Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Brain ultrasound of premature infants is best performed for germinal matrix hemorrhage and hydrocephalus at 10 to 14 days of life and for cystic periventricular leukomalacia at 30 days of life.
The best time to assess for white matter injury of prematurity is at term-equivalent age.
Cerebellar hemorrhage is missed if mastoid views of the posterior fossa are not obtained.
High-resolution images are needed to fully study the brain parenchyma for white matter injury.
Multiple acoustic windows are essential for accurate detailed ultrasound imaging of the brain.
Doppler evaluation is key for any cystic lesion to exclude vascular malformations.
Computed tomography of the newborn brain is not indicated except in the case of a traumatic birth.
Congenital anomalies are well evaluated with ultrasound and, if further information is needed, are an indication for magnetic resonance imaging.
Neonatal sonography of the brain is an essential part of newborn care, particularly in high-risk and unstable premature infants. Current ultrasound technology allows for rapid evaluation of infants in the intensive care nursery with virtually no risk. The advantages of sonography over computed tomography (CT) or magnetic resonance imaging (MRI) include portability, lower cost, speed, no ionizing radiation, no sedation, and real-time visualization with video clips saved for later review. Screening of premature infants for intracranial hemorrhage has proven highly sensitive and specific. Daneman and colleagues have reported that optimal imaging of the neonatal brain requires delineation of structures in both the near and far fields. Thus magnification of selected areas with focal zone and higher resolution megahertz adjustments are essential for these detailed regional assessments. Real-time assessment is essential to detect subtle changes in echogenicity of the brain parenchyma, especially common in white matter injury of prematurity (WMIP). MRI is very sensitive and specific with regard to identification of brain injuries but, because of the cost and need for transfer of very unstable premature infants, is typically performed at term-equivalent age if concerns regarding neurodevelopmental outcomes are present.
Ultrasound is essential to the neonatal evaluation and follow-up of hydrocephalus and periventricular leukomalacia (PVL). Prenatal ultrasound and MRI diagnosis of central nervous system (CNS) malformations, infection, or masses is now followed up by ultrasound in the neonatal period. When major anomalies are present, associated anomalies may need evaluation by neonatal MRI. CT is not indicated in premature infants because of the lack of good gray versus white matter differentiation from the high water content in the newborn brain. For the same reason, CT is rarely used for term infants unless there is a history of birth trauma.
Ultrasound can be useful for the follow-up of ventricular shunt therapy or possible complications. Color and spectral Doppler ultrasound of cranial blood flow may prove valuable, particularly for cystic lesions when the differential diagnosis includes a vascular lesion, or for possible subdural hematomas, and to separate normal vascular structures from clot. Doppler ultrasound is also useful in infants receiving extracorporeal membrane oxygenation (ECMO) or when decreased blood flow is a risk for infarction (see Chapter 46 ).
In the premature infant, a 7.5-MHz or higher transducer is recommended to obtain the highest resolution possible. A 5-MHz transducer may be necessary to allow for adequate sound penetration of a larger infant head. Electronic phased array transducers with a 120-degree sector angle and multifocal zone capabilities are generally used for imaging through the anterior fontanelle. Small-footprint, linear array, high-frequency transducers (up to 12 MHz) can provide high-quality images for scanning of near-field pathology through the anterior fontanelle. These transducers are best for subdural hematomas, meningitis, superior sagittal sinus thrombosis, and cerebral edema, and in some cases, migrational abnormalities, or for scanning over the mastoid fontanelle, posterior fontanelle, and foramen magnum. The squamosal portion of the temporal bone is thin but may require a 5-MHz transducer if not imaged through the mastoid fontanelle. The multifocal zone capability provides excellent resolution throughout the field of view, but requires a cooperative patient because the frame rate is slowed significantly. Compound imaging, allowing for multiple angles of insonation, is also useful when imaging through small spaces such as the fontanelles. Video clip capabilities are invaluable in an uncooperative infant or when documenting motion, such as blood flow.
It has become routine to save video clips for later review, to prevent repeating an examination if there is a questionable finding and to be certain that each view has been completely evaluated. Clips can greatly improve the understanding of the pathology. Areas of increased or decreased echogenicity may be extremely subtle on single images, but they become much more apparent when integrated with cine or video that captures real-time ultrasound findings and the relationship to normal structures.
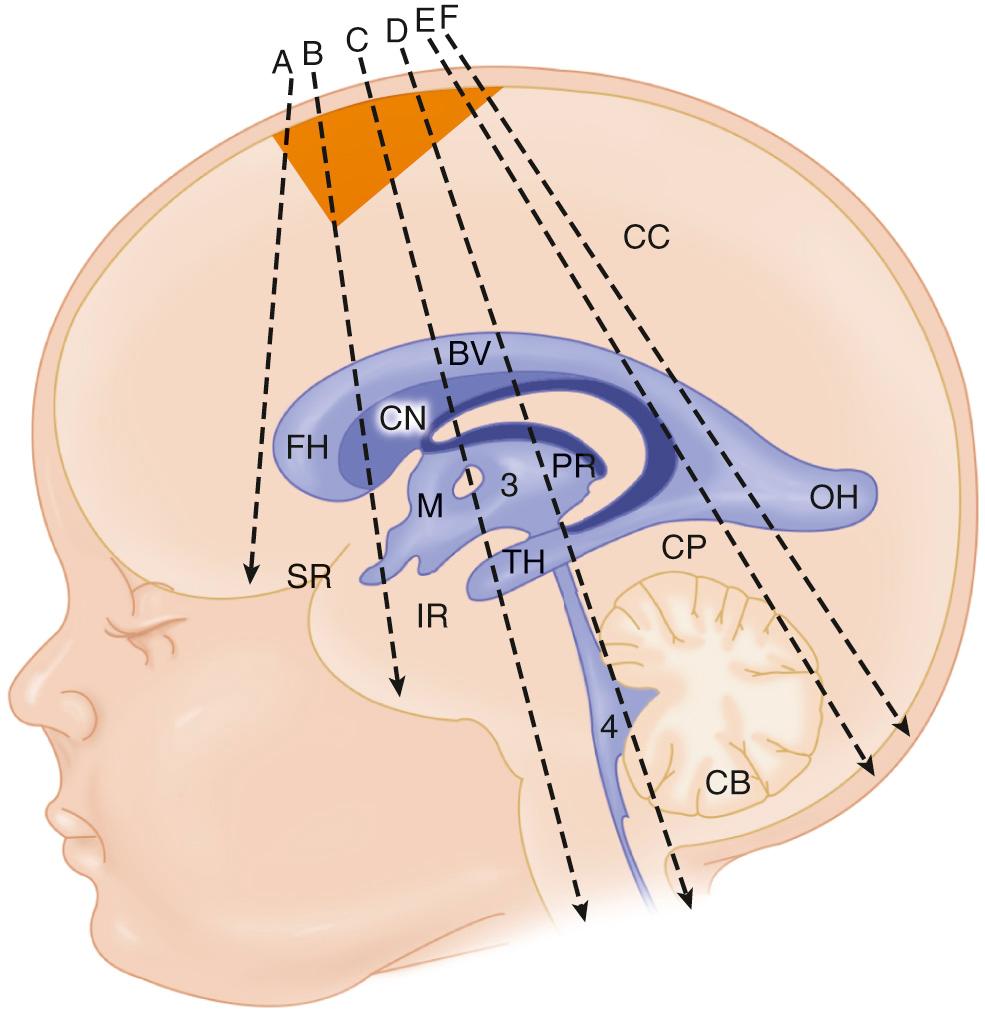
Currently, most brain sonographic examinations are performed through the anterior fontanelle in both the coronal and the sagittal planes. It has become clear that the neonatal brain is not fully examined unless the posterior fossa is evaluated through the posterior and mastoid fontanelles. In fact, cerebellar hemorrhage has been proven by MRI correlation to be missed without the mastoid views of the posterior fossa. Posterior fossa malformations may not be well understood without these highly detailed views of the cerebellum, fourth ventricle, and cisterna magna. Thus the posterior fossa views from the mastoid fontanelle are extremely important in the evaluation of cerebellar hemorrhage or posterior fossa anomalies, which are quite common. Good skin-to-transducer coupling can be achieved by an acoustic coupling gel. Occasionally, a standoff pad can be useful to evaluate superficial abnormalities such as subdural hemorrhage, but a higher resolution transducer is a better option to evaluate the near field in detail.
It is very important to use color Doppler ultrasound imaging to evaluate fluid collections because some cystic areas are actually vessels. If extracerebral fluid collections are expected, they are better evaluated with CT or MRI. Axial ultrasound scanning has been used extensively in utero, particularly for accurate measurements of fetal ventricular dimensions. In the newborn, axial scanning is used in evaluation of the posterior fossa through the mastoid fontanelle and to evaluate the circle of Willis with color Doppler ultrasound. Doppler evaluation of the major cerebral arteries may be indicated with particular attention to the resistive index changes that can indicate cerebral blood flow abnormalities. Color and spectral Doppler may show hyperemia, and they can be used to depict patency and flow in the major dural sinuses (see Chapter 46 ). The posterior scanning techniques are the best approach to evaluate the occipital horns for ventricular clot. The foramen magnum approach may be useful when evaluating the upper spinal canal, as in patients with a Chiari malformation.
The anterior fontanelle remains open until approximately 2 years of age but is suitable for scanning only until about 12 to 14 months. The smaller the fontanelle, the smaller is the acoustic window and the more difficult the examination will be.
Every effort should be made to maintain normal body temperature in premature infants when performing ultrasound. Their small size results in a high surface-to-volume ratio and rapid heat loss when they are exposed. Overhead warming lamps, blankets, and warmed coupling gel should be routinely used. If the infant is in an Isolette, heat loss may be minimized by using access side holes as an entry site for the transducer.
Handwashing and cleansing of the transducer between patients are of paramount importance to avoid the spread of infection in the intensive care nursery. Simple cleansing of the transducer head with a manufacturer-approved disinfectant should be adequate. When absolute sterility is required, such as during operative sonography, the transducer can be placed inside a sterile surgical glove or sterile transducer cover with coupling gel. Sterile aqueous gel or saline solution can be used as a coupler outside the sterile cover.
