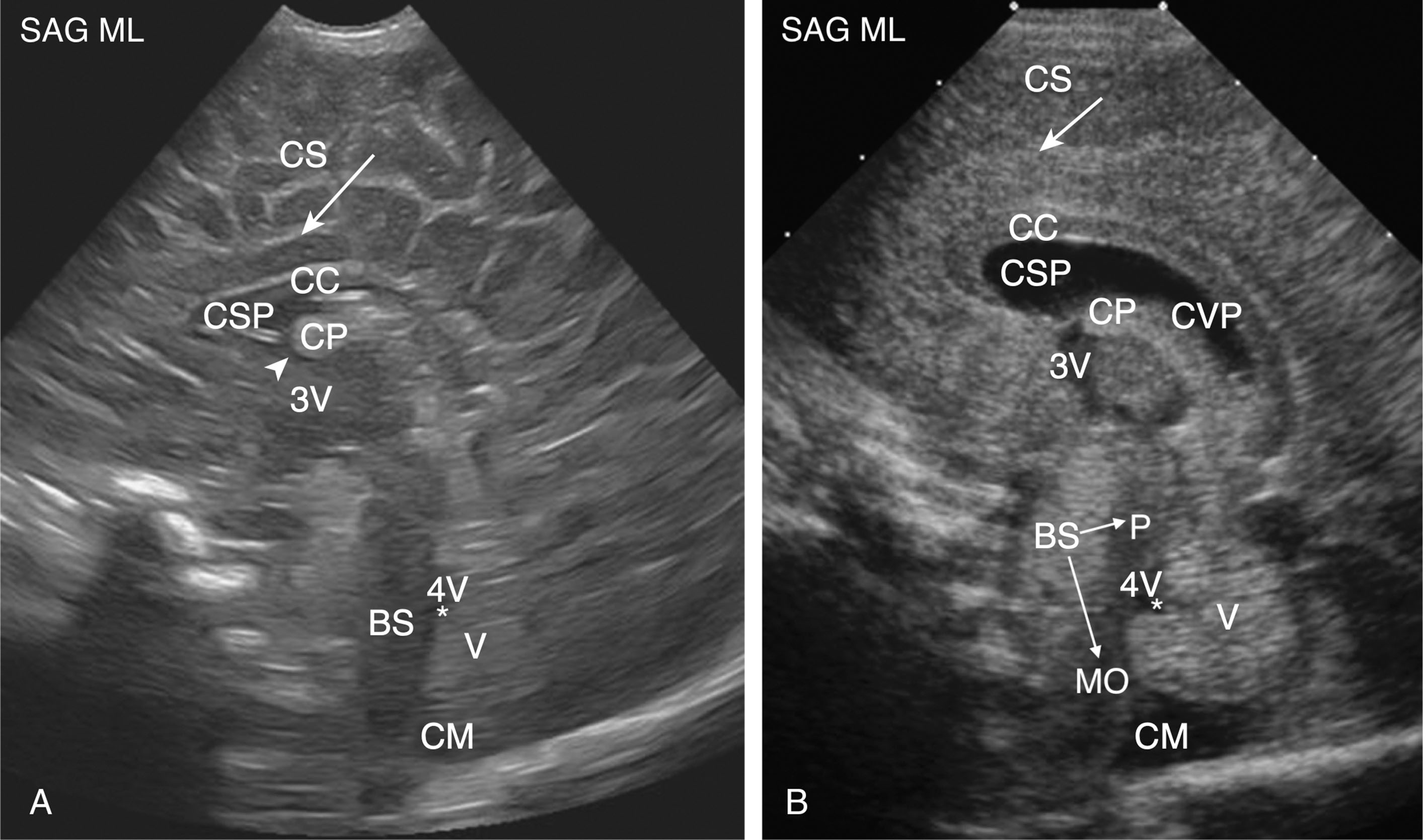
The cerebrovascular system consists of the internal cerebral arteries, vertebral arteries, the circle of Willis, and its major branches. The anterior cerebral artery (ACA) is one such major branch of the circle of Willis and can be visualized in a midsagittal plane. Branches of the ACA are routinely evaluated and include the pericallosal artery and callosomarginal artery. The pericallosal artery is frequently used to evaluate blood flow to the infant brain, and, as the name suggests, it flows up and around the corpus callosum. The cerebrovascular system is discussed in more detail in Chapter 38, Chapter 60 .
