Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Despite meticulous adherence to presently known principles of myocardial protection, perioperative myocardial damage related to ischemia-reperfusion injury continues to occur after cardiac operations that have been performed in a technically adequate manner. Ischemia-reperfusion injury associated with surgically induced myocardial ischemia secondary to aortic cross-clamping results from the attenuation or cessation of coronary blood flow such that oxygen delivery to the myocardium is insufficient to meet basal myocardial requirements to preserve cellular membrane stability and viability. Recovery after surgically induced ischemic arrest involves (1) resumption of normal oxidative metabolism and the restoration of myocardial energy reserves; (2) reversal of ischemia-induced cell swelling, loss of ion gradients, and adenine nucleotide losses; and (3) repair of damaged cell organelles such as mitochondria and the sarcoplasmic reticulum.
After the initiation of open heart surgery with use of extracorporeal circulation by Gibbon, it soon became obvious that aortic cross-clamping was necessary to provide a bloodless field to facilitate the precise repair of intracardiac defects, to prevent air embolism when the left side of the heart was opened, and to avoid a turgid myocardium resistant to retraction. To overcome the difficulties of operating on a rheumatic mitral valve in a patient with aortic regurgitation, Melrose and colleagues introduced the concept of “elective cardiac arrest” by rapidly injecting into the aortic root, after aortic cross-clamping, a 2.5% potassium citrate solution in warm blood to arrest the heart. Soon thereafter, experimental and clinical evidence demonstrated the development of severe myocardial necrosis associated with the Melrose technique.
During the 1960s, two distinct technical pathways for management of ischemic arrest evolved. The “rapid operators” performing uncomplicated cases with short ischemic times adopted the use of normothermic ischemic arrest, until operative mortalities from the “stone heart” syndrome related to ischemic contracture of the myocardium associated with low levels of high-energy phosphate moieties became apparent. Intermittent aortic cross-clamping, involving reperfusion of the coronary circulation for 5 minutes after 15 minutes of ischemic arrest, is an empirical technique, still in use, and was based on the concept that after 15 minutes of ischemia, a sufficient concentration of high-energy phosphate moieties remained in the myocardium to allow replenishment of myocardial stores during the reperfusion period. Later studies demonstrated that there was no functional or metabolic advantage to intermittent reperfusion for normothermic ischemic periods up to 60 minutes. Nevertheless, intermittent cross-clamping accompanied by ventricular fibrillation continues to be used for coronary artery bypass surgery with results comparable to those of cardioplegic techniques.
Electrically induced ventricular fibrillation with coronary perfusion was introduced by Glenn and Sewell and Senning as a means of avoiding air embolism. However, Buckberg and Hottenrott and Hottenrott and coworkers demonstrated subendocardial ischemia and necrosis with this technique, particularly in the hypertrophied ventricle. Later studies showed that if ventricular distention is avoided and coronary perfusion is maintained, postischemic fibrillation is not deleterious in the nonhypertrophied heart. Further validation of this technique, modified by mild hypothermia and the avoidance of aortic cross-clamping, has produced comparable clinical results for coronary revascularization.
In an attempt to mimic the physiologic state, continuous coronary perfusion with a beating heart at normothermia or mild hypothermia at 32° C to prevent the onset of ventricular fibrillation became the preferred technique of myocardial preservation in the late 1960s and early 1970s, particularly after the report by McGoon and colleagues of 100 consecutive aortic valve replacements with no deaths. When aortic valve regurgitation was present or aortic root surgery was performed, the heart was kept beating by perfusing the individual coronary arteries with cannulas inserted into the ostia. However, continuous perfusion became intermittent as coronary perfusion was often discontinued to achieve better visualization of the operative field during critical portions of the procedure. In addition, problems with the coronary cannula, such as poor fixation, leaking associated with calcified ostia, early division of the left main coronary artery resulting in high perfusion pressure necrosis, and damage to the coronary artery such as dissection and late stenosis, continued to occur. Nevertheless, the technique of continuous coronary artery perfusion, either during the performance of complex aortic root surgery or for special situations, such as re-do mitral valve surgery through a right thoracotomy incision after previous coronary artery revascularization with arterial conduits, continues to be used.