Standard brain scanning includes sagittal and coronal planes through the anterior fontanelle and should also routinely include at least two axial views: through the posterior fontanelle and the mastoid fontanelle. Coronal images acquired through the posterior fontanelle may be useful as well, to compare ventricular size. Magnified views with high-frequency transducers are essential to study near-field pathology such as extraaxial fluid for hemorrhage or infection and dural venous sinuses. Whenever possible, the transducer should be held firmly between the thumb and index finger, and the lateral aspect of the hand should rest on the infant's head for stability. Video clips should be obtained routinely for any abnormality to improve ultrasound diagnosis, avoid repeating a scan on an unstable newborn, and allow review of complex images and prompt diagnosis without delaying the patient workflow. Real-time imaging enables the appreciation of subtle changes in echogenicity more easily than static images. Lesions that affect gray-white matter differentiation as well as focal nonhemorrhagic infarcts may cause mild changes in echogenicity.
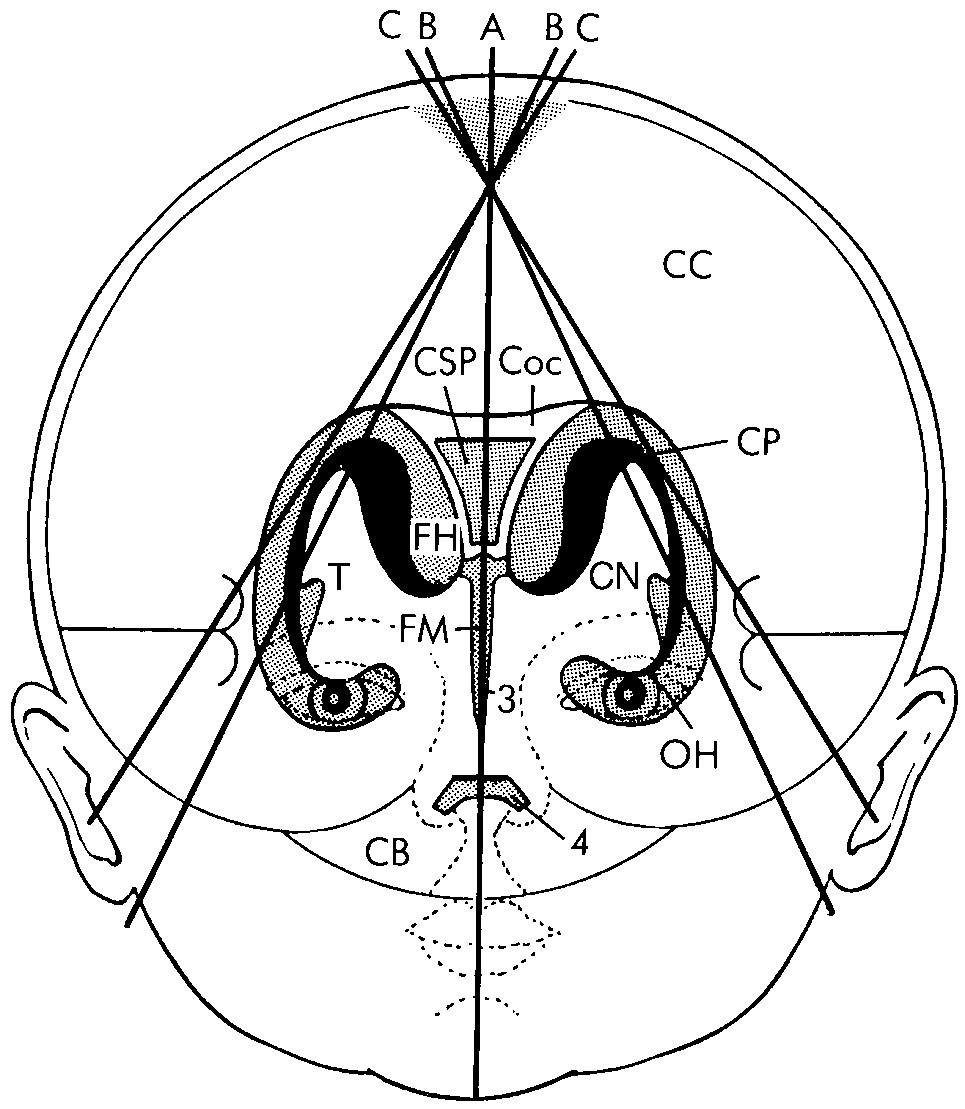
Coronal images are obtained by placing the scan head of the transducer transversely across the anterior fontanelle ( Fig. 45.1 , ). The plane of the ultrasound beam should then sweep in an anterior-to-posterior direction, completely through the brain. Care must be taken to maintain symmetrical imaging of the brain and skull. An initial sweep of the brain to obtain parallel alignment of the thick glomus of the choroid plexus in each trigone is a good method to obtain symmetry. At least six standard coronal images should be obtained during this anterior-to-posterior sweep.
Interhemispheric fissure
Cingulate sulcus
Corpus callosum
Cavum septi pellucidi
Cavum vergae (when present)
Third ventricle
Fourth ventricle
Brainstem
Vermis of cerebellum
Frontal lobe
Parietal lobe
Occipital lobe
Frontal horn of lateral ventricle
Body of lateral ventricle
Temporal horn of lateral ventricle
Trigone of lateral ventricle
Choroid plexus
Glomus of choroid plexus
Caudate nucleus
Internal capsule
Thalamus
Lentiform nucleus
Tentorium cerebelli
Cerebellar hemisphere
Sylvian fissure
Cisterna magna
The most anterior image is acquired just anterior to the frontal horns of the lateral ventricles ( Fig. 45.2A ). Visualization of the anterior cranial fossa is obtained, including the frontal lobes of the cerebral cortex with the orbits deep to the floor of the skull base.
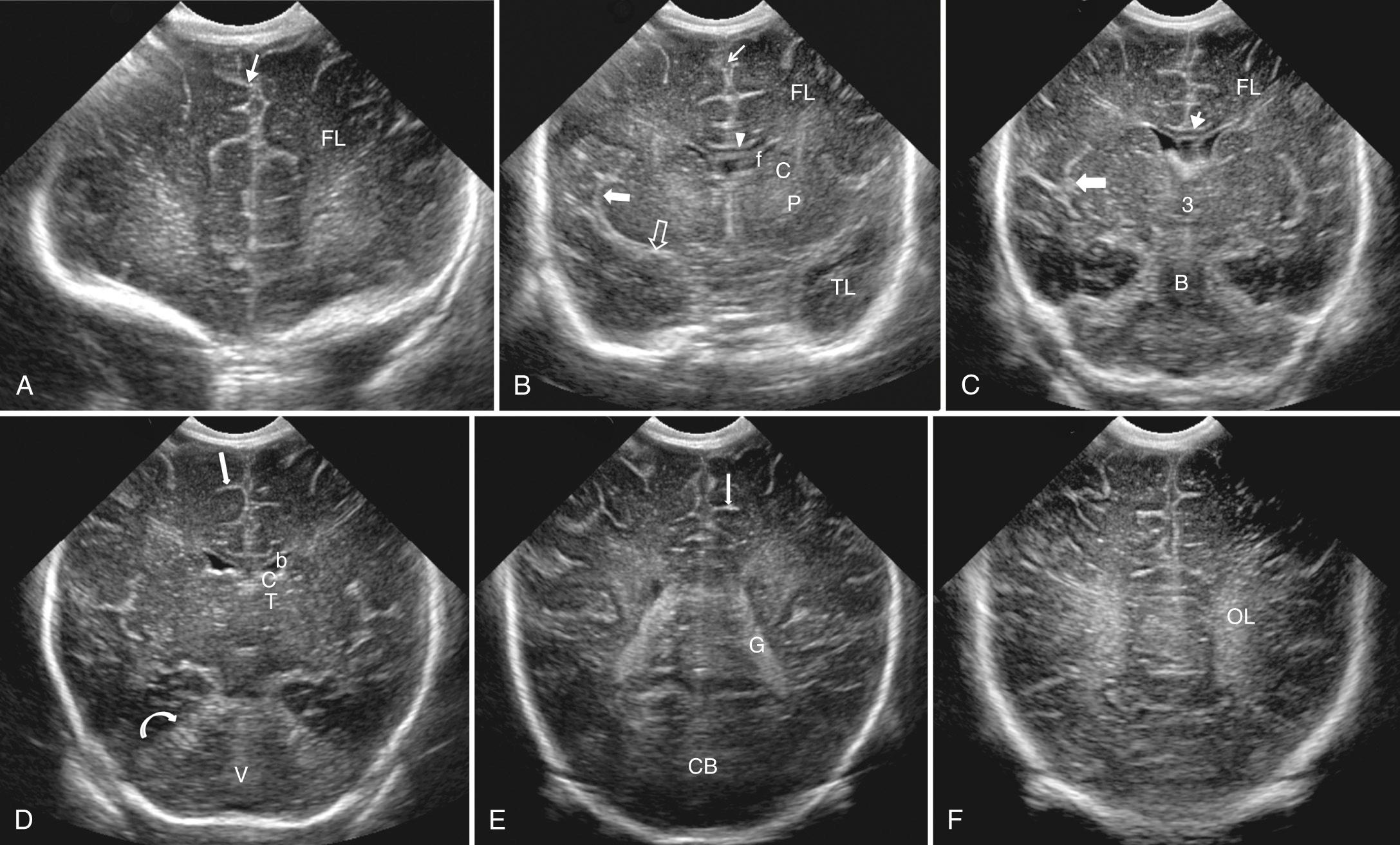
Moving posteriorly, the frontal horns of the lateral ventricles appear as symmetrical, anechoic, comma-shaped structures with the hypoechoic caudate heads within the concave lateral border (see Fig. 45.2B ). Structures visualized from superior to inferior in the midline include the interhemispheric fissure, cingulate sulcus, genu and anterior body of the corpus callosum, and septum pellucidum between the ventricles. Moving laterally from the midline, the caudate nucleus is separated from the putamen by the internal capsule. Lateral to the putamen, the sylvian fissure is echogenic because it contains the middle cerebral artery (MCA). The sylvian fissure separates the frontal from the temporal lobe. Inferiorly, the internal carotid arteries bifurcate to form the echogenic anterior cerebral artery and MCA.
Progressing farther posteriorly to the level above the midbrain, the body of the lateral ventricles is visible on either side of the cavum septi pellucidi (see Fig. 45.2C ). Below this, the thalami lie on either side of the third ventricle, which is usually too thin to visualize in normal infants. Deep to the thalami, the brainstem begins to be visualized. Lateral to the midline, the thalami are separated from the lentiform nuclei (caudate and putamen) by the internal capsule. Lateral to the lentiform nuclei is the deep white matter region of brain called the centrum semiovale. Again, the sylvian fissures are visible.