For the premature or sick infant, most neurosonography examinations are performed portably in the neonatal intensive care unit. Only well, term, or older infants may be examined in the ultrasound laboratory, either on the examination table, in a car seat, or on the parent’s lap. For inpatients, the sonographer must be aware of the infant’s condition before the examination, and therefore should never begin an examination before first contacting the infant’s nurse.
Two key concerns are keeping the premature infant both safe and warm. They can lose a potentially dangerous amount of body heat quickly, so a small amount of warm gel (ideally from an individual packet) should be used while scanning through the isolette portholes. If a large amount of cold gel is applied to the fontanel, or if the isolette is open for an extended period, the infant’s temperature may drop, which will often set off the infant’s thermal regulation (and alarms). Likewise, applying too much pressure while performing the examination may bring about bradycardia (and more alarms). Additionally, the sonographer should be acutely aware of any lifesaving wires, tubes (e.g., endotracheal tubes) and monitors, limit head and neck movement, and practice good infection control techniques to minimize the spread of disease in this vulnerable population.
Neurosonology is performed primarily through the anterior fontanel or easily felt “soft spot” on top of the head. If a good view is not readily obtained in the general area, palpating a fontanel beforehand may be helpful. Additionally, when possible, it is best to rest the hand to keep steady, even if just a single finger atop the infant’s head is achievable. Performing the exam requires staying stationary over the scanning window, using only very small movements.
A curved-array or sector high-frequency transducer of 7 to 10 MHz is best for imaging most preterm infants and neonates, whereas lower frequencies of 5 to 7 MHz may be needed for older infants with closing fontanels or infants with thick hair. A 3- to 5-MHz transducer may also be necessary to visualize deeper structures.
