Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ischemic myocardial injury may be defined simply as those changes that occur in the heart muscle as a result of an imbalance in oxygen and substrate supply and demand. The degree, time course, and type of injury depend on the severity and duration of the ischemic insult. When ischemia is of sufficient duration and magnitude, such that restitution of flow does not prevent irreversible ischemic injury, myocardial necrosis, that is, infarction occurs. The clinical disease that results from the imbalance in myocardial perfusion is often designated as coronary artery disease (CAD) or coronary heart disease (CHD) because coronary artery lesions typically are implicated in the clinical manifestations. However, from a pathophysiological viewpoint, the condition is best designated as ischemic heart disease (IHD).
In 1698 before human myocardial infarction (MI) was recognized at autopsy, experimental coronary occlusion was attempted in dogs by Chirac, who recorded the first gross pathologic description of myocardial infarct; he noted that the heart stopped beating immediately . In 1880 Weigert examined human myocardial infarcts and described the gross and pathologic changes in detail . Now, acute MI (AMI) is classified clinically based on the presence or absence of ST-segment elevation on the electrocardiogram (ECG): unstable angina/non-ST-elevation MI (NSTEMI) and evolving ST-elevation MI (STEMI) . The most common cause of AMI is rupture or erosion of an atherosclerotic plaque with superimposed thrombosis . The less common causes include vasospasm , vasculitis , myocardial bridging , coronary artery anomalies, and thromboembolism associated with a variety of disorders. In patients with severe three-vessel CAD, a relatively minor increase in oxygen requirement (demand) or reduction in oxygen delivery (supply) can precipitate ischemic injury without a change in the coronary arteries .
In 2000 the European Society of Cardiology and American College of Cardiology issued a joint consensus paper establishing a universal definition of MI (UDMI) ( Table 10.1 ) . The UDMI redefined AMI as myocardial injury detected by elevated serum cardiac biomarkers, mainly cardiac troponin (cTn), in the setting of acute myocardial ischemia. Subsequently, a second UDMI was introduced in 2007 that established a five-category classification of AMI, followed by a third UDMI with amendments relating to coronary interventions . A fourth update, published in 2018, addressed the introduction and widespread adoption of high-sensitivity (hs)-cTn assays . While the purpose of the UDMI is to formalize criteria used to render a clinical diagnosis of MI, the five categories established by the UDMI may be applied by pathologists as well .
| Category | Definition |
|---|---|
| Myocardial injury | cTn above the 99 th percentile upper reference limit; myocardial injury is deemed acute if there is a rise or fall in cTn |
| Acute MI | Acute myocardial injury with evidence of ischemia or coronary thrombosis |
| Type 1 MI | Athero/thrombotic occlusion due to plaque rupture or erosion |
| Type 2 MI | Oxygen demand/supply mismatch unrelated to acute athero-thrombosis (e.g. coronary artery spasm, anemia, hypotension, etc…) |
| Type 3 MI | Cardiac death with symptoms of myocardial ischemia before cTn values become available (e.g. death before blood samples can be obtained) |
| Coronary procedure-related MI | |
| Type 4a MI | MI associated with percutaneous coronary intervention (≤ 48 hours) |
| Type 4b MI | MI associated with stent thrombosis |
| Type 4c MI | MI associated with restenosis |
| Type 5 MI | MI associated with coronary artery bypass surgery (≤ 48 hours) |
Per the fourth UDMI, the clinical definition of MI is acute myocardial injury detected by abnormal cardiac biomarkers associated with evidence of acute myocardial ischemia. The classification of MI is then divided into two categories, AMI and MI associated with coronary interventions, and further divided into five types ( Table 10.2 ): MI secondary to a primary atherothrombotic coronary event (type 1), MI secondary to oxygen supply/demand mismatch (type 2), sudden cardiac death (SCD) (type 3), MI in the setting of percutaneous coronary intervention (PCI) (type 4), and MI after coronary artery bypass surgery (type 5).
| Coronary arteries | Myocardium | Mechanisms | Clinical manifestations |
|---|---|---|---|
| Moderate coronary atherosclerosis | Adequate perfusion and function | Outward coronary remodeling; coronary flow reserve | No symptoms |
| Focal severe coronary atherosclerosis with >75% narrowing (cross sectional area) of one or more coronary arteries | Intermittent regional ischemia and contractile dysfunction | Stress or exertion-induced increase in myocardial oxygen demand | Typical angina pectoris; stable angina pectoris |
| Coronary plaque(s) with acute alterations | Sustained regional ischemia and contractile dysfunction | Coronary plaque erosion, rupture, platelet aggregation, vasospasm, mural thrombus, thromboemboli | Unstable angina pectoris |
| Severe coronary atherosclerosis, often with coronary plaque(s) with acute alterations | Ischemic myocardium with ectopic electrical activity | Marked coronary luminal narrowing; coronary plaque with acute alterations as above; ventricular fibrillation | Sudden cardiac death |
| Coronary plaque(s) with variable acute alterations | Irreversible injury and necrosis of ischemic myocardium limited to inner 1/2 of LV wall | Coronary plaque erosion or rupture with mural thrombus or stable coronary plaque with markedly decreased coronary perfusion | Acute subendocardial myocardial infarct |
| Coronary plaque(s) with acute alterations | Irreversible injury and necrosis of ischemic myocardial extending from subendocardium into subepicardium of LV | Coronary plaque erosion or rupture with occlusive thrombosis | Acute transmural myocardial infarction |
| Severe coronary atherosclerosis | Variable myocardial functional impairment | Healed coronary plaque rupture or erosion; inward coronary remodeling; myocardial remodeling | Chronic ischemic heart disease; heart failure; ischemic cardiomyopathy |
A type 1 MI is the result of acute atherothrombotic coronary artery occlusion with critical flow reduction caused by atherosclerotic plaque rupture or erosion. In the clinical setting, type 1 may be further classified based on ECG findings as either STEMI or NSTEMI.
Type 2 MI is the result of myocardial oxygen supply/demand mismatch not due to coronary artery atherothrombosis. Coronary arteries will be widely patent or show low-grade (<50%) stenosis. Clinically this may be referred to as MI with nonobstructive coronary arteries (MINOCA) and may be caused by (1) nonatherosclerotic CAD, such as spontaneous dissection, vasospasm, vasculitis, embolism, etc.; (2) noncoronary cardiac diseases, such as sustained tachyarrhythmias, bradyarrhythmias, and left ventricular hypertrophy; or (3) extracardiac pathology such as respiratory failure, hypotension/shock, and severe anemia. Stable atherosclerotic plaques are frequently present and may contribute to noncoronary causes of oxygen supply/demand mismatch.
Type 3 MI is a designation used when MI is clinically suspected (or discovered at autopsy), but death occurs before an increase in cardiac biomarkers is identified. Per the UDMI, if an autopsy reveals the MI was caused by acute or recent atherothrombosis, the type 3 MI should be reclassified as type 1.
MI related to percutaneous coronary revascularization carries the designation of type 4 MI. If MI occurs within 48 h of the index procedure, it is classified as type 4a. This includes MI due to procedural complications such as coronary dissection, distal embolization, disruption of collateral flow, or occlusion of an epicardial or side branch coronary artery. Beyond the 48-h period, MI related to stent thrombosis is designated as type 4b MI. Type 4b MI may be temporally categorized as acute (0–24 h), subacute (24 h to 30 days), late (30 days to 1 year), or very late (>1 year). Finally, MI that occurs due to in-stent restenosis or restenosis following balloon angioplasty is designated type 4c MI.
Type 5 MI is infarction that occurs within 48 h of coronary artery bypass grafting (CABG). The etiology may be directly procedure-related, or related to reperfusion injury, low-flow, or poor run-off.
Impaired interaction between the coronary circulation and the myocardium can result in a spectrum of clinical disease, including typical angina pectoris, unstable angina pectoris, AMI, SCD, and chronic IHD ( Table 10.3 ). Acute coronary syndrome (ACS) is a broad designation comprised by a spectrum of clinical manifestations, including ST-segment elevation transmural MI (30%), nonST-segment elevation subendocardial MI (25%), unstable angina pectoris (38%) and, in some schemes, SCD .
| Alteration | Time |
|---|---|
| Myofibrillar relaxation | Immediate |
| Glycogen loss | 5–10 min |
| Mitochondrial swelling | 15–30 min |
| Intracellular edema | 15–30 min |
| Mitochondrial matrix granules | 20–30 min |
| Disruption of cell membranes | 20–30 min |
| Margination and clumping of nuclear chromatin | 30–40 min |
In patients presenting with ACS, documentation of a clinical diagnosis of AMI combines multiple clinical tools . Acute or recent MI is recognized by ischemic symptoms, biochemical markers in the blood, and electrocardiographic and echocardiographic changes. The clinical symptoms of AMI vary; possible symptoms include not only chest pain but also epigastric, arm, wrist, or jaw pain, shortness of breath, and/or nausea. Sometimes the patient is asymptomatic—so-called silent ischemia . The symptoms associated with MI usually last at least 30 min, as opposed to the symptoms of angina pectoris that last fewer minutes. Serological biomarkers enable detection of very small and early infarcts only hours old. Cardiac troponins and creatine kinase MB (CK-MB) are most often used. Current biomarkers are sensitive and specific, rarely resulting in false positives or false negatives for MI. Biomarkers are discussed in detail in the “Biochemical changes with ischemia and biochemical markers” section.
ST-elevation or diagnostic Q waves in regional leads are specific electrocardiographic findings for AMI . STEMI shows new ST-segment elevation at the J point in two or more contiguous leads with cut-off points ≥0.2 mV in leads V1, V2, or V3 and ≥0.1 mV in other leads. NSTEMI displays ST-segment depression and T-wave abnormalities. Q waves or QS complexes in the absence of QRS confounders are usually pathognomonic of a prior MI. Two-dimensional echocardiography is often used to evaluate both acute and established MI . The echocardiogram can detect regional wall motion abnormalities within minutes of onset of ischemic injury. Estimation of left ventricular function by echocardiogram is also a valuable prognostic indicator. The echocardiogram is useful to evaluate coexisting complications due to ischemia, such as mitral valve dysfunction, intracardiac mural thrombus, pericardial fluid, and cardiac rupture. Established MI is clinically defined by the development of pathologic Q waves on the ECG, or evidence of regional thinning and dysfunction in the left ventricle by imaging studies. Evidence-based and guideline-based protocols have been increasingly used for diagnosis and treatment. Further reading provides the current protocol for AMI .
Clinical and public health efforts have reduced the incidence and mortality of cardiovascular diseases. However, more than 1 million people in the United States and more than 17 million people in the world die annually due to cardiovascular diseases .