A slightly more posterior transducer angulation results in a plane that includes the cerebellum. The body of the lateral ventricles becomes somewhat more rounded as the size of the caudate nucleus decreases once posterior to the foramen of Monro (see Fig. 45.2D ). At this level in the midline, the body of the corpus callosum is deep to the cingulate sulcus, and the third ventricle is located between the anterior portions of the thalami. Echogenic material visualized in the floor of the lateral ventricles is the choroid plexus. Echogenic choroid plexus is also seen in the roof of the third ventricle, resulting in three echogenic foci of choroid. The thalami are now more prominent on either side of the third ventricle. Midline structures are unchanged, except that deep to the thalami, the tentorium covering the cerebellum can be visualized. Below this, in the posterior fossa, the vermis is the echogenic structure in the midline surrounded by the more hypoechoic cerebellar hemispheres. When the septum pellucidum is cystic posteriorly, it is called the cavum vergae. Because the cystic center of the septum pellucidum closes from posterior to anterior as the brain matures, late-gestation neonates often have only the more anterior cavum septi pellucidi. The lentiform nuclei may no longer be seen at this level. The temporal horns of the lateral ventricles may be seen lateral and inferior to the thalami, but are usually not seen unless there is hydrocephalus.
Further posteriorly, the trigone or atrium of the lateral ventricles and occipital horns are visualized (see Fig. 45.2E ). The extensive echogenic glomus of the choroid plexus nearly obscures the lumen of the cerebrospinal fluid (CSF)–filled ventricle at the trigone. In the midline—the visualized portion of the corpus callosum deep to the cingulate sulcus—is the splenium. Inferiorly, the cerebellum is separated from the occipital cortex by the tentorium cerebelli.
The most posterior section visualizes predominantly occipital lobe cortex and the most posterior aspect of the occipital horns of the lateral ventricles that do not contain choroid plexus (see Fig. 45.2F ). This section is angled posterior to the cerebellum.
Normal premature brain ultrasound images in the same planes are shown in Fig. 45.3 . The lateral ventricles are slightly larger; the cavum septi pellucidi extends back to become the cavum vergae between the lateral ventricle bodies and occipital horns. There are only a few sulci, and the sylvian fissures are wider and typically appear boxlike rather than as thin fissures. The basal ganglia in premature infants are normally diffusely homogeneously echogenic, an appearance that is more prominent than in the thalami and typically more echogenic than the cerebral cortex. Basal ganglia and thalami do not show increased echogenicity at term-equivalent age.
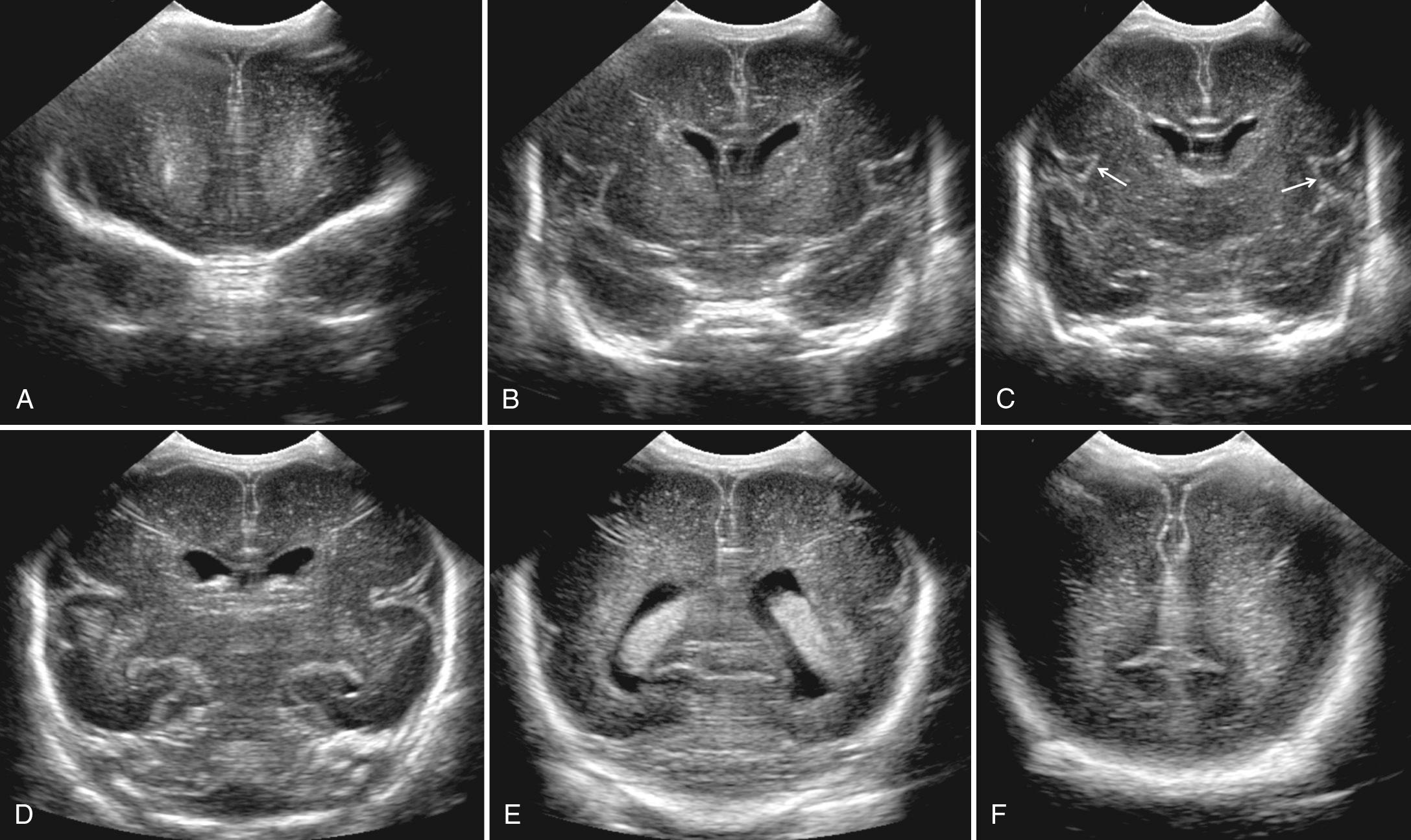
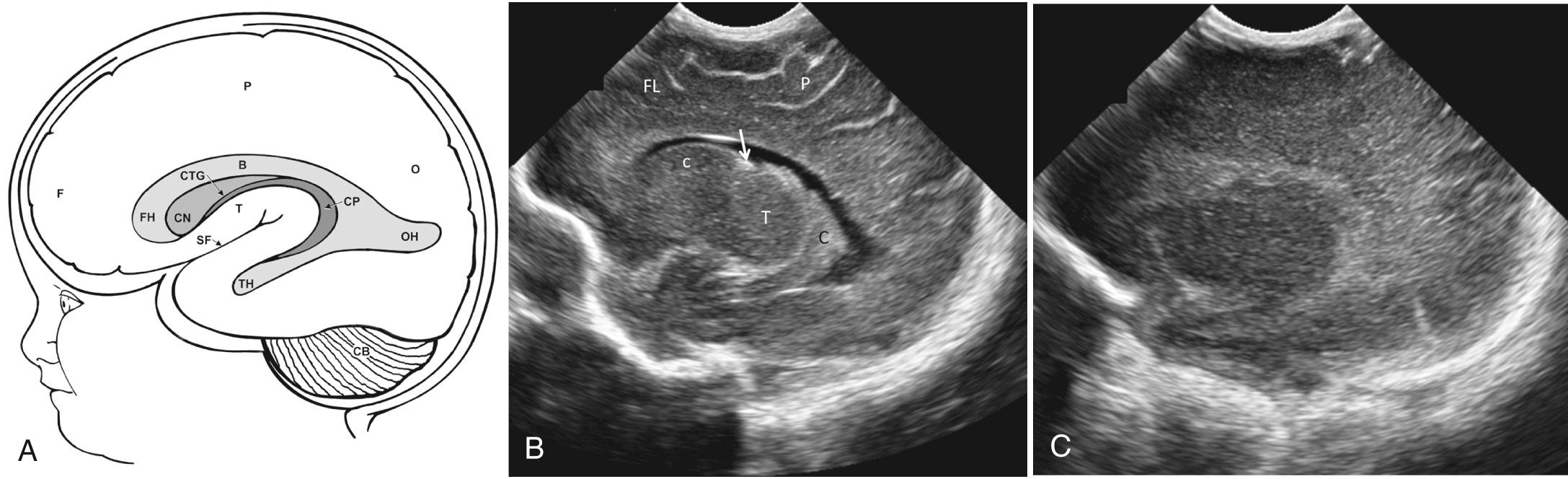
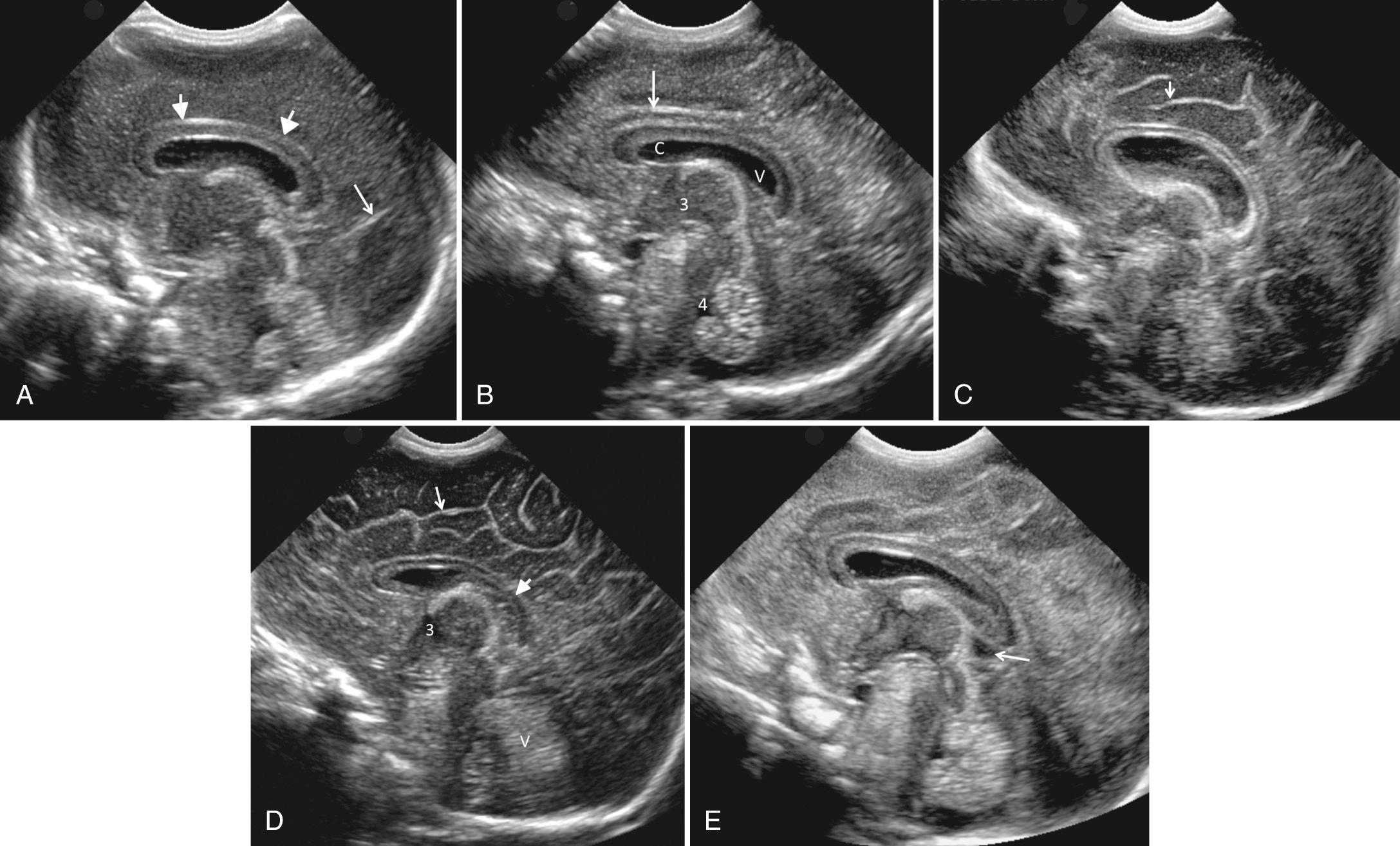
The sagittal images are obtained by placing the transducer longitudinally across the anterior fontanelle and angling it to each side ( Fig. 45.4 , ). The midline is first identified through the interhemispheric fissure by recognition of the curving line of the corpus callosum above the cystic cavum septi pellucidi and cavum vergae. Below the cavum lies the choroid plexus in the roof of the third ventricle. If there is third ventricular enlargement, the massa intermedia will be outlined by CSF and the aqueduct of Sylvius may be visualized. The fourth ventricle is identified as a notch in the anterior surface of the highly echogenic cerebellar vermis ( Fig. 45.5 ).