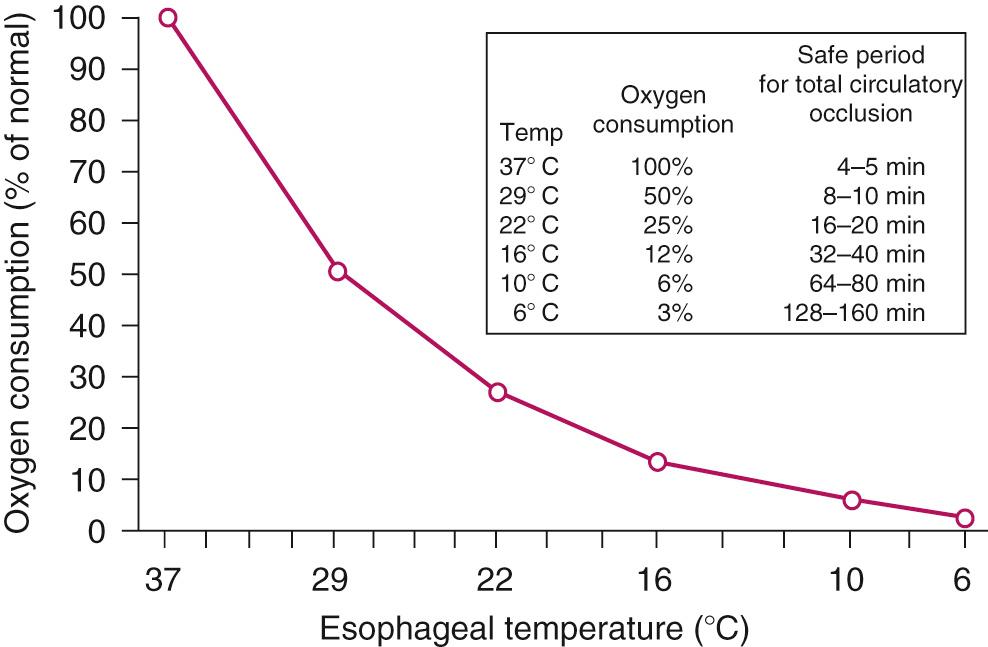
The earliest attempts to perform open heart surgery before the advent of the heart-lung machine used systemic hypothermia produced by surface cooling not only to protect the heart but to protect the brain and other organs during circulatory arrest. Hypothermia protects the ischemic myocardium by decreasing heart rate, slows the rate of high-energy phosphate degradation, and decreases myocardial oxygen consumption ( Fig. 65-1 ). However, uniform cardiac hypothermia is difficult to achieve solely by the introduction of cold intracoronary perfusates, and systemic hypothermia is necessary, particularly in the presence of coronary obstruction, ventricular hypertrophy, rewarming of the right ventricle by the liver's acting as a “heat sink,” and environmental rewarming. In an attempt to overcome this problem, Shumway and associates introduced the concept of profound local (topical) hypothermia by filling the pericardial sac with ice-cold saline. Although this technique is still used as an adjunct to other methods of myocardial protection, it is rarely used as the sole protective method because of the problem of warm bronchial collateral flow reaching the heart cavity, resulting in transmyocardial temperature gradients and resultant ischemia.
Whereas cardioplegia had been abandoned for alternative techniques in the United States after the adverse experience with the Melrose potassium solution, in Germany, Bretschneider continued studying induced cardiac arrest with use of a histidine protein buffer, sodium-poor, calcium-free, procaine-containing solution (Bretschneider solution). Clinical application soon followed, with Kirsch and asssociates using a magnesium aspartate–procaine solution. Hearse and coworkers introduced the concept of using an extracellular rather than an intracellular solution (St. Thomas' solution), which was first applied clinically by Braimbridge and coworkers. On the basis of improved clinical outcomes, North American investigators initiated experimental studies using potassium cardioplegia followed by clinical reports demonstrating the efficacy of cardioplegia. During the next 3 decades, numerous investigators have continued their “quest for ideal myocardial protection.”