In the United States, the incidence of MI and the hospital mortality rate of AMI markedly declined after the late 1990s, primarily due to the decline in STEMI. The risk of SCD has decreased both in patients with and without known heart disease . These trends are seen in all age and sex groups, though women and men with AMI show different clinical profiles, presentation, and outcomes; women are typically older and have significantly higher rates of diabetes, hypertension, and prior congestive heart failure . The reduced mortality of patients hospitalized for STEMI was also reported in a Swedish nationwide study . These reductions are associated with an increase in the prevalence of evidence-based treatments and the development of more effective thrombolytic agents, the introduction of a variety of revascularization procedures, and improvements in medication by more effective platelet inhibition, β-blockade, angiotensin-converting enzyme (ACE)-inhibition, and lipid-lowering . In contrast, the prevalence of NSTEMI has increased since 1990. This increase may be explained by a new and sensitive biomarker, troponin, that had become widely available after the mid-to-late 1990s. Routine use of troponin may have increased the sensitivity for the diagnosis of NSTEMI. Mortality after MI has decreased; however, the incidence of heart failure has been increasing . One could speculate that the advanced treatments have salvaged high-risk MI patients, but resulted in greater incidence of heart failure in those who now survive after MI. In the United States, the incidence of MI and the hospital mortality rate of AMI markedly declined after the late 1990s, primarily due to the decline in STEMI. The risk of SCD has decreased both in patients with and without known heart disease . These trends are seen in all age and sex groups, though women and men with AMI show different clinical profiles, presentation, and outcomes; women are typically older and have significantly higher rates of diabetes, hypertension, and prior congestive heart failure . The reduced mortality of patients hospitalized for STEMI was also reported in a Swedish nationwide study . These reductions are associated with an increase in the prevalence of evidence-based treatments and the development of more effective thrombolytic agents, the introduction of a variety of revascularization procedures, and improvements in medication by more effective platelet inhibition, β-blockade, ACE-inhibition, and lipid-lowering . In contrast, the prevalence of NSTEMI has increased since 1990. This increase may be explained by a new and sensitive biomarker, troponin, that had become widely available after the mid-to-late 1990s. Routine use of troponin may have increased the sensitivity for the diagnosis of NSTEMI. Mortality after MI has decreased; however, the incidence of heart failure has been increasing . It is tempting to speculate that the advanced treatments have salvaged high-risk MI patients, but resulted in greater incidence of heart failure in those who now survive after MI.
Abrupt coronary occlusion causes profound biochemical changes in the ischemic myocardium ( Table 10.2 ). Oxygen tension decreases. There is a reduction in oxidative phosphorylation; adenosine triphosphate (ATP) production virtually stops . This causes a shift to anaerobic metabolism and an increase in glycolysis. Protein synthesis decreases and severe mitochondrial dysfunction occurs along with alterations in calcium homeostasis. Membrane pumps cease functioning so that potassium and magnesium leak out of the cell, while calcium, sodium, and water enter. Actin-myosin interaction fails and contractility becomes impaired. All of these changes occur almost instantaneously .
Once irreversible ischemic injury occurs, metabolic function virtually stops; however, certain biochemical changes continue to occur. Cell constituents leak into the extracellular space and can be measured in the patient’s blood. Different proteins will be released into the circulation and can be recognized in the blood once myocardial necrosis occurs. The most commonly used markers include myoglobin, cardiac troponin T (cTnT) and I (cTnI), and CK-MB . cTn are sensitive and specific biomarkers . cTnT and cTnI are cardiac-specific contractile proteins with greater sensitivity and specificity for myocardial necrosis than total CK or CK-MB. A study using coronary angioscopy in AMI patients showed that serum cTn level was significantly associated with the prevalence of intracoronary thrombus and yellow plaque. cTn elevation predicts 6-month mortality and poor clinical outcome, independent of other risk factors . In addition, cTn has led to a 10% increase in AMI diagnoses in patients previously labeled with unstable angina pectoris. Indeed, while cTns are tissue-specific markers of myocardial necrosis, elevated cTn levels are not necessarily specific to ischemia. Other etiologies, such as myocarditis, aortic dissection, pulmonary embolism, congestive heart failure, and renal failure, should be considered when these biomarkers are elevated. Serial cTn measurements demonstrating characteristic rise and/or fall patterns over time are clinically useful to accurately diagnose AMI ( Fig. 10.1 ). Newer assays, such as high-sensitivity cTn, can detect subtle changes over short intervals, allowing for quicker identification of true AMI . Nonspecific serologic tests also have diagnostic and prognostic utility; high white blood cell count, for example, is associated with a greater chance of severe three-vessel disease, compromised hemodynamics, and worse prognosis.
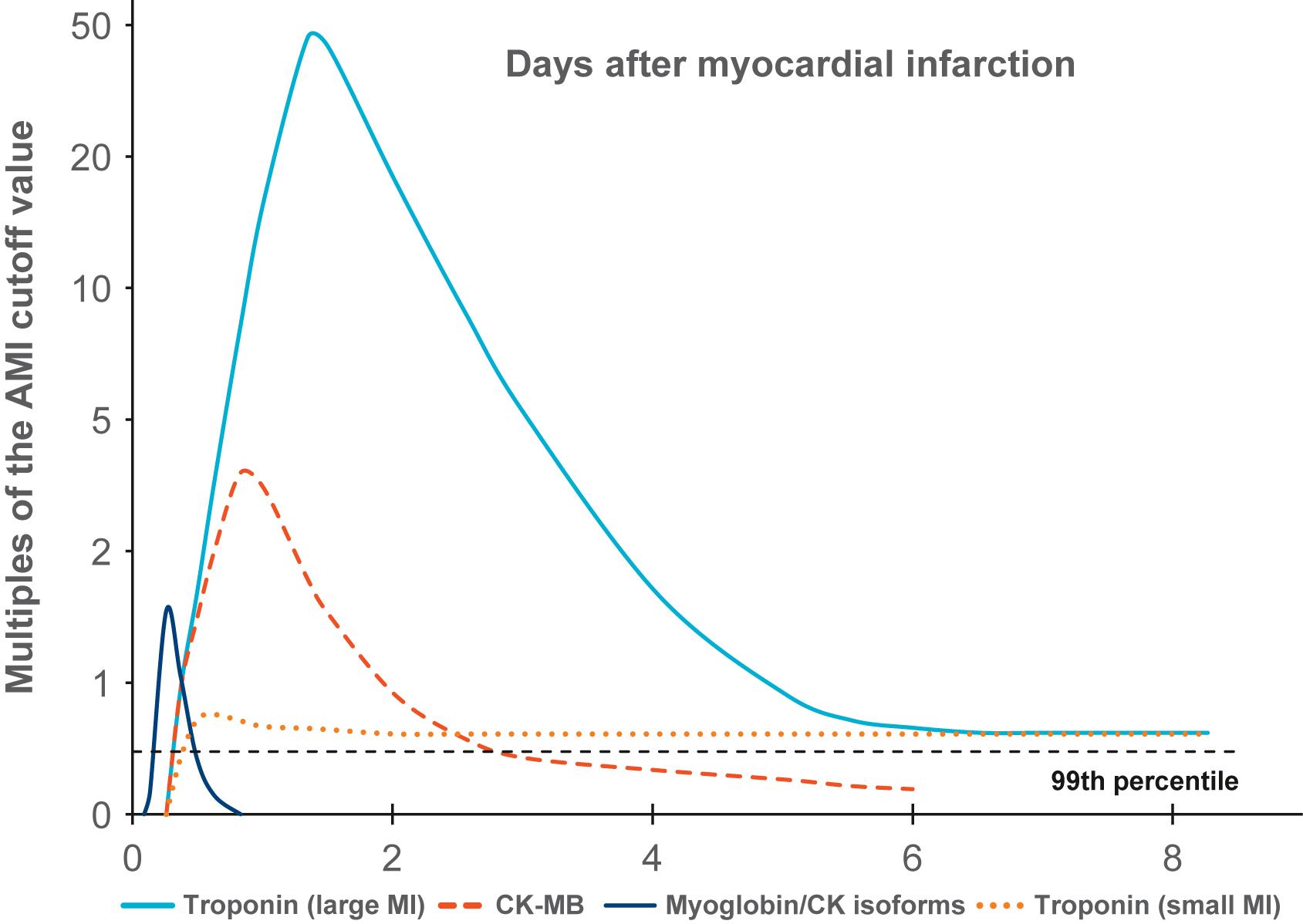
In experimental animals, if certain interventions are administrated early after coronary artery occlusion, particularly reperfusion, the resulting MI will be smaller than would be expected without intervention. This suggests that within the ischemic myocardium there is a jeopardized but salvageable zone of myocardium. This tissue is somewhat ischemic but not irreversibly injured and not necessarily destined to undergo necrosis. Presumably, a beneficial intervention preserves ischemic, yet viable myocardium until effective collateral circulation to the damaged tissue develops. If cell degeneration occurs at different rates in different zones within an area of ischemic myocardium, then the progression of biochemical changes within the ischemic myocardium should also be nonuniform . Over the past 25 years, there has been intensive research to attempt to exploit this phenomenon to reverse ischemic changes before they become irreversible, salvaging myocardium that might otherwise die.
The ultrastructural changes after coronary occlusion have been studied in detail and are quite similar in a wide variety of animal models ( Table 10.4 ). During the reversible phase of ischemic injury, electron microscopy (EM) of cardiomyocytes shows glycogen loss due to the shift from aerobic to anaerobic metabolism, wide I bands due to myofiber stretching (systolic bulge), and mild mitochondrial swelling. During the first 30–40 min of ischemia, the changes are visible only at the ultrastructural (electron microscopic) level .
| Alteration | Time |
|---|---|
| Myofibrillar relaxation | Immediate (min) |
| Glycogen loss | 5–10 |
| Mitochondrial swelling | 15–30 |
| Intracellular edema | 15–30 |
| Mitochondrial matrix granules | 20–30 |
| Disruption of cell membranes | 20–30 |
| Margination and clumping of nuclear chromatin | 30–40 |
Irreversible injury to cardiomyocytes will continue to evolve even if the flow is eventually restored. The severely injured cardiomyocytes show additional changes: glycogen becomes totally depleted, myofibrils become more relaxed or stretched, intracellular edema progresses, nuclear chromatin becomes clumped and marginated, and mitochondria undergo more changes, including swelling and disruption, decrease in matrix density, and disorganization of cristae and linear inclusions (fused cristae). Amorphous or “flocculent” densities appear in the mitochondrial matrix space ( Fig. 10.2 ). Physical defects (holes) develop in the sarcolemmal membrane.

In dogs, the above changes occur focally with 20 min of severe ischemia; in rats, the changes occur as early as 5 min after coronary occlusion . At approximately the same time that irreversible myocardial cell injury is occurring, there are also microvascular changes. Capillary endothelial cells swell, lose their pinocytotic vesicles, and leak. This vascular damage may play a role in determining irreversibility and causing the striking changes associated with reperfusion to an irreversibly injured ischemic zone . In dogs, the above changes occur focally with 20 min of severe ischemia; in rats, the changes occur as early as 5 min after coronary occlusion . At approximately the same time that irreversible myocardial cell injury is occurring, there are also microvascular changes. Capillary endothelial cells swell, lose their pinocytotic vesicles, and leak. This vascular damage may play a role in determining irreversibility and causing the striking changes associated with reperfusion to an irreversibly injured ischemic zone .