The cingulate sulcus lies parallel to and above the corpus callosum. The highly echogenic falx can be difficult to scan directly through and obtain a clear midline sagittal image; therefore a very slight movement to either side of the falx may be necessary to adequately visualize midline brain structures. In this view, the size of the cerebellar vermis has been used to assess gestational age. However, the degree of sulcal development is the most reliable method for gestational age based on established pathologic standards. Shallow angulation to each side of about 10 degrees will show the normally small lateral ventricles ( Fig. 45.6A-B ). The ventricles are not located in a perfectly straight plane anterior to posterior. The transducer must be angled so that the anterior portion of the sector is directed more medially and the posterior portion more laterally, to include the entire lateral ventricle in a single image.
Frontal lobe
Parietal lobe
Occipital lobe
Cingulate sulcus
Pericallosal artery
Corpus callosum
Cavum septi pellucidi
Cavum vergae a
a Not always visible.
Cavum velum interpositum a
Third ventricle
Fourth ventricle
Tentorium
Choroid plexus, third ventricle
Aqueduct
Occipitoparietal fissure
Brainstem
Vermis of cerebellum
Frontal lobe
Parietal lobe
Occipital lobe
Frontal horn of lateral ventricle
Body of lateral ventricle
Atrium of lateral ventricle (trigone)
Temporal horn of lateral ventricle
Occipital horn of lateral ventricle
Choroid plexus
Caudate nucleus
Thalamus
Caudothalamic groove
Cerebellum
Above the lateral ventricle is the cerebral cortex, and below it is the cerebellar hemisphere. The caudate nucleus and the thalamus surround the third ventricle and are within the arms of the ventricle (see Fig. 45.6B ). The caudothalamic groove at the junction of these two structures is an important area to recognize, because this is the most common site of germinal matrix hemorrhage (GMH) in the subependymal region of the ventricle.
More pronounced lateral angulation will demonstrate the peripheral aspect of the ventricles and the more lateral cerebral hemisphere, including the temporal lobes (see Fig. 45.6C ) where the MCA branches extend toward the ventricle.
Sagittal sonography almost always reveals a normal hyperechoic peritrigonal blush in the parietal lobe just posterior and superior to the ventricular trigones on parasagittal views (see Fig. 45.6B ). It is caused by the interface of numerous parallel fibers that are perpendicular to the longitudinal angle of the ultrasound beam passing through the anterior fontanelle. This is an anisotropic effect artifact that occurs when the beam is perpendicular to the fibers through the anterior fontanelle. A similar area of increased echogenicity is not seen on sonograms obtained through the posterior fontanelle, because with that angulation, the long axis of the ultrasound beam and the fiber tracts are almost parallel.
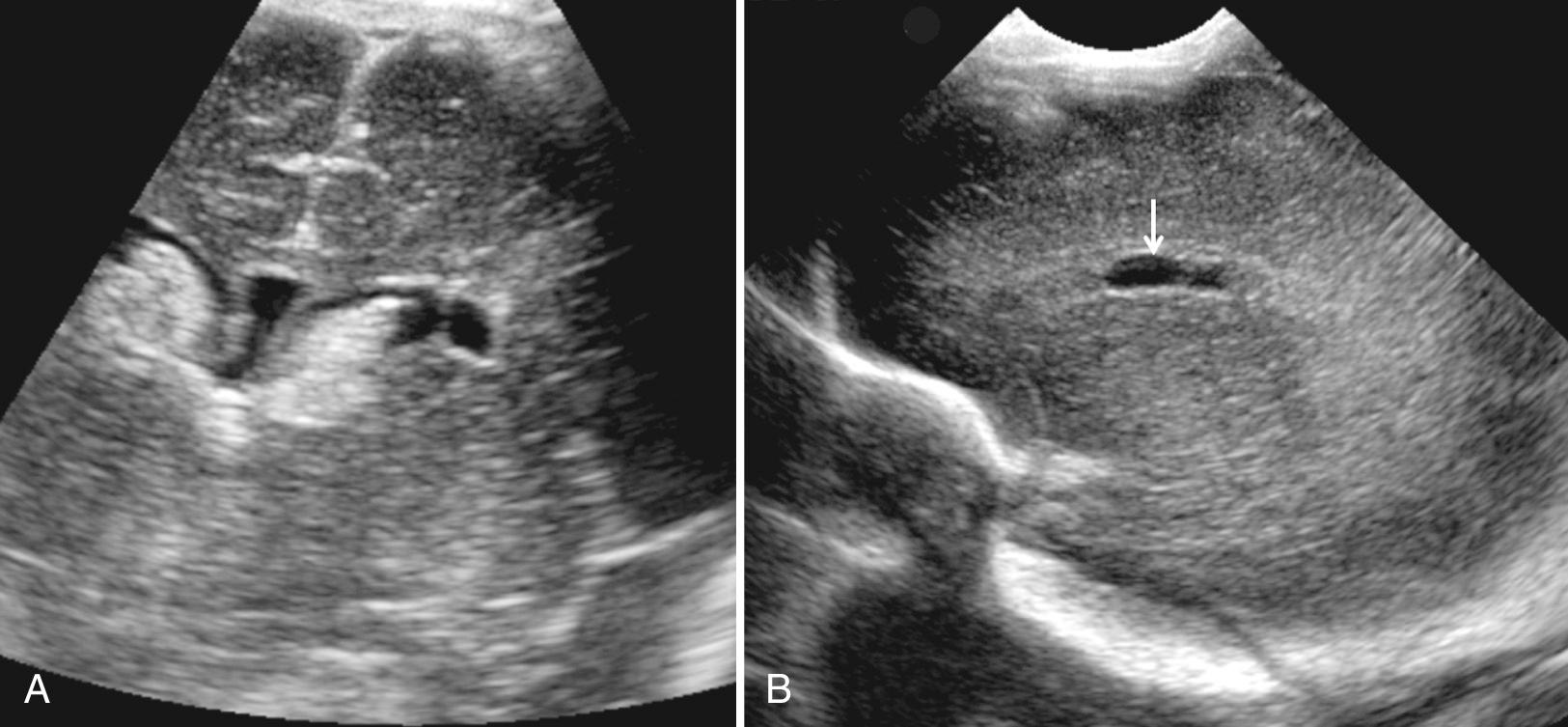
Posterior fontanelle imaging is very useful to evaluate the occipital horns for the diagnosis of intraventricular hemorrhage (IVH). The posterior fontanelle lies in the midline at the junction of the lambdoid and sagittal sutures; it is open only until about 3 months of age ( Fig. 45.7 ). The transducer should be angled slightly off midline with the anterior portion of the probe directed slightly medially, to demonstrate the lateral ventricular trigone with its occipital horn in the near field ( Fig. 45.8 ). The choroid glomus will be seen with extensions into the ventricular body and temporal horn. The occipital horn does not contain choroid plexus and should be completely anechoic. Angling the transducer into the left and right parasagittal planes will display each occipital horn. These planes are extremely useful for detecting dependently layering clot and clot attached to the choroid plexus.