More than one transducer is often necessary and recommended. First, a dedicated neonatal head probe will provide the much-desired small footprint and 110- to 130-degree angle needed to obtain excellent contact and brain visualization. Second, a small linear array, high-frequency transducer (10 to 15 MHz), with or without a standoff pad, may be used to image the extra-axial fluid space and any abnormalities in the near-field. Specialized settings with low grayscale contrast and multiple focal zones throughout the depth of the image help to optimize the lateral resolution of brain parenchyma to detect any subtle changes.
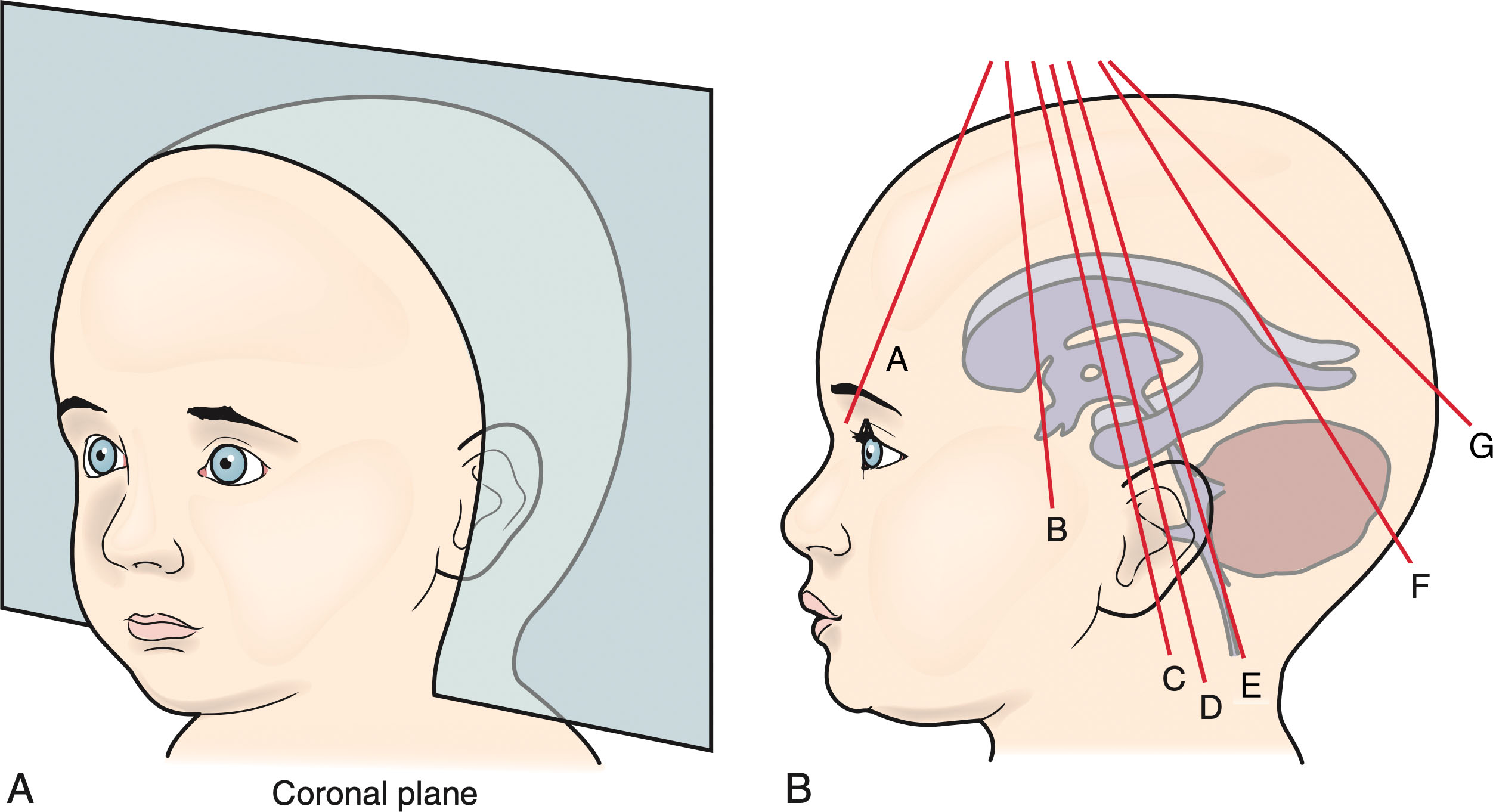
Sonography of the neonatal brain is initiated through the anterior fontanel in coronal and sagittal views to study the supratentorial and infratentorial compartments ( Box 27.2 ). Since the structures in the infratentorial compartment are located relatively far from the transducer, the alteration of a deep focal placement and lowering the transducer frequency are recommended. In older infants, two transducers may be needed, one for the supratentorial structures and another for the infratentorial structures. The posterior cranial bones should always be present in the standard images to ensure the entire brain is being visualized.
The mastoid fontanel is used to better visualize the cerebellum and infratentorial compartment, or posterior fossa, in younger infants. Cine clips should be used when possible to show the coronal and sagittal sweeps through the neonatal head. Additional magnified views are also encouraged to provide better resolution of any abnormal or high-risk areas (e.g., the caudothalamic groove in a preterm infant). Color Doppler evaluation (and pulsed wave Doppler, when indicated) of the pericallosal artery in the midsagittal view is also encouraged.
Knowing the gestational age of the premature infant when performing a neonatal sonogram of the head is essential. Table 27.2 summarizes the general sonographic findings in the term versus preterm neonate.
| Term (> 37 Weeks) | Preterm (<37 Weeks) | |
|---|---|---|
| Sulci and gyri |
|
|
| Ventricles |
|
|
| Third ventricle |
|
|
| CSP |
|
|
| Cavum vergae |
|
|
| Choroid plexus |
|
|
| Caudate nuclei |
|
|
| Periventricular white matter |
|
|
a The glomus is a common site for intraventricular bleeding in the full-term neonate.
b Not to be confused with the caudothalamic groove, which is the most common site for intracranial hemorrhage, often in preterm infants <34 weeks.
c The periventricular area is where most white matter necrosis or periventricular leukomalacia occurs in the preterm infant.