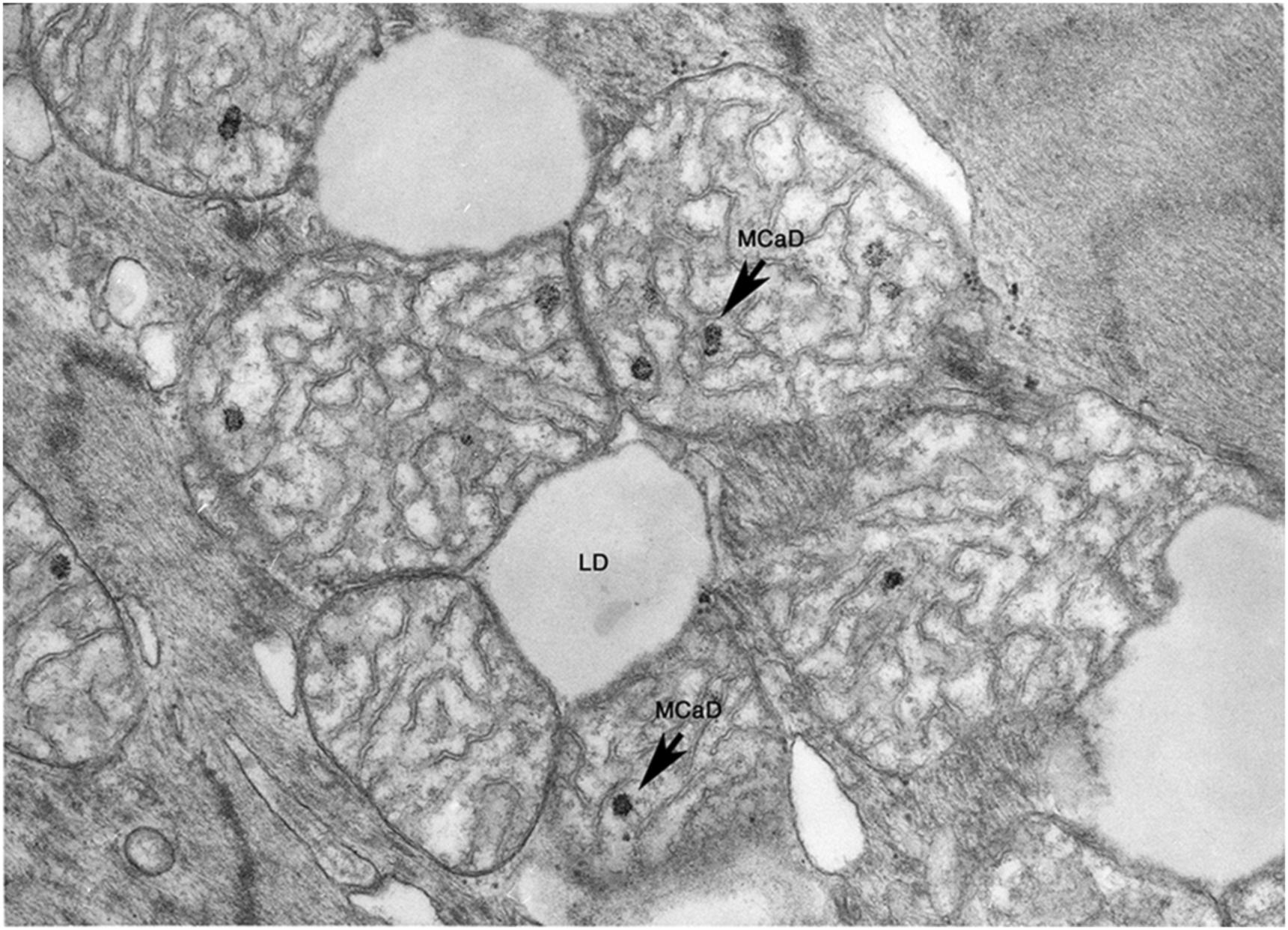
If there is reperfusion after irreversible damage has occurred, massive cell swelling, vacuolization, and subsarcolemmal blebs are apparent. Instead of relaxation, spasm occurs with the appearance of “contraction bands” that represent hypercontraction of adjacent sarcomeres. Intracellular and intramitochondrial calcium increases with reflow to irreversibly injured cells and more electron-dense granular (as opposed to amorphous) densities which are crystals of calcium phosphate appear within swollen mitochondria ( Fig. 10.3 ).
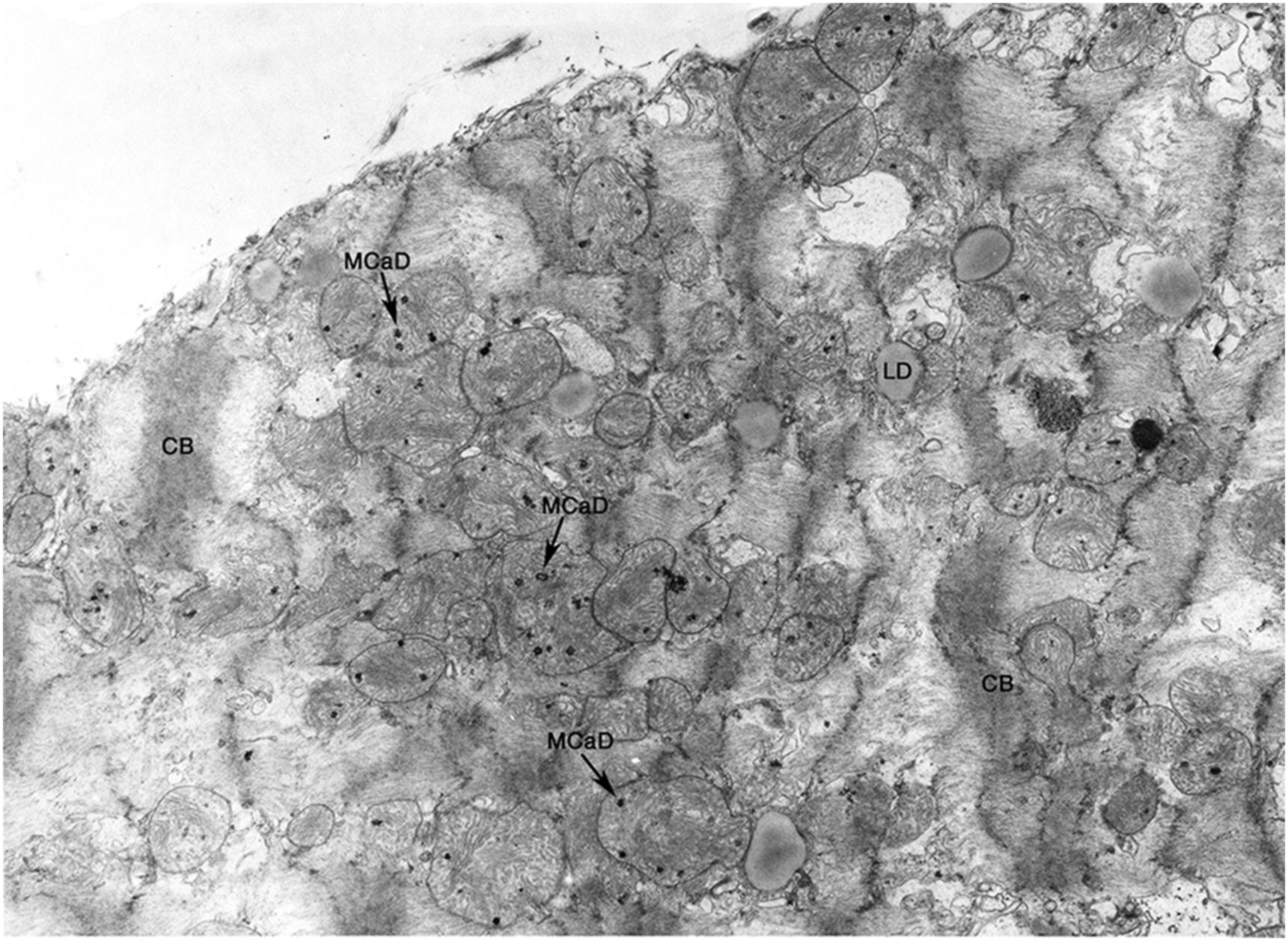
Since mitochondrial amorphous bodies appear at about the time that reflow no longer prevents cell death, mitochondrial injury with failure is thought to have an important role in the irreversibility of ischemic injury. But, membrane damage-causing failure of cell volume regulation, as manifested by severe cell swelling and electrolyte shifts, is also prominent, and may also be of primary importance. Substrate depletion, ATP loss, intracellular acidosis, and lysosomal rupture with release of acid hydrolases have also been implicated and also may play a role in the pathogenesis of irreversible ischemic injury ( Fig. 10.4 ). In general, the most reliable ultrastructural features of irreversible injury are the amorphous matrix densities in the mitochondria and the sarcolemmal defects.
The biochemical and ultrastructural changes described above are characteristic of severely ischemic myocardium. These findings need to be considered in the context of current information that several modes of cell injury and death can lead to necrosis, the final common pathway of cellular degradation following irreversible injury ( Fig. 10.5 ). For myocardial ischemic injury, the two processes of significance are oncosis and apoptosis . Oncosis (also referred to as oncotic necrosis or necrosis) is characterized by cellular and organellar swelling secondary to rapid depletion of ATP and progressive membrane damage and energy depletion with rapid loss of ATP. The membrane injury progresses from ionic disturbances induced by alterations in ionic transport systems followed by increasing permeability of the phospholipid bilayer and finally discrete defects (holes) in the membrane. The progressive membrane damage is mediated by activation of phospholipases and proteases and accumulation of toxic lipid species, and free radicals. In contrast, classical apoptosis as observed in isolated cell models is identified by cellular and organellar shrinkage, with membrane preservation, and subsequent fragmentation into apoptotic bodies. The process is initiated by stimulation of extrinsic or intrinsic pathways leading to activation of a caspase enzyme cascade and subsequent activation of endonucleases producing double-stranded DNA fragmentation. Apoptosis requires at least partial preservation of ATP since steps in the process are energy-dependent.