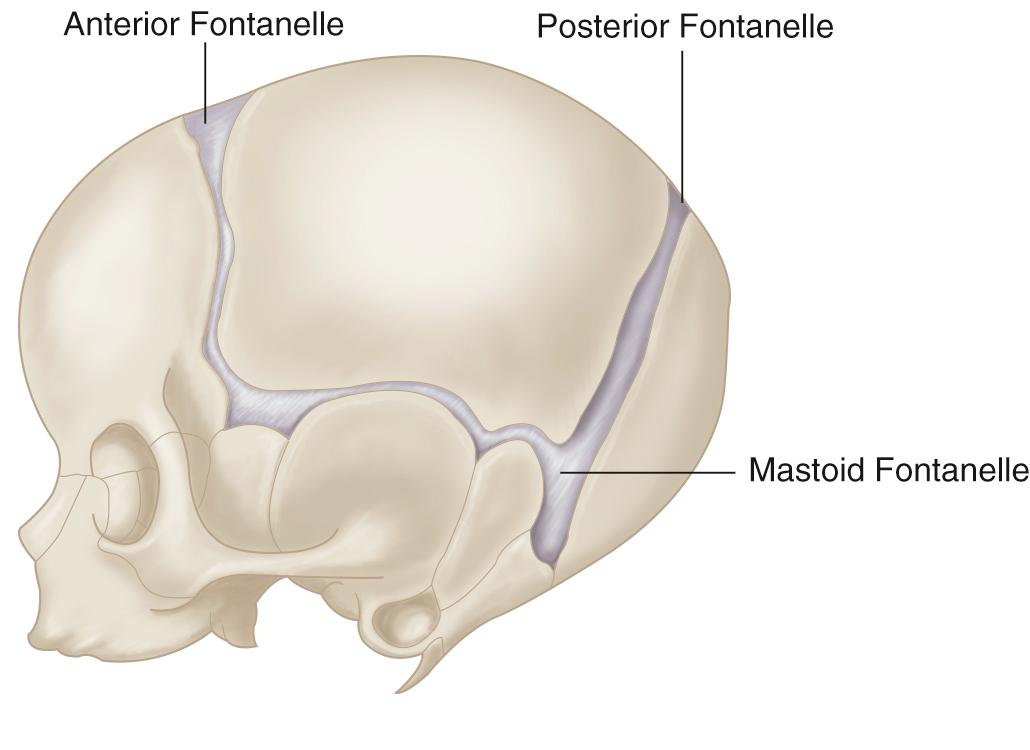
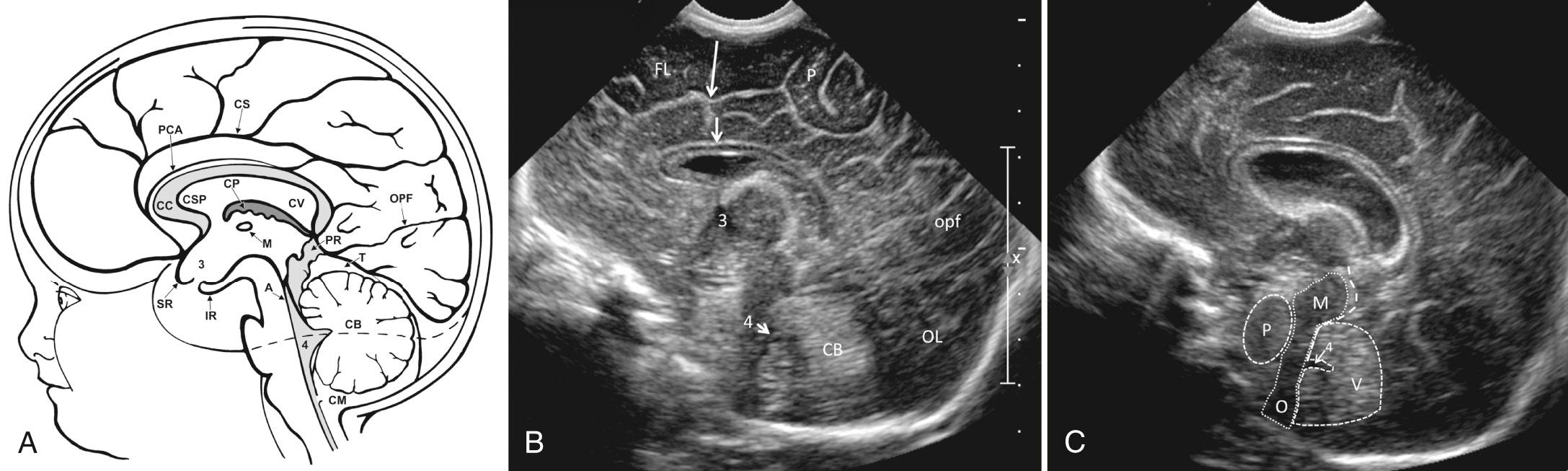
The mastoid fontanelle allows assessment of the brainstem and posterior fossa, which are not well demonstrated in the standard planes through the anterior fontanelle. The ultrasound transducer is placed about 1 cm behind the helix of the ear and 1 cm above the tragus. The mastoid fontanelle is located at the junction of the squamosal, lambdoidal, and occipital sutures (see Fig. 45.7 , ). Posterior fossa axial images, with the anterior portion of the transducer angled slightly cephalad, will demonstrate the fourth ventricle, posterior cerebellar vermis, cerebellar hemispheres, and the cisterna magna. These axial images should be displayed with the top of the head to the left ( Fig. 45.9 ). The radiating folia of the cerebellar hemispheres contain relatively hypoechoic neural tissue and are surrounded by echogenic leptomeninges in the multiple cerebellar fissures. In the roof of the fourth ventricle lies the normal echogenic fourth ventricular choroid plexus. Behind the fourth ventricle is the echogenic midline vermis, which appears less echogenic in the axial view compared with midline sagittal scans. When angled axial images are obtained through the lower parts of the cerebellum below the fourth ventricle, the (normally thin) vallecula may be seen in the midline as a space between the cerebellar hemispheres (see Fig. 45.9C ), particularly in the presence of hydrocephalus. In steeply angled axial scans, the foramen of Magendie can be seen in the midline as a thin sonolucent line between the cerebellar hemispheres extending from the fourth ventricle to the cisterna magna. It should not be mistaken for a Dandy-Walker variant. The presence of an intact vermis on higher images and the marked caudal angulation required to see the vallecula allow differentiation of this normal variant. Color Doppler ultrasound in this view allows evaluation of flow in the transverse and straight sinuses to exclude venous thrombosis.
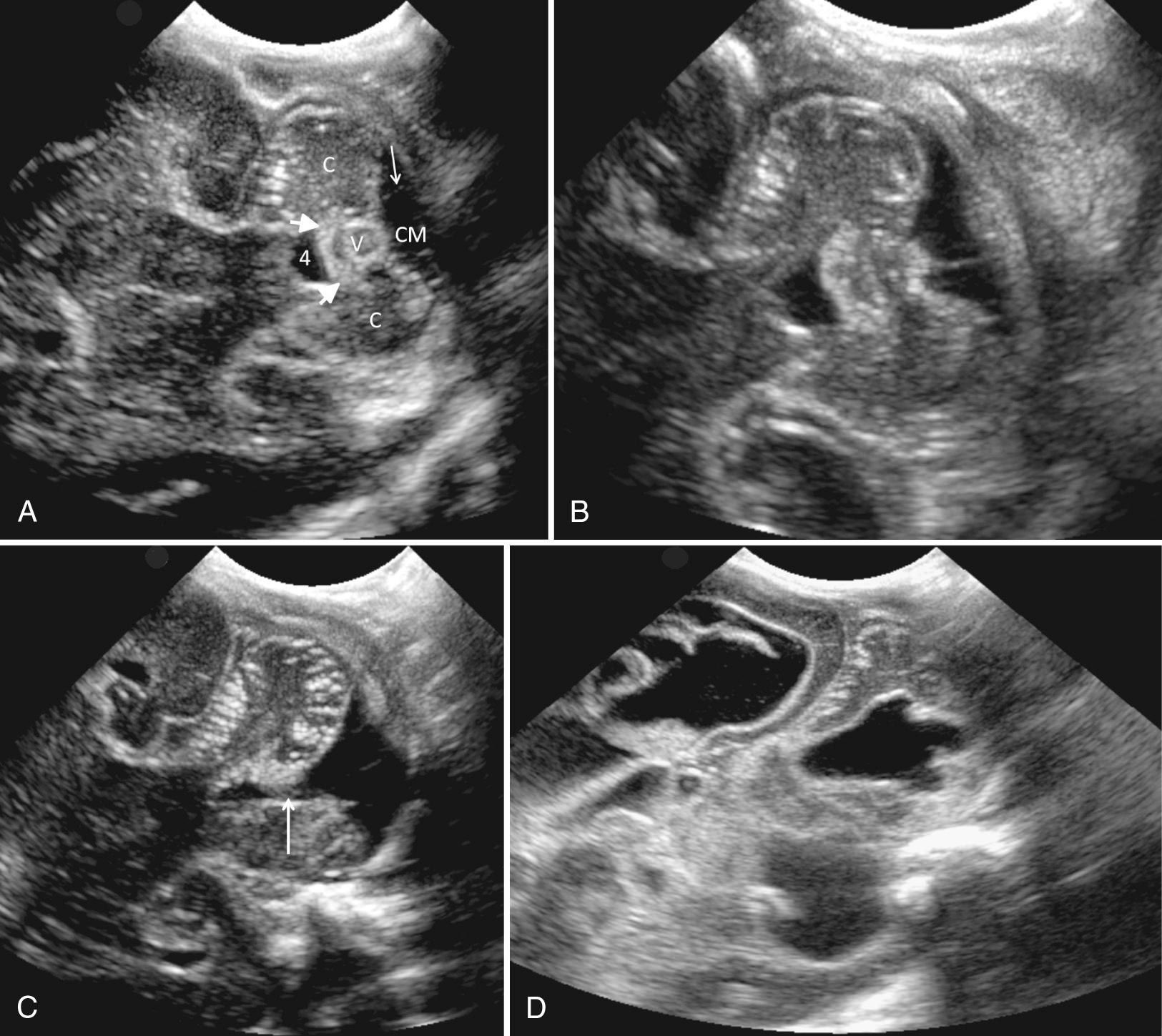
A slightly higher axial scan should include the thalami, midbrain, third ventricle, aqueduct of Sylvius, and quadrigeminal plate cistern, with the transducer angled from the standard axial plane and placed cephalad to the external auricle ( Fig. 45.10 ). At this level, the thalami are hypoechoic, inverted, heart-shaped structures. The midbrain, including the cerebral peduncles and corpora quadrigemina, consists of paired hypoechoic lenticular structures just caudal to the thalami. The third ventricle is usually a thin cleft, barely visible between the thalami. The aqueduct is usually a thin echogenic line but may occasionally be a thin slit in the midbrain. The quadrigeminal cistern is echogenic and surrounds the midbrain.
Three-dimensional (3-D) ultrasound may be a useful adjunct to standard two-dimensional (2-D) imaging of the neonatal brain. A volume of brain can be acquired in a few seconds, and then reconstructed displays of three octagonal slices at any angle can be viewed, in addition to coronal, sagittal, and axial planes. Brain lesions can be tracked in three views at once, which can better identify the position in the brain and the most likely diagnosis. Some authors recommend 3-D volume measurement of the ventricles ; the standard method is still a qualitative assessment based on typical normal ventricles of different age groups.
Brain anatomy images should be displayed in a consistent manner so that comparisons can be easily made. At our institution, routine sagittal images are always shown with the infant's face to the left. Labels on sagittal scans should be “left” or “right.” Video clips are more difficult to label and might be best done separately on the left and right, in addition to a full sweep through the entire brain from side to side.