While the posterior fontanel is often not useful beyond the neonatal period, or first four 4 weeks of life, it can provide more up-close sagittal and coronal views of the occipital horns, periventricular blush, and cerebellum in premature infants and neonates. It may be used routinely in this population or when pathology is suspected. It may also be used if imaging is restricted due to overlapping bones in the area of the anterior fontanel. The posterior fontanel approach is most helpful for any critical neonate on extracorporeal membrane oxygenation (ECMO) , where the mastoid view may be unattainable.
Pathology should always be evaluated in multiple planes and with color and/or power and spectral Doppler. Additional views of pathology may be obtained from the posterior or mastoid fontanel, the foramen magnum, or thin areas over the temporal and parietal bones. Clot in the occipital horns is best evaluated from a posterior approach (mastoid or posterior fontanel), and the foramen magnum is useful to evaluate the proximal end of the spinal canal, often in the setting of known or suspected Chiari malformation (type I or II). Additionally, any open suture, burr hole, or craniotomy defect can also serve as an acoustic window.
High-frequency linear array transducers used in the near-field can identify superior sagittal sinus thrombosis, cerebral edema, and subdural hematomas or subdural abscess secondary to meningitis. It is also used in evaluating for possible craniosynostosis, holding it perpendicular to the anticipated course of the coronal, sagittal, lambdoid, and metopic sutures.
Finally, although standard protocols are provided, additional oblique or axial views are very helpful and encouraged. This is particularly true in patients with ventricular shunt tubes, in which oblique angles help to identify the shunt and tip. When clinically indicated, the circle of Willis and its major branches are evaluated with pulsed wave and color Doppler ultrasound from a transtemporal approach in determining cerebral blood flow patterns.
Three-dimensional (3D) sonography is showing promise as an emerging method in imaging the neonatal head. This technique can reduce inter-operator variability and significantly reduce the time required to perform a neurosonology examination (about 15 minutes down to 1 to 2 minutes.) Research shows this is achievable without compromising diagnostic quality in comparison to the standard two-dimensional approach. It also allows for later 3D reconstructions and may be useful in better evaluating brain anomalies. The 3D assessment of fluid volume in the setting of ventriculomegaly may be more accurate.
Additionally, ultrafast Doppler is a newer Doppler technique capable of quickly mapping cerebral vascular resistivity in neonates. It has superior temporal resolution, surpassing functional magnetic resonance imaging (MRI), sending out greater than 100,000 frames per second. For comparison, conventional color Doppler sends out around 50 frames per second. It has been called ultrasound angiography. It offers the evaluation of blood flow speed at a subcardiac cycle time scale, which other angiographic modalities are unable to assess. This may be useful for monitoring hypoxic-ischemic injuries (HIIs) in the future, as well as opening up knowledge about cerebrovascular regulation in infants in general.
Contrast-enhanced ultrasound allows for detailed perfusion studies of the infant head in the setting of post-cardiac arrest. Finally, shear-wave and strain elastography are also emerging in providing more information about hypoxic-ischemic events and the periventricular gray-white matter softening that can follow. All the above techniques provide clinicians a portable, relatively low-cost, and quick evaluation without sedation, providing valuable information, which may be used in serial monitoring of pathology.
To obtain the coronal views, the transducer is placed with the notch to the infant’s right side on the anterior fontanel with the scanning plane following the coronal suture. When looking at the coronal sections, the vertex of the skull is at the top, and the left side of the brain is to the right of the image and should be annotated as such. The middle of the transducer must be centered in the coronal suture to reduce bone interference and to procure the most extensive image of the brain.
Symmetric images must be obtained; this is accomplished by using the skull bones and the middle cerebral arteries at the sylvian fissure as landmarks. The skull bones and the arteries should be the same size bilaterally. In the coronal plane, the transducer is angled from the anterior to the posterior skull to completely visualize the lateral and third ventricles, the deep subcortical white matter, the basal ganglia, as well as the peripheral brain parenchyma. The coronal view orientation and angles are summarized in Fig. 27.14 , with corresponding protocol images and annotations found in Fig. 27.15 and Table 27.3 , respectively.