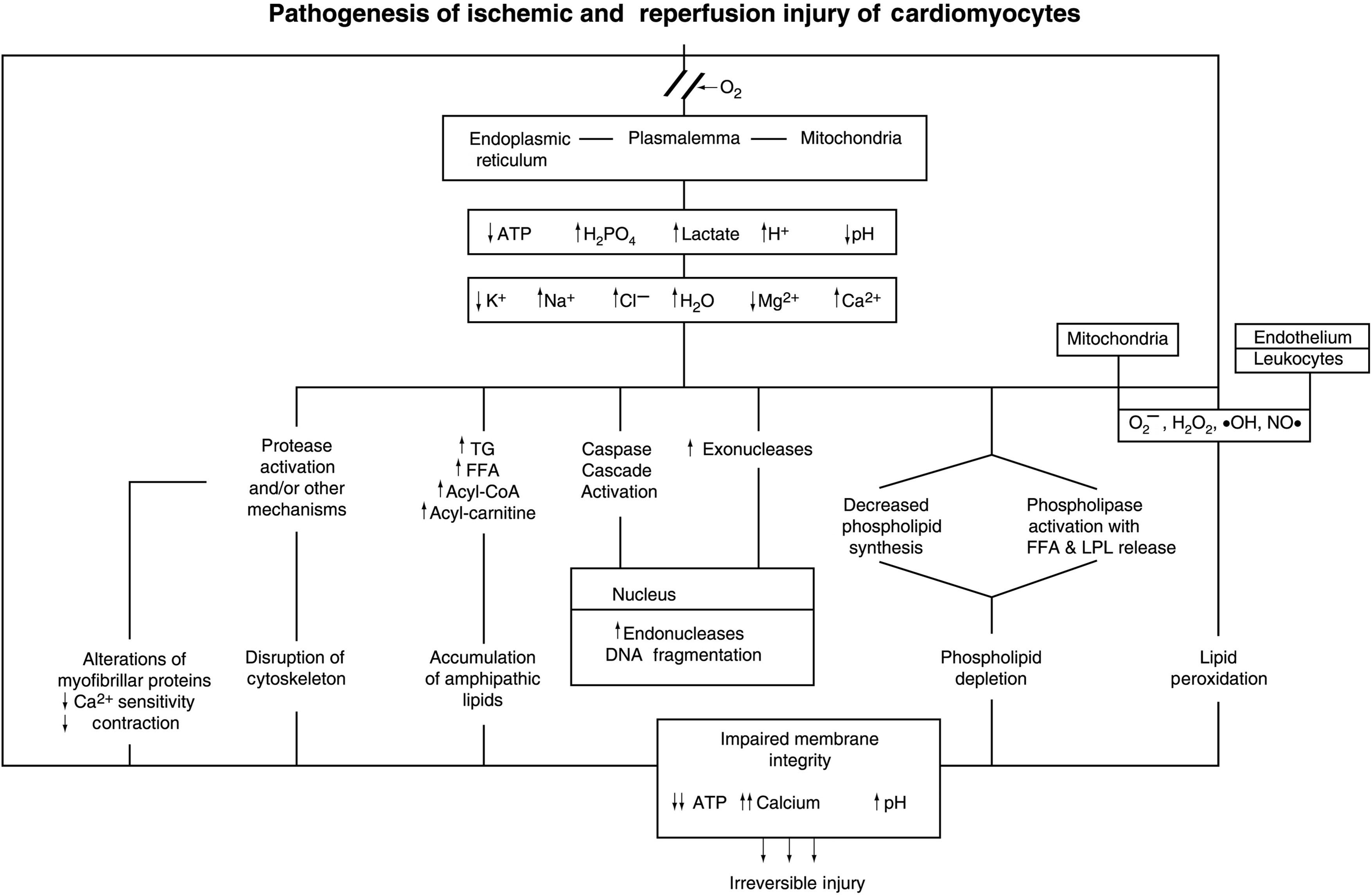
Cardiomyocytes have been shown to have the molecular components of both pathways of oncosis and apoptosis . Manipulation of experimental models of MI using caspase inhibitors and mutant mouse models with knockout or increased expression of apoptosis components have shown moderate reduction of infarct size, with a residual significant amount of infarction. These results indicate that initial injury of ischemic cardiomyocytes can involve both oncosis and apoptosis. The rapidity and magnitude of ATP depletion is a key factor in determining whether ischemic injury in a given myocyte initially proceeds by the oncotic or apoptotic pathway. Since ischemic cardiomyocytes are subject to simultaneous activation of both modes of cell death, myocardial ischemic injury can be considered to be a hybrid pattern of cell injury and death. Although the apoptotic pathway may be operative in the initial phase of ischemic injury in some cardiomyocytes, the ultrastructure of myocardial ischemic damage is dominated by the changes of oncotic necrosis.
With both oncosis and apoptosis, the mitochondria have a critical role in the pathogenesis of cell injury . The high energy requirement of cardiomyocytes is met by the large number of mitochondria with tightly packed cristae reflecting their ability to conduct vigorous oxidative phosphorylation. Preservation of the electrical potential difference (ΔΨ m ) across the inner mitochondrial membrane is critical for oxidative phosphorylation and ATP generation.
In perturbed cells, deleterious and protective influences on mitochondria are mediated by death channels and salvage pathways. The mitochondrial death channels involve the mitochondrial permeability transition pore (mPTP) and the mitochondrial apoptosis channel (mAC). The mPTP is a voltage-dependent channel that is constituted by three proteins—the voltage-dependent anion channel (VDAC), the adenine nucleotide translocator (ANT), and cyclophilin D (CypD). The proteins are localized as follows: VDAC in the outer membrane, the ANT in the inner membrane, and CypD on the matrix side of the inner membrane. Thus the protein complex of the mPTP spans the two mitochondrial membranes and provides a path for molecules to flow selectively between the mitochondrial matrix and the cytoplasm. Calcium and oxidative stress regulate the function of the mPTP. The second channel, the mAC, is highly regulated by the Bcl-2 family of proteins and is important in many forms of apoptosis.
Apoptosis can be triggered by an extrinsic pathway involving membrane receptors with death domains and intrinsic pathways involving the endoplasmic reticulum and the mitochondria. Interactions between these pathways occur. Many forms of apoptosis are mediated by mitochondrial outer membrane permeabilization (MOMP) induced by activation of the mAC. Opening of the MOMP results in release of cytochrome c and other molecules which bind to cytoplasmic components, including procaspase 9, apoptotic protease activating factor-1, and dATP to form the apoptosome. The apoptosome then triggers the activation of caspase 3 and other effector caspases in the cytoplasm which then activate endonucleases producing double-stranded DNA breaks in the nucleus. The mitochondrial pathway rather than the extrinsic pathway appears to be dominant in the apoptosis component of myocardial ischemic injury.
A key event in oncosis is early opening of the mPTP in the inner mitochondrial membrane, without cytochrome c release. The opening of the mPTP leads to immediate loss of the electrical potential difference across the inner membrane, with resultant cessation of ATP synthesis, influx of solute, and mitochondrial swelling. Apoptosis and oncosis have been shown to be capable of interactions in series and parallel. Initial activation of apoptosis can be followed by oncosis and, conversely, oncosis followed by apoptosis. The oncotic trigger of loss of the mPTP in the mitochondria can occur in close temporal relationship to cytochrome c release via the mAC. Such interactions appear to be operative in myocardial ischemic injury.
Autophagy is an additional mode of cell modulation . The process involves segregation of cellular components, including proteins and mitochondria, in autophagic vacuoles, merger of the autophagic vacuoles with lysosomes to form autophagolysosomes, and followed by degradation of the constituents in the vacuoles. Regulated autophagy can mediate cell survival following stress; however, unregulated autophagy can lead to cell death. Autophagy can function to modulate acute myocardial ischemic injury. Autophagy also contributes to cardiomyocyte survival in the setting of chronic ischemic myocardium associated with decreased myocardial function, a condition referred to as hibernating myocardium.
The sentinel study on which the histologic criteria for establishing the age of myocardial infarcts in man are based on a study of 72 autopsied patients by Mallory et al. in 1939 . Over the years, the treatment of patients with acute ischemic syndromes has changed drastically from a passive approach associated with weeks of in-hospital bed rest to active acute interventions with hospital discharge in less than 1 week in uncomplicated cases. Now, timely myocardial reperfusion is a well-established therapeutic strategy for AMI. Thrombolysis or mechanical interventions such as PCI with stenting reduce acute myocardial injury and limit size of AMI .
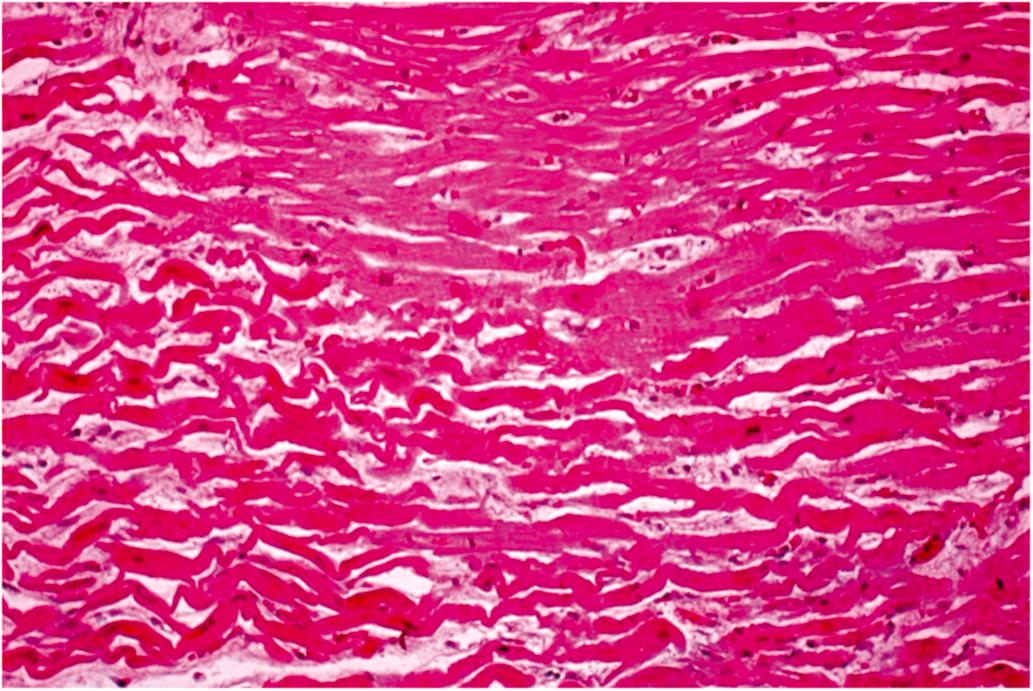
Cardiomyocyte injury and necrosis can exhibit a spectrum of histological features based on the rapidity and severity of the insult ( Tables 10.5 and 10.6 ). The first histologic abnormality associated with ischemia is the presence of myocardium with thinned, wavy fibers separated by spaces representing edema, and microvascular congestion ( Fig. 10.6 ; Tables 10.2, 10.4 and 10.5 ). Wavy fibers may be found clinically in infarcts only 1-h-old and in over 90% of infarcts 24 h or less in age . The wavy fibers probably result from systolic stretching of noncontractile fibers in the severely ischemic tissue. Since these stretched fibers cannot contract like the adjacent myocardium, they appear long, thin, and wavy in histologic sections. However, isolated myocyte waviness is not diagnostic of AMI . The edema seen histologically is probably intracellular in vivo, but moves to the extracellular compartment during tissue processing. The vascular congestion is most likely the result of capillary endothelial cell injury with swelling, occluding capillary lumens. Dilation and congestion are most obvious in the venules. The frequency of wavy fibers in recent myocardial infarcts supports the view that they are related to ischemia; this finding thus serves as a useful criterion to aid in the morphologic recognition of early myocardial injury. However, wavy fibers are not specific for necrosis, as they have been observed experimentally very early after coronary occlusion in myocardium lacking ultrastructural evidence of irreversible injury . The earliest histological change confirmed to be specific for myocardial necrosis by ultrastructural techniques is the so-called “brick-red change” or hypereosinophilia of necrotic myocytes in hematoxylin–eosin (H&E) stained sections. The sensitivity of this finding depends on the quality of the histologic sections and staining technique. Different laboratories vary quite a bit in both hematoxylin and eosin staining. In spite of this, hypereosinophilia can be readily identified by comparing the region suspected to be necrotic with the first two or three layers of subendocardial myocardium in the same section. These subendocardial fibers are almost always spared in acute ischemic injury so they will appear less red than the adjacent necrotic fibers. This subendocardial sparing is thought to be related to either Thebesian venous perfusion or diffusion of oxygen from intraluminal ventricular blood. Masson’s trichrome stain has been advocated as a useful tool to better delineate this early tinctorial change in necrotic myocardium.
| Wavy fibers | Ischemia | 5 min |
| Glycogen loss | Ischemia | 5 min |
| Electron microscopy | Ischemia | 10 min |
| Necrosis | 20 min | |
| Tetrazolium stains | Necrosis | 2–3 h |
| Light microscopy | Necrosis | 3–4 h |
| Cardiomyocyte abnormalities | Settings | Outcome |
|---|---|---|
| Hydropic change, fatty degeneration | Hypoxic, ischemic, or toxic insult | Potentially reversible |
| Myocytolysis (colliquative myocytolysis) | Isolated hypoxic, ischemic, or toxic insult |
|
| Contraction band necrosis (coagulative myocytolysis) | Miliary (microscopic) infarcts with or without stromal necrosis; military lesions due to catecholamines, drugs, toxins, myocarditis; outer zones of confluent myocardial infarcts; predominant pattern with reperfused infarcts | Irreversible |
| Coagulation necrosis (necrosis without hypercontraction) | Inner zones of large myocardial infarcts; sometimes seen in miliary lesions | Irreversible |
Cell death is not immediate after the onset of ischemia. Cell death may take as little as 15–20 min to develop, or considerably longer if ischemia is less severe. In experimental animals, fine changes in the sarcoplasm of some myocardial fibers have been noticed after 90 min of ischemia; the color change and subtle edema are evident 2–3 h after coronary occlusion. No one knows for certain how soon they develop in humans; however, several hours after the infarction, myocardial necrosis can be identified by microscopic examination. Cardiomyocytes do not immediately undergo rapid fragmentation and removal. From 3 to 6 h after coronary occlusion, hypereosinophilia and edema become more pronounced and more easily recognizable. This time may depend on the presence of collateral blood flow. Contraction bands correspond to a focal, highly pathologic spasm, where adjacent sarcomeres fuse. These are an important sign of early infarction, if there has been reperfusion .