With use of the American College of Radiology (ACR) and American Institute of Ultrasound Medicine (AIUM) practice parameters for the performance of neurosonography in neonates and infants, there are specific questions to answer. Developing a template for reports is a useful task to ensure complete reporting.
The ventricular size and configuration are <normal>.
The corpus callosum is <present> <above the cavum septum pellucidi and/or cavum vergae>.
There is <no hemorrhage in the caudothalamic groove or in the ventricular system>.
The cerebellar vermis, fourth ventricle, and cerebellar hemispheres <appear normal>.
Sulcal development is <normal> for gestational age.
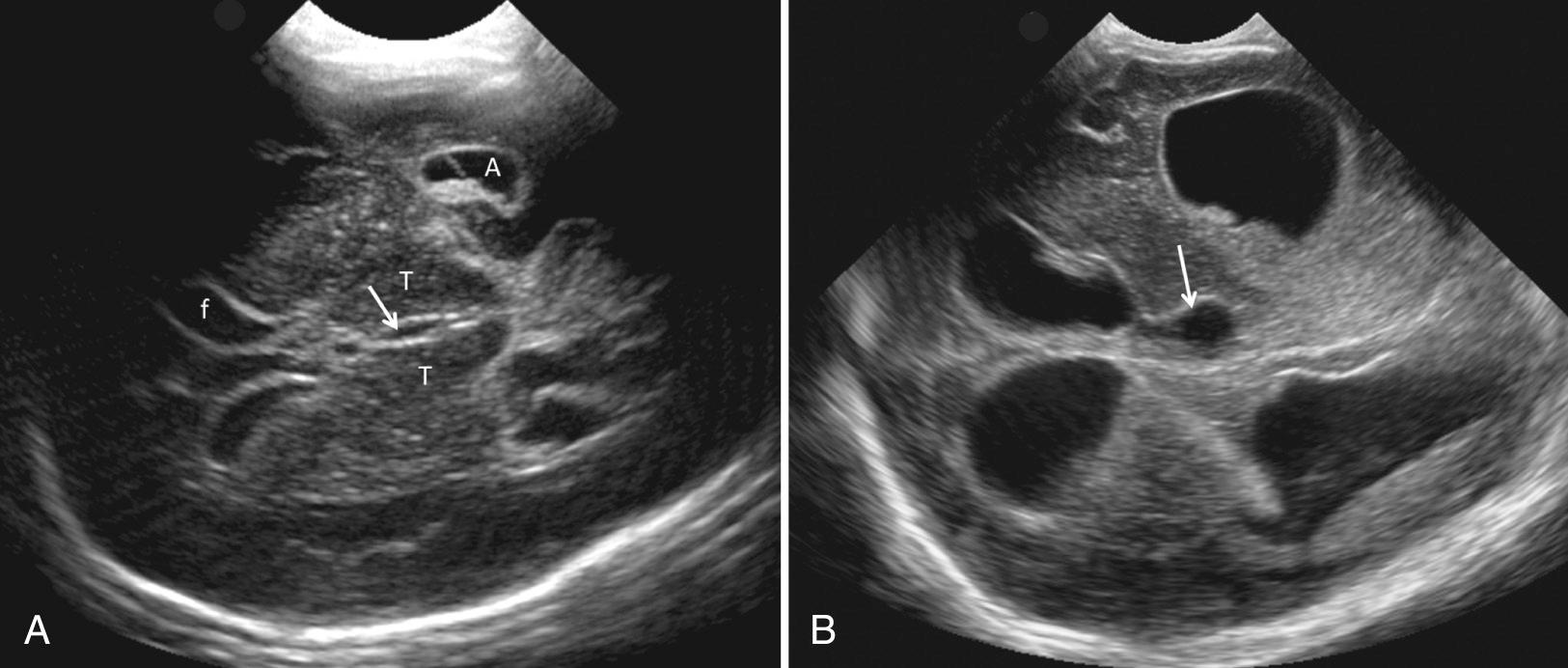
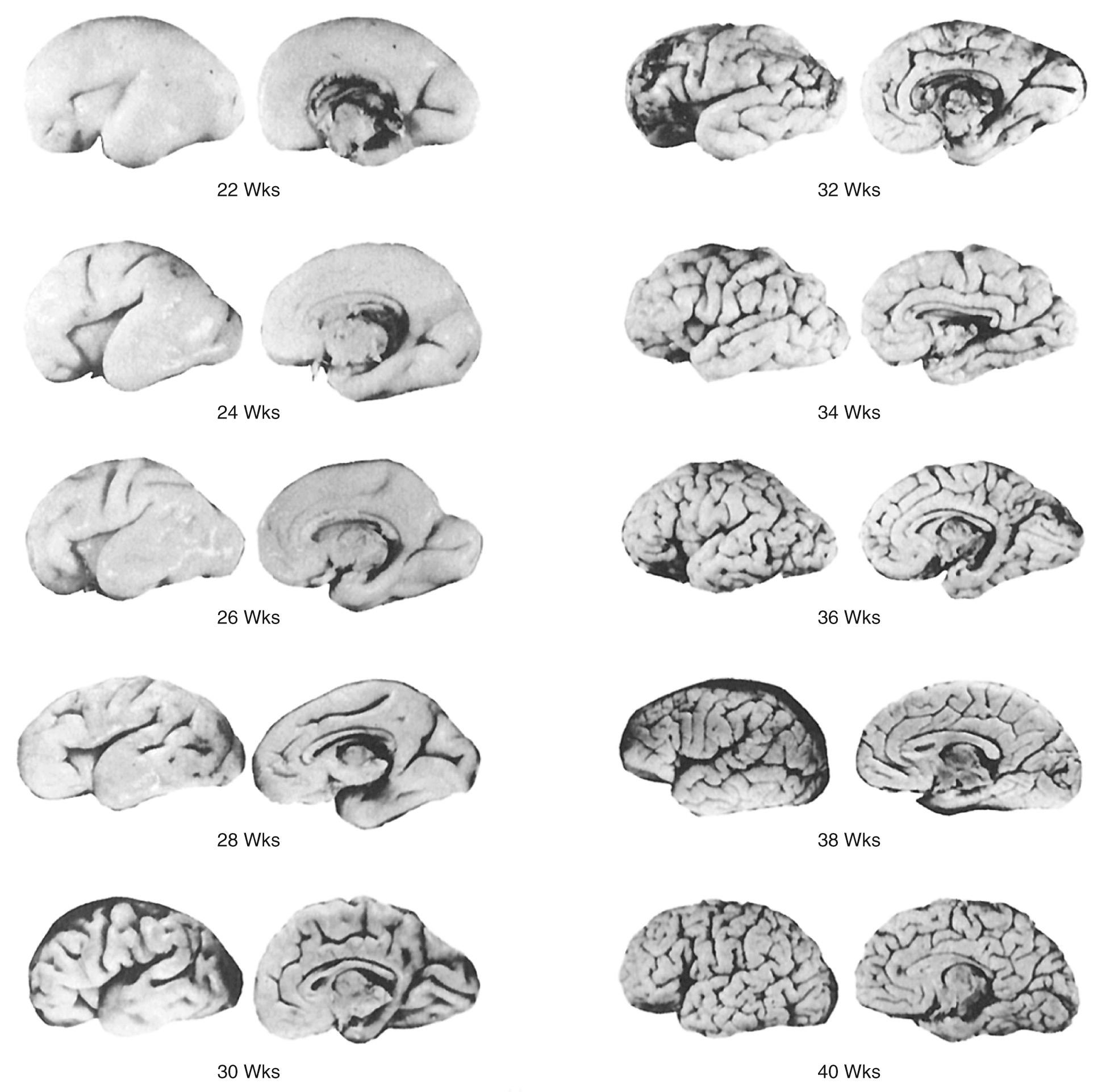
In the very premature infant, the brain sulci are not fully developed and the brain appears quite smooth ( Figs. 45.11 and 45.12 ). The first sulcus to form is the primitive, almost square, sylvian fissure, best seen on coronal images (see Fig. 45.3C ). Later, after infolding of the insula (opercularization), the sylvian fissure becomes a narrow, echogenic fissure filled with MCA branches. Sulcal development, best evaluated on midline sagittal images, then extends to the calcarine fissure as a simple straight line in the fifth gestational month (20 weeks). By 24 to 25 weeks' gestation, the occipitoparietal fissure is present, but no actual sulci. By 28 weeks, the callosomarginal sulcus over the corpus callosum and a simple linear cingulate sulcus superior and parallel to the corpus callosum are seen (see Fig. 45.12B ). By 30 weeks, the cingulate sulcus is branched. Between 33 and 40 weeks' gestation, sulci bend, branch, and anastomose so that a full-term infant has many peripheral branches over the brain surface (see Fig. 45.12D ).
Although not used routinely, measurement of the subarachnoid space on a magnified view of the brain can be done from the triangular sagittal sinus to the surface of the cortex. Armstrong and colleagues have reported that the subarachnoid space is normally less than 3.5 mm in 95% of preterm infants before 36 weeks' gestation. Closer to term, the values tended to be at the higher end of the range, increasing slightly on a weekly basis, which suggests that premature infants have reduced brain growth during extrauterine life.