Global ischemia is not an all-or-nothing phenomenon; rather, it is heterogeneous in that at any moment of time, myocardial cells will have varying extents of damage. These changes affect cellular metabolism, ion transport, electrical activity, contractile function, vascular responsiveness, tissue ultrastructure, changes in nuclear and mitochondrial DNA, release of free radical oxygen species, and activation of inflammatory components.
Because the heart is an obligate aerobic organ, it depends on a continuous supply of oxygen to maintain normal function. Myocardial oxygen reserve is exhausted within 8 seconds after the onset of normothermic global ischemia. Myocardial oxygen consumption (  ) is compartmentalized into the oxygen needed for external work of contraction (80% to 90%) and the unloaded contraction, such as basal metabolism, excitation-contraction coupling, and heat production.
) is compartmentalized into the oxygen needed for external work of contraction (80% to 90%) and the unloaded contraction, such as basal metabolism, excitation-contraction coupling, and heat production.
A unique aspect of myocardial energetics is that 75% of the coronary arterial oxygen presented to the myocardium is extracted during a single passage through the heart; thus, depressed coronary venous oxygen content persists despite a wide range of cardiac workloads. Therefore, the heart is susceptible to the limitations of oxygen delivery, whereby an increase in  can be met only by augmentation of coronary blood flow. This is diametrically opposite to skeletal muscle, in which increased oxygen demand can initially be met by an increase in oxygen extraction. Clinically, a marked increase in coronary blood flow is observed at the beginning of the reperfusion period, after the aortic clamp is removed.
can be met only by augmentation of coronary blood flow. This is diametrically opposite to skeletal muscle, in which increased oxygen demand can initially be met by an increase in oxygen extraction. Clinically, a marked increase in coronary blood flow is observed at the beginning of the reperfusion period, after the aortic clamp is removed.
Under aerobic conditions, the heart derives its energy primarily from mitochondrial oxidative processes, using substrates such as glucose, free fatty acids, lactate, pyruvate, acetate, ketone bodies, and amino acids. However, oxidation of fatty acids provides the major source of energy production and is used in preference to carbohydrates.
As tissue P o 2 falls, oxidative phosphorylation, electron transport, and mitochondrial adenosine triphosphate (ATP) production cease. Early in ischemia, the heart depends on the energy production of glycogenolysis and aerobic glycolysis (Pasteur effect). However, unlike other organs, the heart requires a minimum threshold of ATP to prevent irreversible ischemic contracture. Reduced mitochondrial activity leads to the accumulation of glycolytic intermediaries, reduced NADH, and the reduction of pyruvate to lactate. The resultant severe intracellular acidosis impairs contractile function, enzyme transport, and cell membrane integrity. This results in a cellular loss of potassium and pathologic accumulation of sodium, calcium, and water ( Fig. 65-2 ).