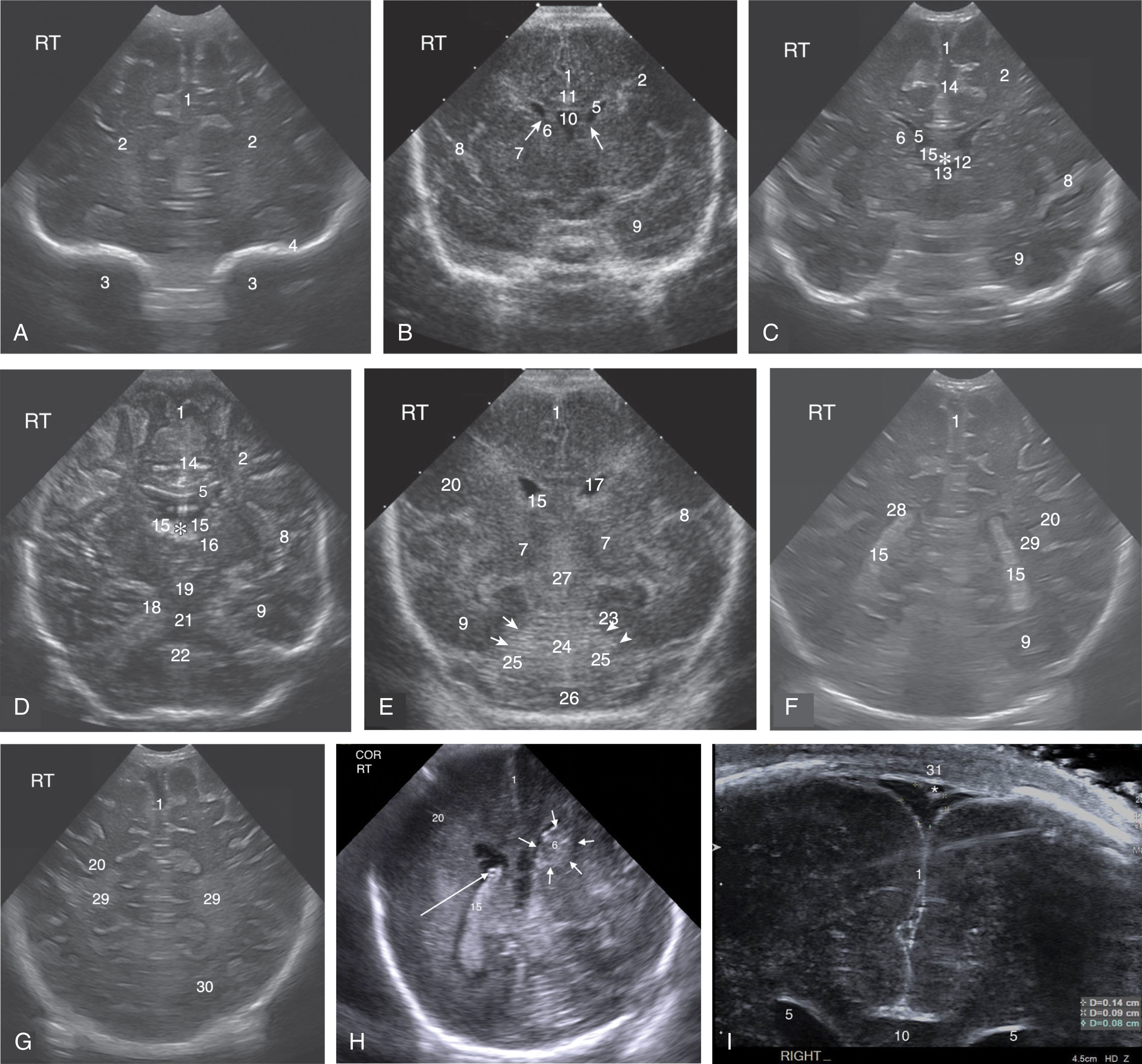
| Coronal Protocol (Corresponds to Images A–I in Fig. 27.15 ) | Labeled Anatomic Structures ( Bold Numbers Also Correspond to Sagittal Image Structures) | |
|---|---|---|
| A. Level of the Orbits | ||
|
1.Interhemispheric fissure2.Frontal lobe | 3.Orbits4.Skull |
| B. Level of the Frontal Horns and CSP | ||
|
1.Interhemispheric fissure 2 .Frontal lobe 5 .Frontal horn of lateral ventricle 6 .Caudate nucleus (area of caudothalamic groove, arrows on image) | 7 .Thalamus 8 .Sylvian fissure 9 .Temporal lobe 10 .CSP 11 .Corpus callosum |
| C. Level of the Third Ventricle | ||
|
1.Interhemispheric fissure 2 .Frontal lobe 5 .Frontal horn of lateral ventricle 6 .Caudate nucleus 8 .Sylvian fissure | 9 .Temporal lobe 12 .Intraventricular foramen (of Monro) 13 .Third ventricle 14 .Cingulate sulcus 15 .Anterior choroid plexus in the roof of the third ventricle (asterisk) |
| D. Level of the Third Ventricle (Angled Posteriorly) | ||
|
1.Interhemispheric fissure 2 .Frontal lobe 5 .Frontal horn of lateral ventricle 6 .Caudate nucleus 8 .Sylvian fissure 9 .Temporal lobe | 14 .Cingulate sulcus 15 .Anterior choroid plexus (asterisk) 16 .Thalamus 18 .Hippocampal fissure 19 .Mesencephalic aqueduct 21 .Pons 22 .Medulla oblongata |
| E. Level of the Cerebellum | ||
|
1.Interhemispheric fissure 7 .Thalamus 8 .Sylvian fissure 9 .Temporal lobe 15 .Choroid plexus 17 .Body of lateral ventricle | 20 .Parietal lobe 23 .Tentorium (arrows) 24 .Cerebellar vermis 25 .Cerebellar hemispheres 26 .Cisterna magna 27 .Quadrigeminal cistern |
| F. Level of the Choroid Plexus | ||
|
1.Interhemispheric fissure 9 .Temporal lobe 15 .Choroid plexus | 20 .Parietal lobe 28 .Trigone of the lateral ventricle 29 .Periventricular white matter |
| G. Level of the Occipital Lobes | ||
|
1.Interhemispheric fissure 20 .Parietal lobe | 29 .Periventricular white matter 30 .Occipital lobe |