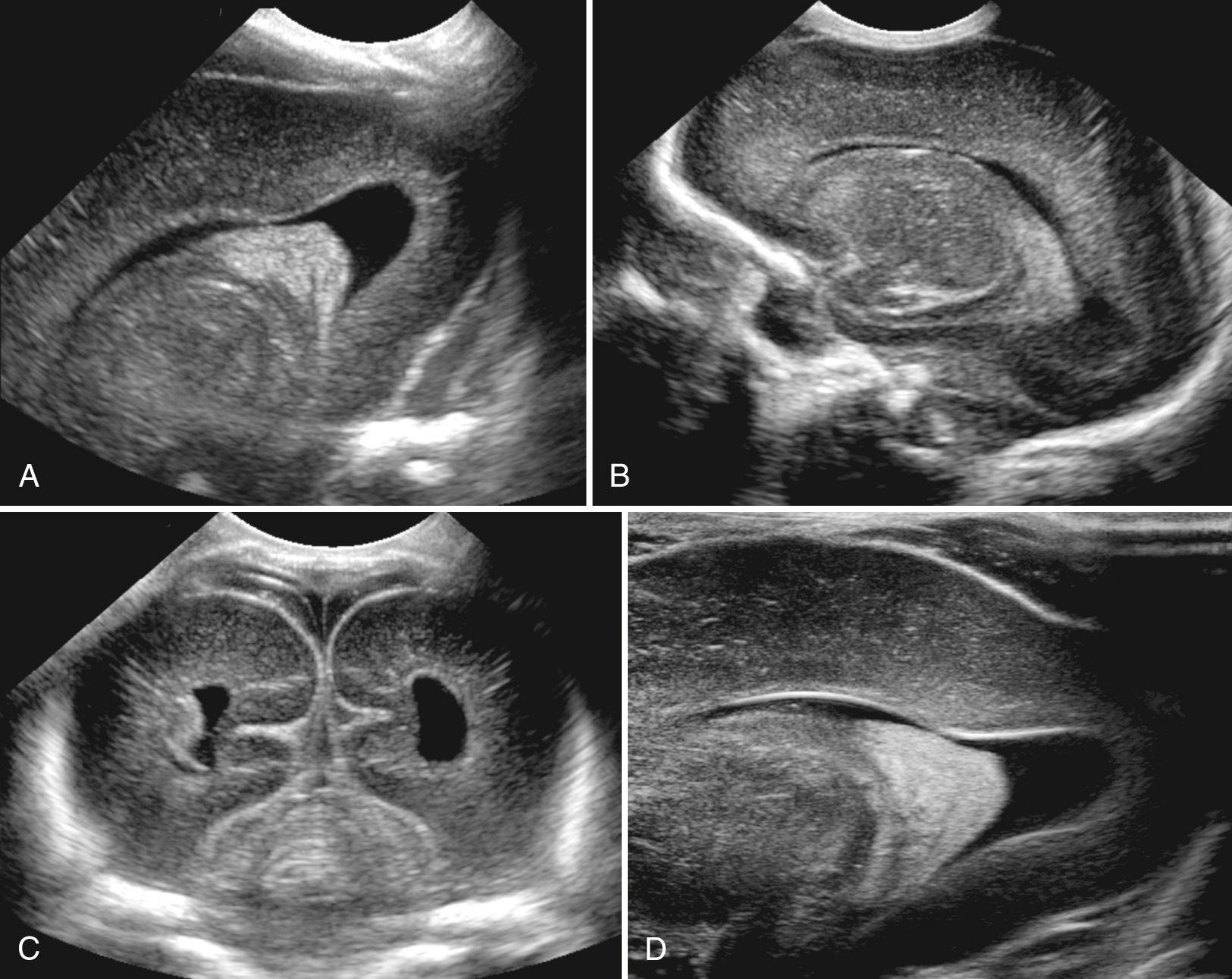
There is one continuous cystic midline structure in the septum pellucidum during fetal life. The septum contains the cavum septi pellucidi anterior to the foramen of Monro (see Fig. 45.12 ) and the cavum vergae posteriorly. Both parts are normally present early in gestation, but they close from back to front, starting at approximately 6 months' gestation ( Fig. 45.13 ). By full term, closure has occurred posteriorly in 97% of infants so that there is only a cavum septi pellucidi at birth. By 3 to 6 months after birth, this septum is completely closed in 85% of infants, although in some the septum remains open into adulthood. In fetal brain imaging, Callen and colleagues reported that the columns of the fornix can be mistaken for the cavum septi pellucidi. On axial views below the frontal horns, the fornix appears as a cystic structure with a central linear echo. Absence of the corpus callosum may not be identified because the fornices are present and simulate the cavum between the two frontal horns. Careful evaluation of the inferior position of the fornices below the frontal horns will avoid this error.
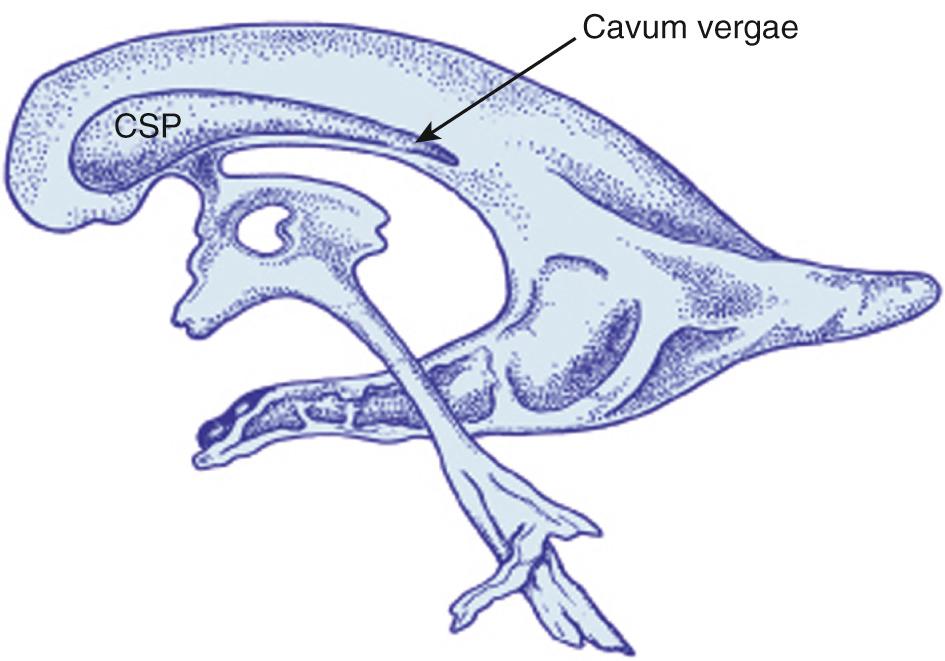
The cavum of the velum interpositum represents a potential space above the choroid in the roof of the third ventricle and below the columns of the fornices. It may appear as an anechoic, inverted, helmetlike space just inferior and posterior to the splenium in the pineal region (see Fig. 45.12E ). Blasi and colleagues have described the prenatal diagnosis of the cavum veli interpositi on 2-D and 3-D ultrasound with color flow Doppler. Careful study of the anatomic location of the cystic structure, its size, and changes over time is required to be certain this is a normal variant, not an arachnoid cyst with mass effect or associated anomaly of the corpus callosum. Chen and colleagues reported that 21% of neonates have a cavum veli interpositi on sonography. By 2 years of age, this cystic area is an uncommon finding and thus is thought to be a normal stage of brain development. Rarely, a cyst is found in this area that causes compression of other structures.
A few newborns have cysts exactly parallel (not above or below) and adjacent to the frontal horns ( Fig. 45.14 ). These cysts are typically bilateral and have septations between the cyst and the frontal horns. Frontal horn cysts, also called coarctation of the frontal horn and connatal cysts, are caused by folding of the frontal horn on itself, resulting in kinking (seen as a septation). Typical normal frontal horns are directly lateral to the cavum septi pellucidi on coronal images, below the corpus callosum. The frontal horns are relatively thin compared with the occipital horns.
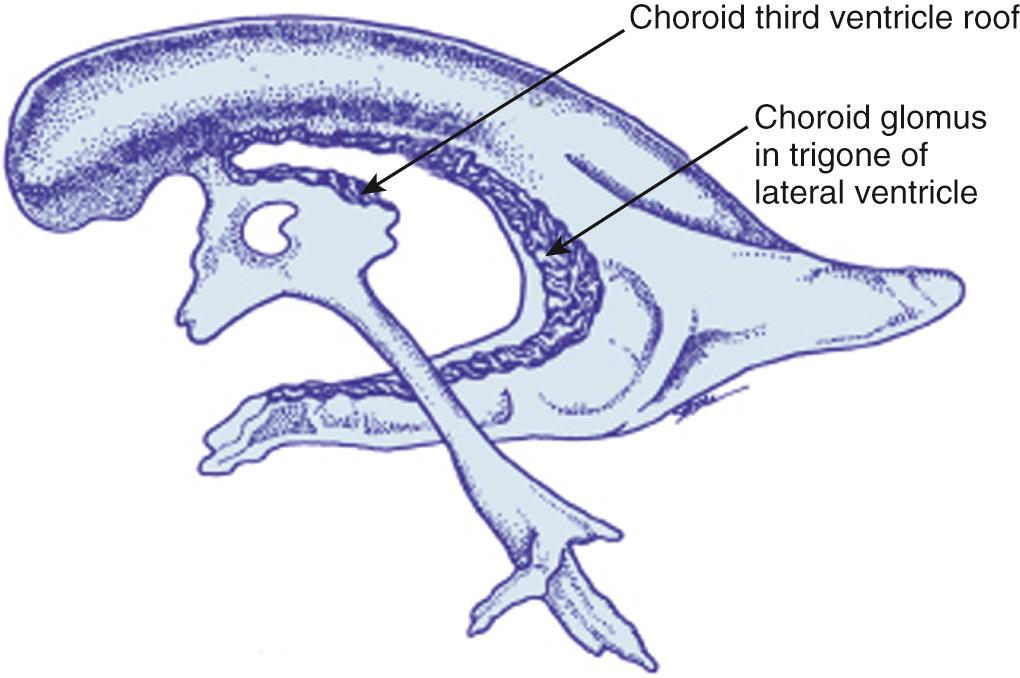
The choroid plexus is the site of CSF production in the ventricles ( Fig. 45.15 ). The largest portion of the choroid plexus is the glomus, a highly echogenic structure attached to the trigone of each lateral ventricle. The choroid tapers as it extends anteriorly to the foramen of Monro and continues from each lateral ventricle into and along the roof of the third ventricle. The choroid plexus tapers laterally as it extends into the temporal horn of the lateral ventricle. The choroid plexus does not extend into the frontal or occipital horn. The choroid plexus is also present in the roof of the fourth ventricle. (see 45.9A ) Small cysts in the choroid plexus are common, but at times areas that appear to be cysts are in fact small vessels.