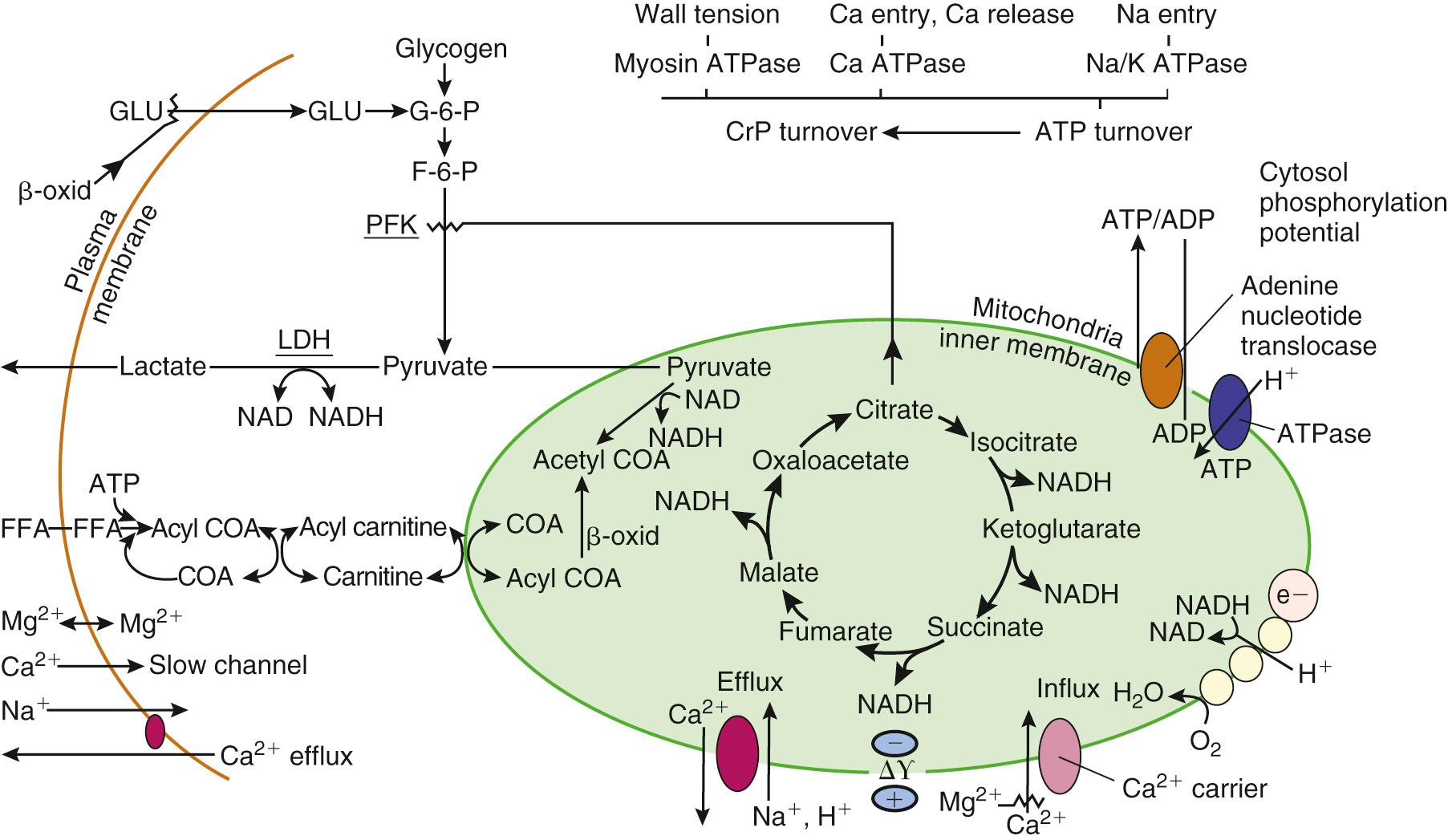
Ischemia-reperfusion injury occurs as the result of attenuation or cessation of coronary blood flow such that oxygen delivery to the myocardium is insufficient to meet basal myocardial oxygen requirements to preserve cellular membrane stability and viability. The initiation of myocardial injury requires an ischemic episode that, by itself, may induce reversible or irreversible cellular injury.
Reversible ischemia-reperfusion injury may be manifested as either stunning or hibernation. Stunning “describes the mechanical dysfunction that persists after reperfusion despite the absence of myocellular damage and despite the return of normal or near-normal perfusion.” A second form of reversible ischemia-reperfusion injury is hibernation, which is a syndrome of reversible, chronically reduced contractile function as a result of one or more recurrent episodes of acute or persistent ischemia, referred to as chronic stunning.