| H. Off-Axis Views of Cerebral Hemispheres/Pathology | ||
|
1.Interhemispheric fissure 6 .Caudate nucleus ( arrows point to a bleed within the caudate, seen as echogenic as the choroid) | 15 .Choroid plexus ( long arrow to small cyst within the choroid) 20 .Parietal lobe |
| I. Magnification View of Extra-axial Spaces | ||
|
1.Interhemispheric fissure 5 .Frontal horn of lateral ventricle 10 .CSP 31 .Sagittal sinus (transverse view of the vein; asterisk ) | |
When the transducer is angled anteriorly, the frontal horns of the lateral ventricles appear as slit-like hypoechoic or cystic formations. As the transducer is angled posteriorly, the ventricles acquire a comma-like shape, and their width increases from the frontal horns to the atria or trigone. The choroid plexus is an echogenic structure inside the ventricular cavities surrounding the thalamic nuclei. It lies along the floor of the lateral ventricles, extending from the temporal horn into the atrium and body of the lateral ventricles. It should not appear extending anterior into the frontal horns or posterior into the occipital horns. Increased hyperechoic areas in the floor of the ventricles, anterior to the third ventricle, would indicate hemorrhage at the caudothalamic groove.
At the intraventricular foramen, the choroid plexus enters the third ventricle. The choroid plexus becomes enlarged at the level of the atria (glomus of the choroid plexus) and can almost entirely fill the ventricular cavity.
The third ventricle is not well visualized in the normal coronal study in the term neonate. An off-axis approach and high-frequency transducer may be helpful to identify it. Occasionally, a thin and very echogenic formation can be seen in the midline immediately below the septum pellucidum. This echogenic image corresponds to the choroid plexus extending into the roof of the third ventricle.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here