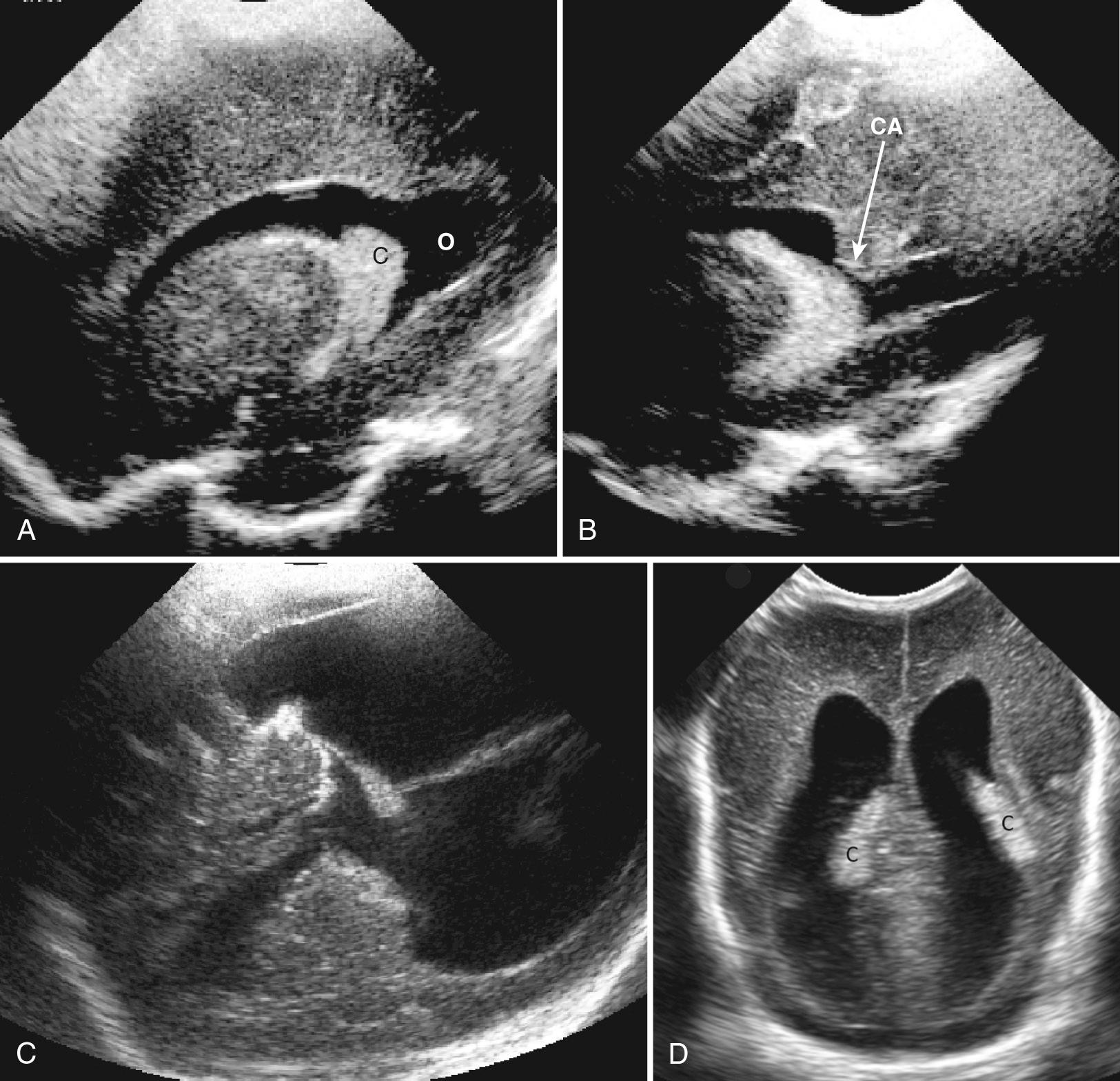
The glomus of the choroid plexus is often doubled and thus appears lobulated ( Fig. 45.16 ). Some authors have termed this appearance a “split” choroid plexus. It may be mistaken for clot adhering to the choroid plexus. Color Doppler ultrasound will differentiate the normal highly vascular choroid from similarly echogenic, but avascular, clot (see Fig. 45.8C ). Coronal views may also show a flattened or truncated choroid plexus at the level of the thickest portion at the trigone, probably related to the angle of the transducer to the choroid. If there is a large ventricle, the choroid will normally “dangle,” or hang down toward the dependent ventricle, when the infants are lying on their side (see Fig. 45.16D ).
Peritrigonal blush on sagittal view (anisotrophic effect)
Choroid plexus shapes affected by position
Normal choroid plexus: split, lobulated, or truncated
Dangling choroid (in hydrocephalus)
Normal choroidal vessels vs. choroid plexus cyst
Normal choroid plexus vs. hemorrhage around the choroid
Asymmetrical normal-sized ventricles
Dandy-Walker malformation false-positive diagnosis due to angled view through the lower cerebellum
The germinal matrix develops deep to the ependyma and consists of loosely organized, proliferating cells that give rise to the neurons and glia of the cerebral cortex and basal ganglia ( Fig. 45.17 ). Its vascular bed is the most richly perfused region of the developing brain. Vessels in this region form an immature vascular rete of fine capillaries, extremely thin-walled veins, and larger irregular vessels. The capillary network is best developed on the periphery of the germinal matrix and becomes less well developed toward the central glioblastic mass. Although the germinal matrix is not visualized on sonography, it is important as the typical anatomic site over the caudate nucleus where GMH occurs in premature infants.
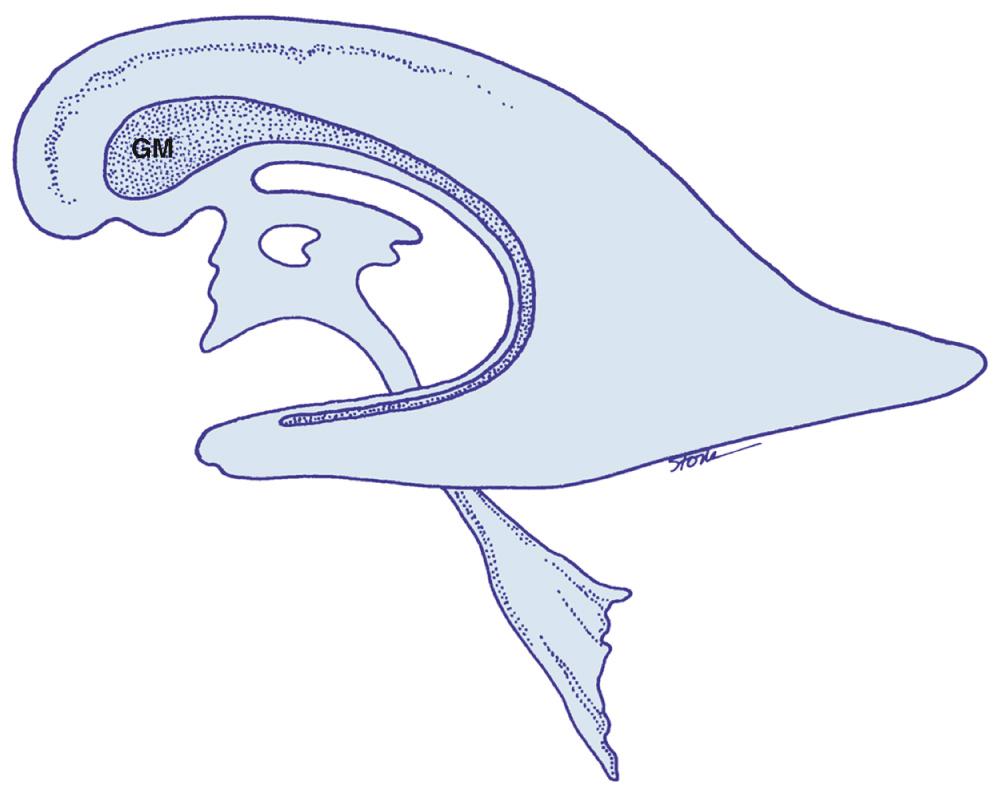
Early in gestation, the germinal matrix forms the entire wall of the ventricular system. After the third month of gestation, the germinal matrix regresses, first around the third ventricle, then around the temporal and occipital horns and trigone. By 24 weeks' gestation, the germinal matrix persists only over the head of the caudate nucleus and to a lesser extent over the body of the caudate. By 32 weeks' gestation, it is unusual to see GMH because these cells migrate out to the cerebral cortex. This regression continues until 40 weeks' gestation, when the germinal matrix ceases to exist as a discrete structure, and the immature vascular rete has been remodeled to form adult vascular patterns.
On posterior fontanelle views, a normal gyrus, the calcar avis, frequently protrudes into the medial aspect of the lateral ventricle at the junction of the trigone and occipital horn (see Fig. 45.16B ). Although this normal brain gyrus may mimic intraventricular clot, slightly turning the transducer will show its continuity with the brain. It can be recognized because of a central echogenic sulcus (calcarine fissure), its continuity with the adjacent brain, and normal vascularity on color Doppler ultrasound.
Because the cerebellum develops late in gestation, a mistaken fetal diagnosis of cerebellar vermian hypoplasia is occasionally made. If axial scans are done below the normal level of the fourth ventricle, the vallecula between the cerebellar hemispheres may be mistaken for a Dandy-Walker variant. Pseudoabsence of the inferior vermis or pseudo–vermian hypoplasia can be appreciated on axial views through the posterior fossa. There may appear to be a small or absent cerebellar vermis and a wide communication between the fourth ventricle and cisterna magna. Slightly more superior axial views will show the normal cerebellar vermis. The appearance of a missing or hypoplastic vermis can be evaluated carefully by moving the transducer superiorly to depict the normal vermis, and by obtaining sagittal midline views. The cerebellar vallecula is a variably sized subarachnoid space below and not continuous with the fourth ventricle. The foramen of Magendie is thinner than the vermian cleft in a Dandy-Walker variant (see Fig. 45.9C ).
Cisterna magna septa are typically seen inferior and posterior to the cerebellar vermis, usually straight and parallel (see Fig. 45.9 ). These septa arise at the cerebellovermian angle and continue to the occipital bone. Robinson and Goldstein proposed that these septa are a remnant of Blake pouch cysts and thus a marker of normal cerebellar development.
Congenital brain malformations are the most common anomalies in humans. Malformations can be classified based on brain development and the types of anomalies that result when development is altered. Brain development can be divided into three stages. Cytogenesis involves the formation of cells from molecules. Histogenesis is the formation of cells into tissues and involves neuronal proliferation and differentiation. Organogenesis is the formation of tissues into organs.
Organogenesis can be subdivided into further stages ( Fig. 45.18 ). The first stage, neural tube formation and closure, occurs at 3 to 4 weeks' gestation. The neural plate folds in on itself, fusing dorsally and giving rise to the earliest recognizable brain and spinal cord. In the next stage, segmentation and diverticulation of the forebrain occur at 5 to 6 weeks. The single central fetal ventricle separates into two lateral ventricles, and the brain divides into two cerebral hemispheres. Anterior diverticulation results in the formation of the olfactory bulbs and optic vesicles and the induction of facial development. The pituitary and pineal gland also develop by diverticulation from the ventricle at this stage.
Chiari malformation
Agenesis of the corpus callosum
Lipoma of the corpus callosum
Dandy-Walker malformation or variant
Posterior fossa arachnoid cyst
Teratoma
Septo-optic dysplasia
Holoprosencephaly (alobar, semilobar, lobar)
Lissencephaly
Schizencephaly
Heterotopias
Pachymicrogyria or polymicrogyria
DISORDERS OF MYELINATION
DESTRUCTIVE LESIONS
DISORDERS OF HISTOGENESIS
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here