Three forms of myocardial necrosis are seen in established myocardial infarcts ( Table 10.5 ). These are typical coagulation necrosis seen in the most severely ischemic central zone, coagulative myocytolysis (contraction band necrosis) seen in the infarct periphery, and colliquative myocytolysis (often referred to simply as “myocytolysis”) sometimes seen adjacent to the infarct proper . The pattern of injury seen in the infarcted periphery characterized by contraction band necrosis is also characteristic of myocardial injury produced by temporary coronary occlusion followed by reperfusion and catecholamines or sympathomimetic drugs.
A well-characterized sequence of events follows the onset of myocardial necrosis and allows for understanding of the progression of changes following onset of MI as well as identification of features useful in determining the age of the infarcts ( Table 10.7 ). The lethally injured cardiomyocytes in AMI trigger exudative inflammation. In the 6–12 h time period, an increased number of neutrophils line up in capillaries at the edges of the infarct, so-called margination of neutrophils. These neutrophils accumulate over the next 12 h with extravasation into the interstitial space. By 24 h, vascular congestion, interstitial edema, and focal areas of hemorrhage are also recognized. After day 1, neutrophils are quite numerous. Loss of myocyte nuclei occurs after necrosis is recognizable by other changes. On day 1, necrotic fibers are recognized in most cases, showing increased eosinophilia, granularity of the cytoplasm and nuclear pyknosis or karyolysis. Loss of cross-striations is often mentioned; however, these may persist long after necrosis is clearly evident and can be seen in mummified myocardium, weeks after an acute infarct occurs . By day 2, all cases show severe necrotic changes, and the region of infarction is surrounded by a distinct zone of neutrophils. On day 3 or 4, degeneration of the neutrophils becomes clearly evident as they undergo apoptosis. The resulting nuclear fragments have been referred to as “nuclear dust.” Lymphocytes do not appear at the edge of the infarction until day 2, but will be seen in all cases by day 6. Pigmented mononuclear cells containing lipofuscin appear after 2–3 days. Plasma cells appear by day 4. Phagocytosis and removal of necrotic fibers begin on days 4–5, and subtle vascular and connective tissue proliferation has begun around the edges of the necrotic muscle, usually beneath the epicardium and in perivascular areas. Macrophages containing hemosiderin and myoglobin pigment are both seen after infarction; hemosiderin deposition will be seen until around 3 months.
| Time | Light microscopic changes | Gross changes |
|---|---|---|
| 0–1 h | Stretched, wavy cardiac myocytes at borders of ischemic myocardium | None |
| 4–12 h | Early changes of cardiomyocyte coagulation necrosis with nuclear pyknosis, cytoplasmic hypereosinophilia and focal contraction bands; interstitial edema and focal hemorrhage; margination of neutrophils; changes accelerated and more extensive contraction band necrosis with reperfusion | None (without reperfusion) |
| 18–24 h | Continuing coagulation necrosis with karyorrhexis and karyolysis of nuclei; thinning of myocytes in central region and contraction band necrosis toward periphery; interstitial infiltration of neutrophils | Pale, dry myocardium with focal, dark red areas of congestion or hemorrhage at edges; congestion and hemorrhage more prominent and extensive with reperfusion |
| 24–72 h | Advanced coagulation necrosis with loss of nuclei and striations; heavy influx of neutrophils in periphery; early degeneration of neutrophils | Pallor and yellowish discoloration more pronounced |
| 3–5 days | Beginning of disintegration of necrotic myocytes; continued degeneration of neutrophils producing basophilic granular debris; influx of macrophages; phagocytosis of myocyte debris; onset of granulation tissue at edge of infarct | At 4 days, a fine yellow line or zone (produced by neutrophilic infiltrate) appears in the periphery. This zone becomes progressively broader |
| 7 days | Granulation tissue and early fibrosis at edge of infarct | Thin rim of gelatinous tissue at edge of infarct |
| 10 days | Extensive phagocytosis by macrophages; prominent granulation tissue with fibrovascular reaction in margins | Thickness of the wall is reduced; center of the infarct is yellow and surrounded by a red-purple depressed band of granulation tissue |
| 3–4 weeks | Granulation tissue extends throughout the infarcted area | Red-purple sunken granulation tissue extends throughout the infarct |
| 2–3 months | Granulation tissue is less cellular and less vascular, and newly formed collagen is abundant | Infarct area gradually develops a gelatinous gray appearance and then is transformed into a sunken, firm scar |
| After 3 months | Fibrous scar is fully developed in most infarcts | Gray scar becomes progressively whiter and very firm |
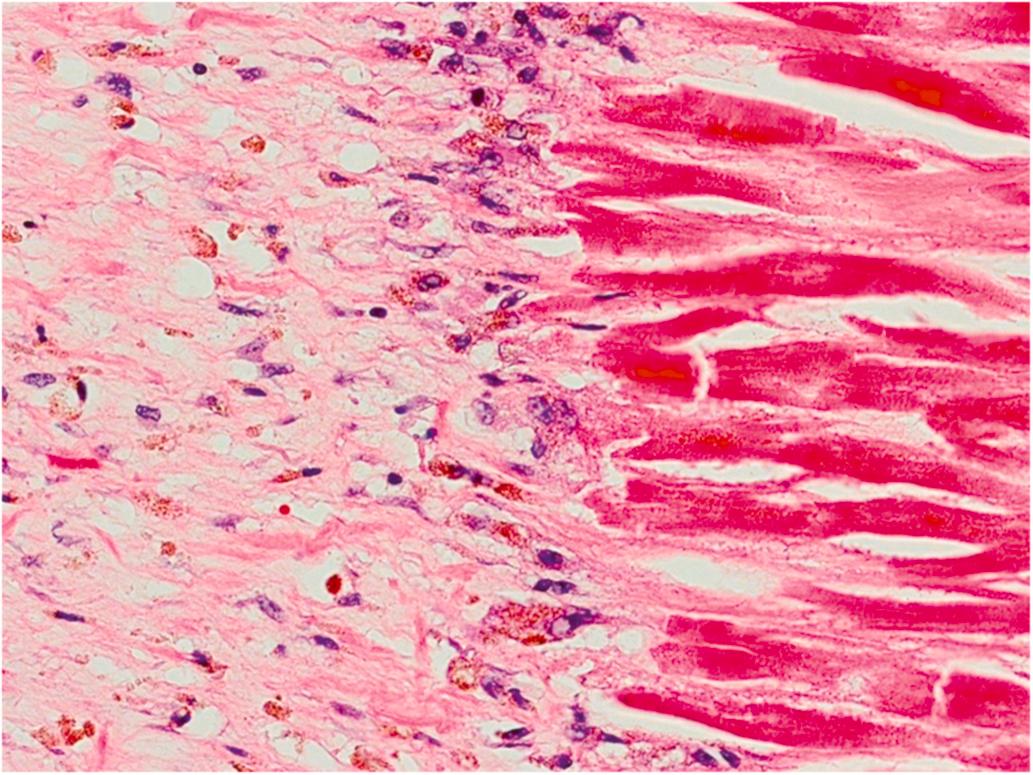
Healing infarction is recognized by the presence of mononuclear cells and fibroblasts and the absence of neutrophils. During the second week, chronic inflammation and removal of peripheral muscle fibers occur. Increased numbers of pigmented mononuclear cells are apparent at the margins of the infarcts where vascular and connective tissue proliferation has become more prominent ( Fig. 10.7 ). The cells are both macrophages and degenerated myocytes; the former contain both hemosiderin and myoglobin pigment. Eosinophils, lymphocytes, and plasma cells are present. Eosinophils may be quite prominent during the subacute phase of infarction. Neutrophils are infrequent by the end of the second week. Dense basophilic granules (calcium salts) may be seen in necrotic myocytes at the periphery of the infarcted tissue after first week. On the second week, Antischkow cells, “caterpillar-like” configuration of nuclear chromatin, become evident at the border between normal myocardium and the healing infarct area . In addition to the myocardial changes, endocardial and pericardial inflammation may be seen. Left ventricular endocardial mural thrombi are often present adjacent to an infarct. Focal pericarditis and pericardial hemorrhage may occur. During the healing process, secondary endocardial fibroelastosis and pericardial fibrosis may develop along with myocardial fibrosis.
During the third week, there is continued removal of necrotic fibers and proliferation of connective tissue, recognized as granulation tissue. Macrophages are still numerous, and plasma cells, eosinophils, and lymphocytes are still present, but there is more substantial fibroblastic proliferation with collagen formation recognizable as pink fibrillar extracellular material.
From this time on, the healing of the infarct is characterized by removal of residual necrotic fibers and continued gradual scar formation with deposition of collagen. Inflammatory cells and vascularity decrease, but a few macrophages, plasma cells, and lymphocytes persist and remain in the scar that eventually forms. Healing occurs from the edges with little activity in the center of a necrotic zone; hence, it is the edge that must be examined to most accurately age an infarct.