As in stunning, hibernating myocardium is viable but not functional and is reversible with coronary revascularization. There is good clinical evidence that despite seemingly adequate application of modern methods of myocardial protection, all patients undergoing cardiac surgery have varying degrees of myocardial stunning. Evidence to support this concept is based on the requirement of inotropic support for separation from bypass for hours or days after surgery in some patients who are eventually weaned from these drugs as the stunning abates, without objective evidence of a myocardial infarction.
Two major theories have been proposed as possible mechanisms leading to ischemia-reperfusion injury ( Fig. 65-3 ). The calcium hypothesis suggests that the inability of the myocyte to modulate intracellular and intraorganellar calcium homeostasis induces a cascade of events culminating in cell injury and death ( Fig. 65-4 ). Ischemia leads to the induction of metabolic acidosis and the activation of the sodium-proton exchanger, resulting in the transport of hydrogen ions to the extracellular space and the movement of sodium into the cytosol. As the sodium-calcium exchanger is activated, sodium is transported to the extracellular space and calcium is taken up into the cytosol, increasing cytosolic calcium concentration ([Ca 2+ ] i ). Increased [Ca 2+ ] i accumulation is also augmented by ischemia-induced depolarization of the membrane potential, which allows the opening of the l -type calcium channels and further calcium entry into the myocyte. Cellular and cytosolic calcium-dependent phospholipases and proteases are in turn activated, inducing membrane injury and the further entry of calcium into the cell. These processes alter myocardial cellular homeostasis, leading to cellular dysfunction or, if they are of sufficient duration or intensity, cell injury or death. Alternative explanations include the concept of reperfusion-induced myocardial contracture resulting from rapid re-energization of contractile cells with persistent calcium overload affecting myofibrillar calcium sensitivity.
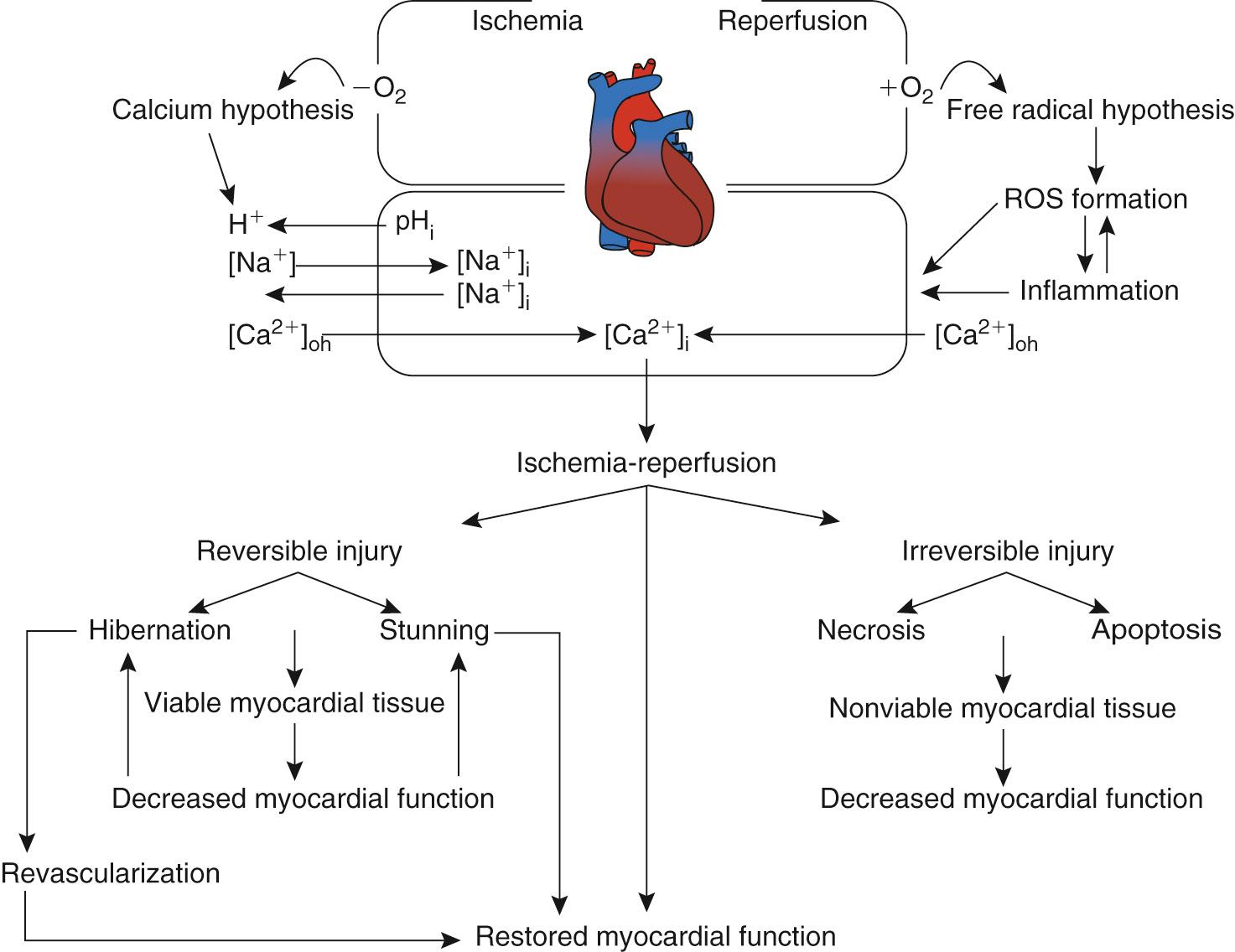