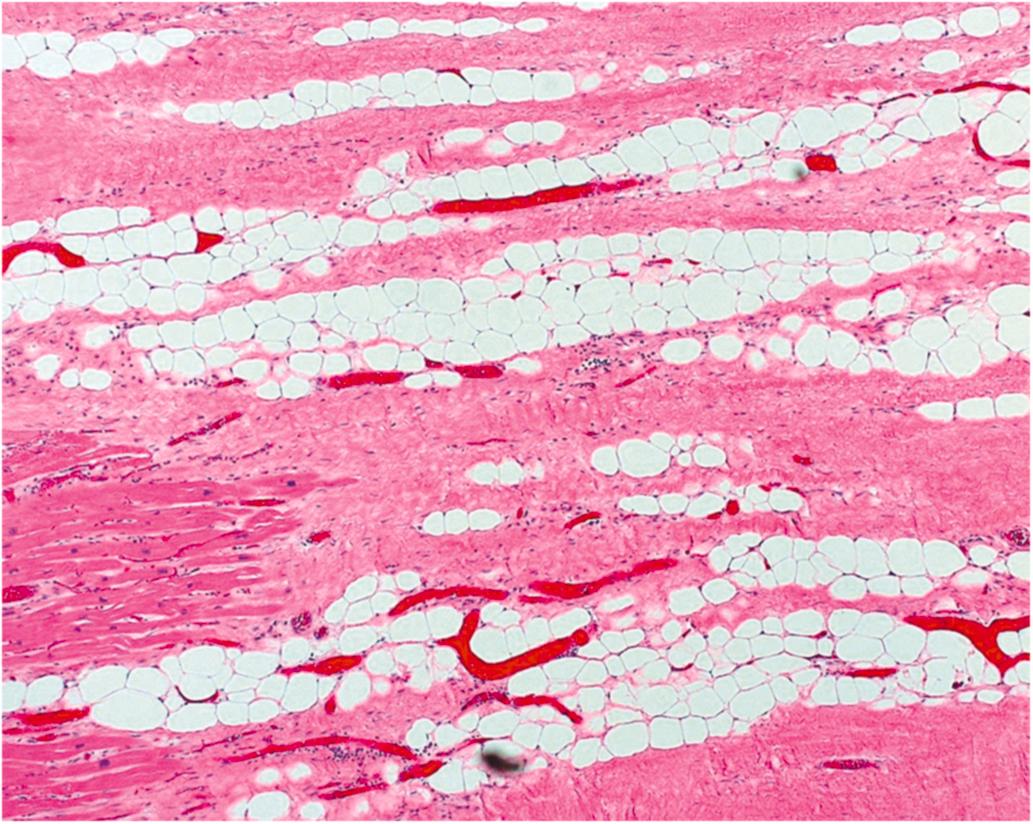
Healed infarction is scar tissue without cellar infiltration and takes at least 5–6 weeks after the infarction. Adipose tissue is a prevalent histological finding in healed infarction ( Fig. 10.8 ). Interestingly, the amount of adipose tissue increases with age, and is greater in males and in those patients who had CABG surgery. The pathogenesis of adipose tissue is unknown, but may be influenced by current medical therapy for AMI since adipose tissue in healed MI was not reported until 1999 . Healed infarction is scar tissue without cellar infiltration and may take 5–6 weeks or more after the infarction. Adipose tissue is a prevalent histological finding in healed infarction ( Fig. 10.8 ). Interestingly, the amount of adipose tissue increases with age, and is greater in males and in those patients who had CABG surgery. The pathogenesis of adipose tissue is unknown, but may be influenced by current medical therapy for AMI since adipose tissue in healed MI was not reported until 1999 .
In summary, necrosis and acute inflammation predominate during the first week; chronic inflammation reaches its peak during the second week, and connective tissue proliferation dominates from the third week until healing is completed. The time required for complete healing of myocardial infarcts in man (i.e., absence of residual necrotic fibers) is quite variable. Complete healing may take considerably longer than 3 weeks depending on the size of the infarct and other factors, such as drugs that affect inflammation and wound healing. Current practice for uncomplicated MI dictates hospital discharge in less than 1 week for most patients.
Other types of cell death, different from the coagulation necrosis described above, occur in MI. Contraction band necrosis, sometimes called “coagulative myocytolysis,” indicates degeneration of myocardial fibers characterized by a hypercontraction or spasm of the fibers with the formation of irregular abnormal transverse bands. These changes have been observed in association with electric shock, potassium deficiency, catecholamine administration, coronary artery reperfusion, and in patients dying after cardiac surgery . Contraction bands are found in some myocytes at the periphery of almost all infarcts, and are widely distributed in infarcts after reperfusion of the necrotic zone (see the “Reperfusion of ischemic myocardium” section).
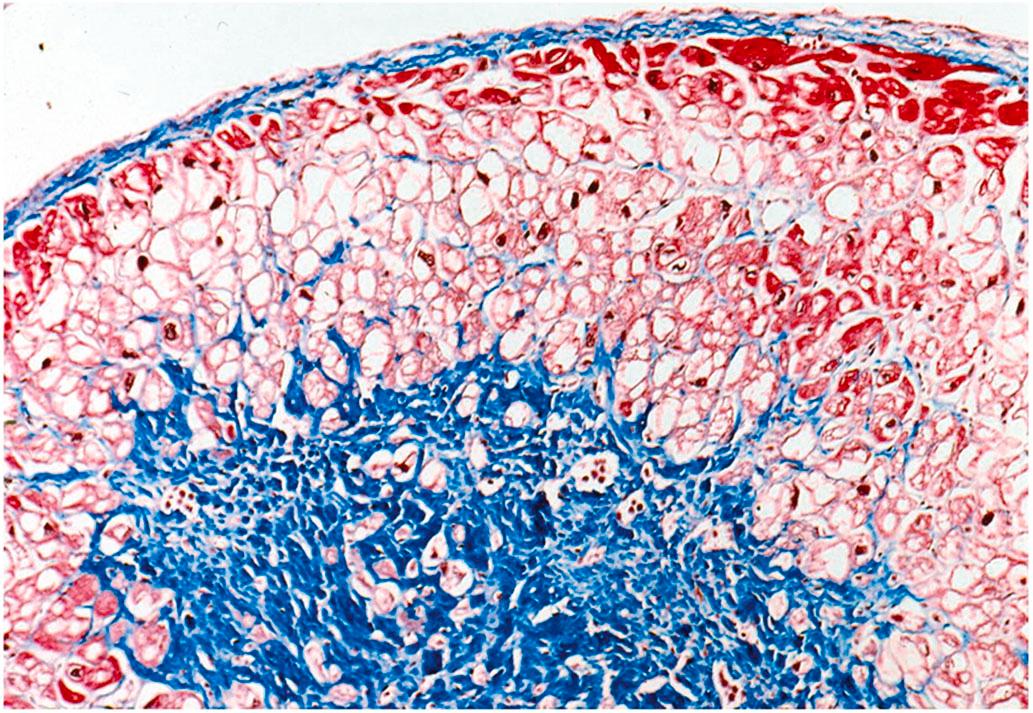
Vacuolar degeneration, often referred to as simply “myocytolysis” or “colliquative myocytolysis,” describes focal lesions, mainly in the subendocardium and in perivascular regions, characterized by progressive vacuolization of fibers with lysis of contractile elements until only empty sarcolemmal tubes remain ( Fig. 10.9 ). The apparently empty spaces are thought to represent water, glycogen, and/or lipids, which are arrhythmogenic. It has been suggested that the lesion results in cell death and scarring, although the vacuolated fibers may remain viable for prolonged periods, as demonstrated by ultrastructural and immunohistochemical techniques. Unlike coagulative necrosis, healing occurs without a prominent inflammatory response, blood vessel necrosis, or granulation tissue proliferation. Myocytolytic lesions can evolve to the point of complete loss of myocytes, leaving behind perimyocytic connective tissue in an alveolar pattern with subsequent fibrosis produced by collapse of the connective tissue matrix (collapse fibrosis). Vacuolar degeneration usually occurs in the left ventricular subendocardium, in perivascular regions, and at the perimeter of myocardial infarcts. This type of focal myocytolysis may not be related to acute ischemia per se but perhaps to hypoxia, drug therapy, or chronic ischemia. Vacuolar degeneration is not specifically seen in acute and chronic IHD; it has also been noted in cardiomyopathies, myocarditis, endocarditis, scleroderma, and uremia .
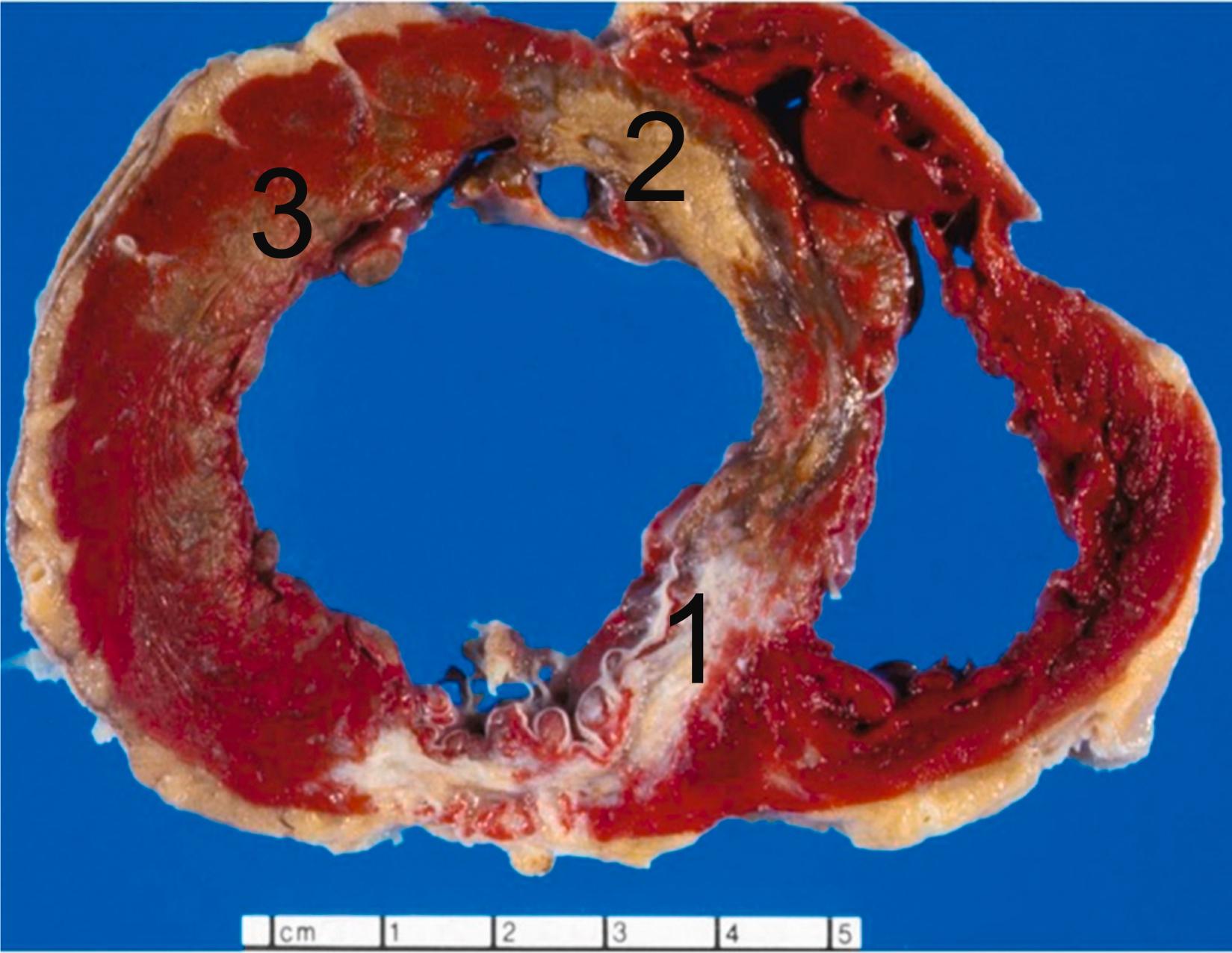
ACSs are most often triggered by acute changes superimposed on coronary atherosclerotic plaques . These changes include erosion or rupture of the plaque surface, intraplaque hemorrhage, and superimposed thrombosis ( Fig. 10.10 ). Lesions prone to these changes are designated as vulnerable plaques. The exact relationship between vulnerable plaques and plaques that have undergone erosion or rupture remains subject to further elucidation. In cases of SCD, plaque erosions are more frequent in women and plaque ruptures in men. More than 75% of MIs are caused by coronary occlusion due to ruptured or eroded atherosclerotic plaques . Atherosclerosis will be discussed in detail in Chapter 3 , Age-Related Cardiovascular Changes and Diseases; however, here we briefly mention the definite cause of MI, culprit coronary artery lesions. Culprit lesions in STEMI frequently are located in the proximal left anterior descending artery (LAD), right coronary artery around the origin of the marginal branch, and left circumflex proximal to and near the origin of the obtuse marginal branch.