![FIGURE 65-4, Sources of calcium regulation. The inability of the myocyte to modulate intracellular and intraorganellar calcium homeostasis during ischemia and during early reperfusion is the basis of the calcium hypothesis for ischemia-reperfusion injury. Increased intracellular calcium ([Ca 2+ ] i ) induces a cascade of events culminating in increased mitochondrial and nuclear calcium accumulation and cell injury and death. FIGURE 65-4, Sources of calcium regulation. The inability of the myocyte to modulate intracellular and intraorganellar calcium homeostasis during ischemia and during early reperfusion is the basis of the calcium hypothesis for ischemia-reperfusion injury. Increased intracellular calcium ([Ca 2+ ] i ) induces a cascade of events culminating in increased mitochondrial and nuclear calcium accumulation and cell injury and death.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MyocardialProtection/3_3s20B978032324126700065X.jpg)
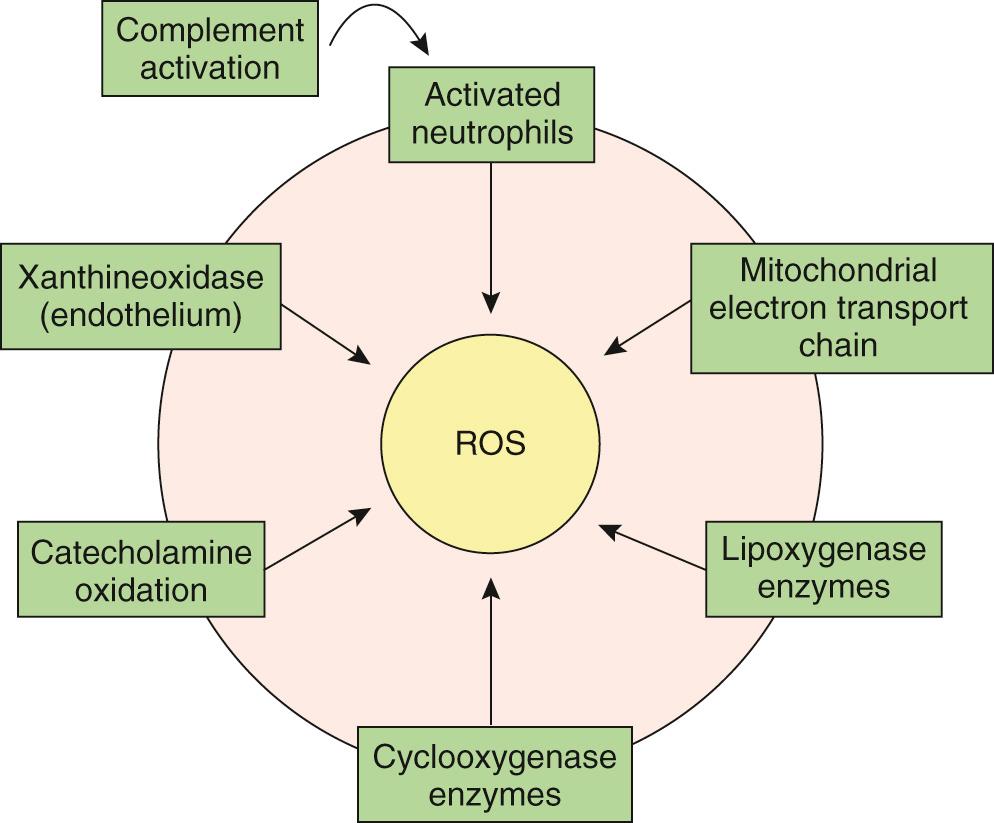
The free radical hypothesis suggests that the accumulation of partially reduced molecular oxygen, collectively known as reactive oxygen species, during the early stages of reperfusion causes myocardial cellular damage and cell death through microsomal peroxidation of the cellular phospholipid layer, leading to loss of cellular integrity and function ( Fig. 65-5 ). The generation of reactive oxygen species is believed to be mediated by xanthine oxidase, activation of neutrophils, or dysfunction of the mitochondrial electron transport chain. It has been suggested that the generation of reactive oxygen species may induce cellular membrane damage, thus facilitating calcium entry and the induction of cellular death. This hypothesis unifies both prevailing theories and is currently considered to be valid. However, therapeutic attempts to control calcium and reactive oxygen species overload have not yielded meaningful advantage.
Alternative explanations include the concept of lethal reperfusion injury, defined as death of myocardial cells that were viable immediately before reperfusion. In an excellent review by Yellon and Hausenloy, alternative cardioprotective strategies are suggested to manage this injury by reperfusion injury salvage kinase pathways and targeting mitochondrial permeability transition pores to avoid mitochondrial calcium overload. Cyclosporine, a potent inhibitor of mitochondrial permeability transition pores, has recently been shown to limit infarct size after percutaneous coronary intervention during acute myocardial infarction.
Irreversible cell injury, described ultrastructurally by Schaper and coworkers, occurs by two morphologically distinct pathways, necrosis and apoptosis. Necrosis is initiated by noncellular mechanisms with cell swelling, depletion of ATP stores, and disruption of the cellular membrane involving fluid and electrolyte alterations. In contrast, apoptosis (programmed cell death) is an evolution-based mode of cell death characterized by a discrete set of biochemical and morphologic events involving the regulated action of catabolic enzymes (proteases and nucleases) that results in the ordered disassembly of the cell, distinct from cell death provoked by external injury.
Inflammation has been implicated as a secondary mechanism contributing to injury after reperfusion. It is initiated through complement activation leading to the sequential formation of a membrane attack complex, which creates a cellular lesion and eventual cell lysis. In addition, cytokines, vasoactive and chemotactic agents, adhesive molecule expression, and leukocyte and platelet activation participate in the inflammatory process, producing cytotoxic molecules that facilitate cell death. Oxygen-derived free radical scavengers have also been used to limit reperfusion injury. Tissue factor, an inflammatory and procoagulant mediator, initiates the extrinsic coagulation cascade, resulting in thrombin generation and fibrin deposition, and may be related to the no-reflow phenomenon. Clinical applicability of anti-inflammatory agents awaits well-designed clinical trials because studies up until now have not shown any “meaningful cardioprotective effect.”
In addition, endothelium-dependent microvascular responses and coronary artery spasm may be related to reduced myocardial perfusion after reperfusion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here