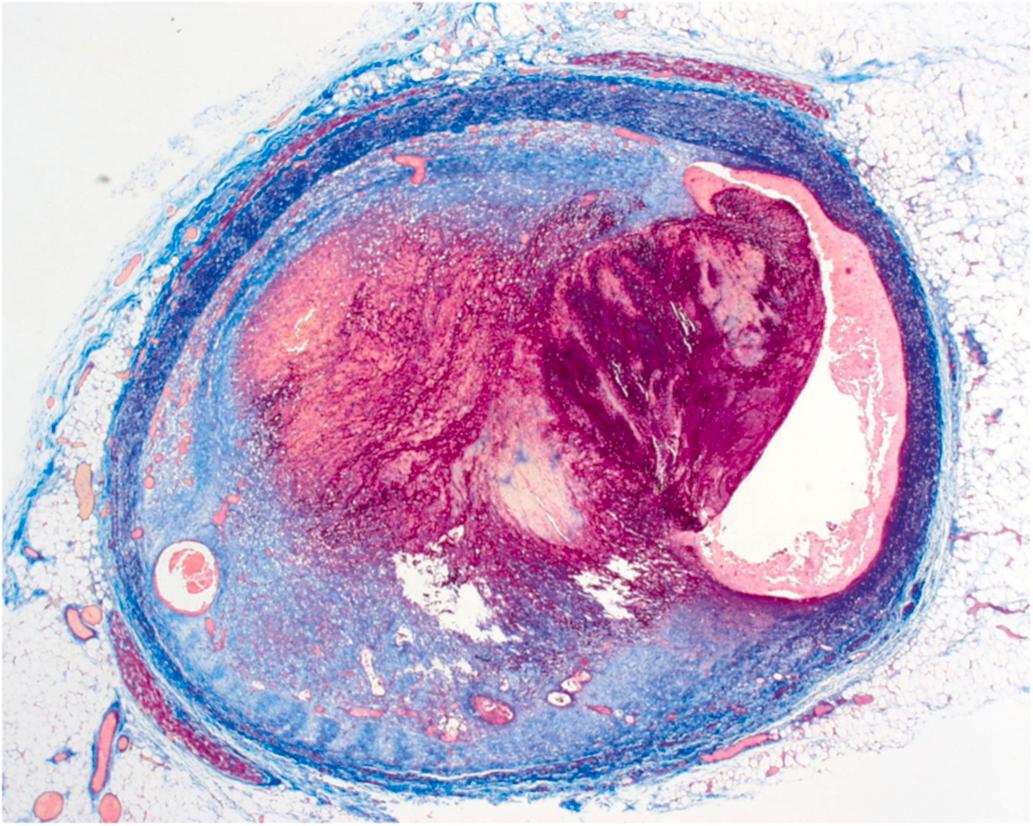
Atherosclerotic plaque rupture and erosion have been identified as the major underlying substrates for “coronary events” . Ruptures of plaque occur in over 75% of fatal MI and/or SCD. The vulnerable plaque is a plaque that is particularly susceptible to disruption. Morphologically, the vulnerable plaque has a lipid-rich necrotic core with a thin fibrous cap (<55 μm) and inflammation in the shoulder region of the plaque. In addition to hemodynamic trauma, inflammation, involving abundant macrophages and T cells and mediators, including interferon-gamma, matrix-metalloproteinases, and CD40 ligand, has an important role in degradation and destabilization of the plaque capsule .
Microscopically, the ruptured plaque displays discontinuity of the fibrous cap, with endothelial injury and overlying mural thrombus ( Fig. 10.11 ). Necrotic material from the core is mixed with thrombotic material in the lumen. Thrombus without rupture may overlie superficial erosion of proteoglycan rich plaques that have less lipid and less inflammation.
In some cases of ACS, progressive stenosis narrows the lumen of an atherosclerotic coronary artery to the point that a small platelet thrombus can completely occlude the vessel. An occlusive thrombus that complicates a high-grade stenosis arrests flow and causes a STEMI. However, a different scenario appears to be operative in many cases of ACS. This view has come about by correlation of pathological findings with the multiple approaches to coronary artery imaging now available, including quantitative coronary angiography, computed tomography (CT) angiography, intravascular ultrasound (IVUS), optical coherence tomography, magnetic resonance imaging (MRI) and Mr angiography, and positron emission tomography (PET) .
Imaging studies have shown that plaque at the site of the culprit lesion of a future AMI often does not cause stenosis severe enough to limit flow. This is due to the phenomenon of outward remodeling of the artery with the culprit atheromatous lesion, the so-called Glagov phenomenon . Angiography following thrombolytic therapy has shown that the culprit lesion often is not the cause of the critical stenosis of the artery, but due to another lesion in the same vessel. By CT angiography, typical culprit plaques of ACS show little or no calcium and outward expansion of the artery wall reflecting a remodeling process that allows plaque growth with minimal luminal narrowing. By IVUS, the culprit lesions often are proximal to the sites of maximal stenosis, with the latter being the traditional targets of revascularization therapies . The lack of correlation between the degree of stenosis and development of instability or rupture of a vulnerable plaque explains the often sudden onset of an AMI without prior angina pectoris or detection on coronary arteriograms.
With survival of an episode of ACS, eroded or ruptured plaques with hemorrhage and thrombosis undergo organization and healing. Stages in the organization of coronary thrombi have been defined, thereby allowing for dating of the age of the thrombi ( Table 10.8 ). Organization of the acute lesions leads to inward remodeling and contributes to progression of atherosclerotic disease. In cases of chronic IHD, coronary arteries frequently show layering of one plaque type on top of plaque of different composition. These features illustrate the subsequent development of new atherosclerotic lesions superimposed on older lesions .
|
|
|
|
|
How does MI occur in the absence of the atherosclerotic plaque? Other mechanisms of occlusion include myocardial bridging (also known as tunneled coronary artery), vasospasm, vasculitis, spontaneous coronary artery dissection, thromboembolic disease, and microvascular coronary disease. Myocardial bridging occurs when myocardial tissue covers part of a coronary artery, resulting in a tunneled arterial segment that gets compressed as the myocardium contracts . Significant bridging exists almost exclusively in the LAD. The prevalence of at least a short bridge is very high, reportedly seen in more than 50% of cases on autopsy. It is rare to find stenosis and severe atherosclerosis in a coronary artery within a myocardial bridge. In contrast, the segment proximal to the myocardial bridge often exhibits severe atherosclerosis. The significance of a myocardial bridge appears to depend on the length and thickness of the myocardial bridge. The presence of myocardial fibrosis in the territory of the bridged artery provides confirmation of episodes of ischemic injury.
Tako–Tsubo cardiomyopathy is discussed here because it may be due to myocardial ischemia. Tako–Tsubo cardiomyopathy, also called stress-induced cardiomyopathy, transient apical ballooning syndrome, and broken-heart syndrome, is a disease characterized by acute but rapidly reversible left ventricular systolic dysfunction in the absence of atherosclerotic CAD . The name Tako–Tsubo refers to the appearance of the heart on echocardiogram, resembling the Japanese octopus fishing pot by that name (Tako, octopus; Tsubo, pot) ( Fig. 10.12 ). Tako–Tsubo has a wide base and a narrow neck, and the echocardiogram during this condition displays transient hypokinesis, akinesis, or dyskinesis in the left ventricular middle segments without apical involvement. The process is considered to be mediated by excessive activation of the sympathetic nervous system .

Tako–Tsubo cardiomyopathy has a clear gender predilection. Females comprise over 90% of reported cases; typically Tako–Tsubo affects postmenopausal, older women. Interestingly, Tako–Tsubo cardiomyopathy is often preceded by emotional or physical stressors, such as death of a close relative, abuse, a quarrel, exhausting work, and after an earthquake. Patients complain of an abrupt onset of angina-like chest pain and dyspnea, resembling AMI. The ECG demonstrates diffuse T-wave inversion, usually in the anterior wall of the left ventricle, and normalizes in a timeframe of hours. Blood sampling shows mild cardiac enzyme elevation. Elevated plasma catecholamine level is also reported. Echocardiogram shows left ventricular dysfunction, especially in the mid-left ventricle. Left ventricular function usually normalizes over days to weeks after onset. Outcome is generally favorable, but rarely left ventricular rupture is seen.
The pathogenesis of Tako–Tsubo cardiomyopathy has been thought to include coronary artery spasm, microvascular dysfunction, catecholamine cardiotoxicity, and neurogenic stunned myocardium. Cellular mechanisms may be different from the ischemia secondary to coronary stenosis. Histologically, one may see contraction bands with or without myocyte necrosis and interstitial inflammation. Myocardial injury also can occur in association with various cerebral lesions apparently mediated by excessive sympathetic nervous system activation and release of catecholamines in the myocardium . A similar phenomenon can be seen in patients with pheochromocytoma.
The biochemical, ultrastructural, and microscopic changes described previously are accompanied by characteristic gross changes that are useful in determining the age of an MI. During the first 24 h after coronary occlusion, infarcts are better felt than seen in that there is palpable softening of the normally firm myocardium, probably due to edema . The myocardium without reperfusion may appear pale or “anemic.” If a patient dies after reperfusion, the infarct is hemorrhagic . With time, the endocardium also becomes opaque and white due to fibrosis. In transmural infarcts, fibrinous pericarditis, which may be observed after 24 h, causes thickening of the epicardium. When death occurs before the time necessary for coagulative necrosis to become visible histologically, the diagnosis of a fatal coronary event relies on the detection of occlusive coronary thrombosis of an epicardial coronary artery.
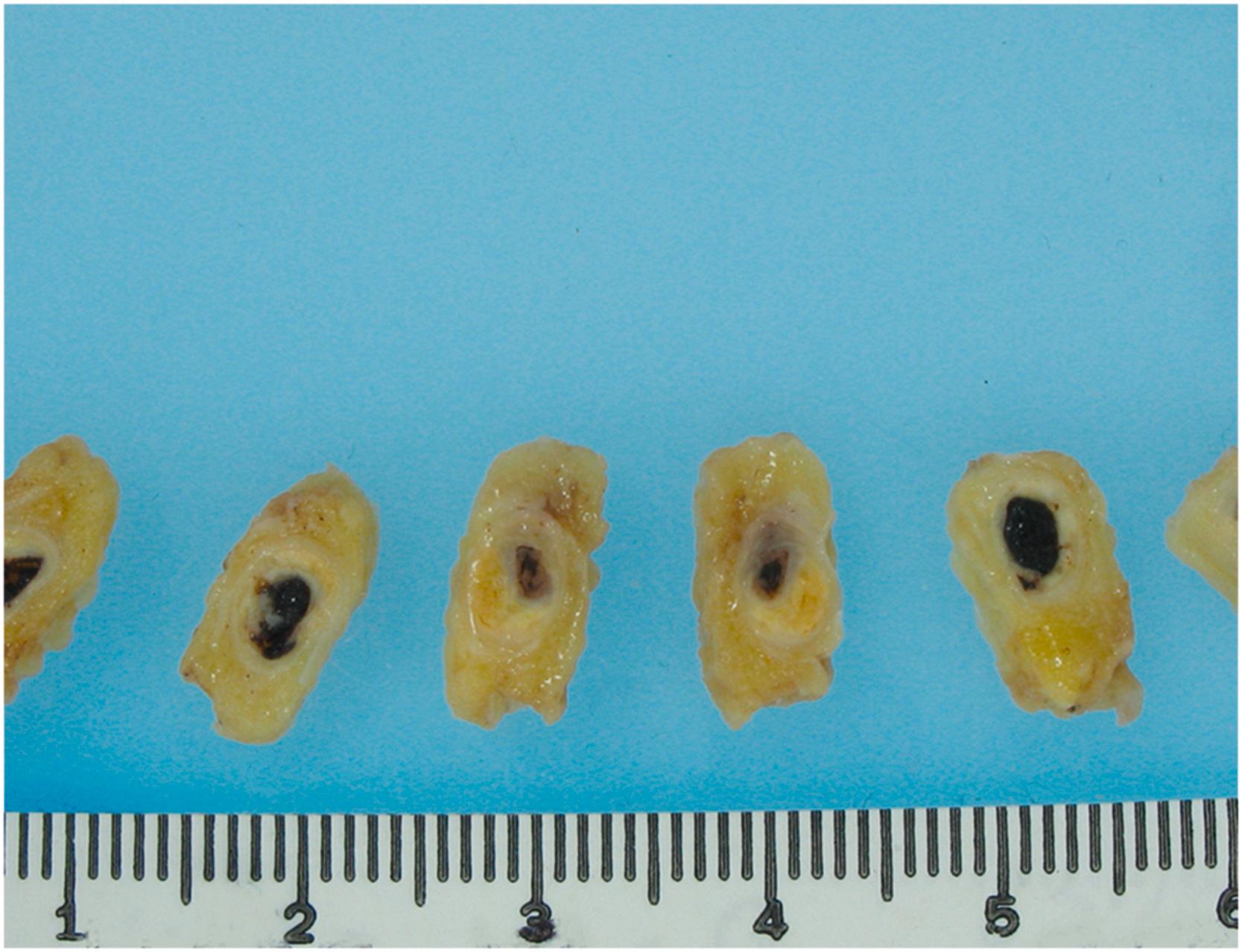
A useful tool to make a macroscopic early diagnosis (within a few hours from onset) of MI at autopsy is the triphenyltetrazolium chloride (TTC) method. A fresh cross-section of the ventricles is placed in TTC solution for 20–30 min. This histochemical stain imparts a brick-red hue to the noninfarcted area where the dehydrogenase enzymes are preserved; in contrast, the infarcted myocardium fails to stain ( Fig. 10.13 ).
Two-day-old infarcts may appear focally hyperemic with patchy, reddish discoloration due to small hemorrhages that occur often and spontaneously even in anemic infarcts. The mottling is often around a yellow-tan core. Hyperemia and hemorrhage will be more pronounced if there has been therapeutic and/or spontaneous reopening of the culprit occluded coronary artery. By 3 or 4 days, the infarcts are gray to grayish-yellow due to increased infiltration of inflammatory cells with degeneration of myocardium and leukocytes. The periphery may be red due to vascular congestion, hemorrhage, and/or early neovascularization. At the end of the first week, if the infarct is transmural, thinning of the wall will be seen . During the second week, the infarcted lesion becomes surrounded by a gelatinous, hyperemic zone composed of granulation tissue. This zone progressively enlarges as healing occurs at the expense of the central necrotic area. Usually, by the end of 3 weeks, most of the necrotic tissue is removed; from 3 to 6 weeks, the gelatinous granulation tissue is replaced by firm, white scar tissue. After 2 months, the scarring process is usually completed.
The term “myocardial infarct” refers to necrosis of myocardium as a result of a critical reduction in the arterial blood supply to the affected region . The pattern and extent of the necrosis depends upon a number of variables: (1) the severity of ischemia; (2) the duration of ischemia; (3) the anatomy of the coronary arteries; (4) the pattern of collateral blood flow; and (5) coexisting myocardial disorders.
It has been demonstrated in experimental animals and humans that with coronary occlusion blood flow decreases more severely in the subendocardial than the subepicardial myocardium . Therefore ischemia is most severe in the subendocardium and as a result, necrosis occurs there first, sparing only a few layers of periventricular fibers, spreading toward the epicardium and to some extent the endocardium . This “wave-front phenomenon of cell death” has been described and elucidated by the elegant work of Jennings, Reimer, and colleagues, using a left circumflex occlusion, posterior papillary muscle infarction model in the open-chest dog . Interestingly, the evolution of ischemic injury depends to a great degree upon the animal being used and the conditions of the model. Coronary anatomy, extent of collateral flow, type of anesthesia, and open-chest versus closed-chest models of coronary occlusion will all differ in the extent and rate of progression of ischemic injury following coronary occlusion. Nevertheless, the evolution of AMI in man mirrors that of most experimental models. The evolution of AMI takes place rapidly, and the full extent of myocardial necrosis is complete by 6 h in most cases. In the presence of some collateral flow, the evolution may be slower.
In light of the aforementioned, it is easy to understand why infarcts vary in size and distribution. In attempts to correlate gross pathology with electrocardiographic findings, pathologists have characterized infarcts involving less than half of the wall thickness as subendocardial, and more than half as transmural . Subendocardial infarcts were often considered to be “mild” infarcts, associated with low in-hospital mortality . However, because of the likelihood of arrhythmia and/or extension of infarction due to persistence or recurrence of obstruction in the offending coronary artery, this concept has changed. Indeed, data indicate that patients with “subendocardial infarcts” are at risk for recurrent ischemic events .
Infarcts are characterized by (1) the chamber involved (usually left ventricle); (2) the wall involved; (3) the apical-basal location; and (4) the size of infarct. The locations are anterior or anteroseptal, lateral, posterior or posteroseptal, or septal, or a combination of locations. Anterior infarcts are more often apical than basal, but not infrequently involve the mid-ventricular level. Posterior infarcts tend to be basal, and may extend all the way up to the atrioventricular groove. Both anterior and posterior left ventricular infarcts usually involve some portion of either the interventricular septum and/or lateral wall of the left ventricle. In a patient with a long LAD that curves around the apex, supplying the posteroapical wall, occlusion of the artery may lead to circumferential infarction at the apex. Although atrial and right ventricular infarcts occur, they are less common and are more difficult to detect clinically and pathologically .
If a left anterior, descending occlusion is very proximal, before the takeoff of the first septal perforating branch, a substantial portion of the anterior two-thirds of the septum may become ischemic resulting in massive infarction involving most of the anterior and septal walls of the left ventricle, often leading to death; hence, the name “widow maker” for such a coronary lesion. Posterior infarcts usually involve only the posterior 1/3 of the interventricular septum. Infarcts associated with certain electrocardiographic changes involving leads II, III, and AVF are called “inferior.” When examined by a pathologist, such infarcts usually involve the most apical portion of the posterior wall of the left ventricle; however, which wall, and which part of which wall of the heart that is “inferior” depends upon the body habitus of the individual.
In patients with multivessel CAD with all possible combinations of collateral flow, there is not always an obvious association between the site of coronary occlusion and the site and extent of necrosis. For example, if a patient has had a previous occlusion of the LAD without infarction, due to development of collateral flow from the right coronary artery, a subsequent right coronary artery occlusion may in fact cause an acute anterior infarct. The term applied to this phenomenon, “paradoxical infarct,” is somewhat of a misnomer, since knowledge of the collateral flow pattern shows that there is nothing paradoxical about the infarct location. If myocardial necrosis is due to severe hypoxia or hypotension, necrosis does not correspond to the distribution on any single coronary artery. Instead, it is circumferential and usually subendocardial.
In general, anterior infarcts are considered more serious and more lethal than posterior infarcts, as they are more often larger and associated with congestive heart failure and/or arrhythmias . Documenting the location of infarcts is also important for other reasons. Right ventricular infarcts occur in association with anteroseptal and posteroseptal infarcts, but much less frequently do they complicate anterior infarcts. Isolated right ventricular infarction is a rare, but recognized occurrence, usually in the setting of acute elevation of pulmonary artery pressure due to a pulmonary thromboembolus . Focal right ventricular necrosis may complicate up to 20% of left ventricular infarctions. Right ventricular infarction can lead to tricuspid regurgitation, right ventricular failure, and even right ventricular rupture. Preexisting right ventricular hypertrophy makes the right ventricular myocardium more susceptible to ischemic injury after coronary occlusion. Even if the right ventricle is not infarcted, right ventricular remodeling is often associated with left ventricular remodeling.
Atrial infarction is almost always a postmortem diagnosis. Atrial infarction may be associated with atrial fibrillation (AF); however, since AF is a common arrhythmia in the absence of infarction, it is not helpful in the premortem diagnosis of atrial infarction. Ruptures of atrial infarctions have been reported; the right atrium ruptures three to five times more frequently than the left, perhaps owing to the thicker left atrial endocardium .
The phenomena to be discussed include: (1) stunned myocardium; (2) conditioned myocardium; (3) hibernating myocardium; (4) infarct expansion; (5) ventricular remodeling; and (6) compensatory hypertrophy of noninfarcted myocardium . Some of these conditions contribute to “chronic ischemic injury” characterized by changes not associated with the initial ischemic insult and myocyte necrosis, but rather subsequent alterations in both the initially ischemic and newer ischemic tissue.
If ischemic myocardium is reperfused early (15 min or less in some canine models) before irreversible injury occurs, the affected myocardium will return to normal structure and function. There is, however, usually a long delay before normalcy is reestablished. Systolic and diastolic dysfunction and metabolic changes may persist for 1 week. Hence, the myocardium is “stunned.” It should be noted that brief periods of occlusion followed by reperfusion may have a cumulative effect and cause myocardial necrosis, as occurs in patients with repeated angina episodes . On the other hand, if the ischemic periods are brief enough, and the reperfusion periods long enough, prolonged myocardial viability may not only be sustained, but also prolonged. The term “preconditioning” has been used to describe the myocardium that has prolonged resistance to ischemic injury if a severe ischemic insult is preceded by a mild one.
Biochemical changes implicated in myocardial stunning include (1) depression of ATP content by as much as 50%; (2) abnormal calcium transport; (3) interruption of the creatine phosphate shuttle; and (4) abnormalities of cardiac sympathetic innervation.
The morphologic changes observed in stunned myocardium range from none, to those observed in early reversible ischemic injury: nuclear chromatin clumping and margination, glycogen depletion, edema, evidence of I bands, and only minimal mitochondrial swelling. Interestingly, scanning electron microscopic studies of stunned myocardium has shown marked disruption of the extracellular collagen matrix, with destruction and loss of extracellular matrix components associated with an increase in myocardial compliance . These findings in the extracellular matrix early after ischemic injury provide a structural explanation for the abnormal contractile function of stunned myocardium.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here