Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Myeloma and leukemia share a common origin as hematologic malignancies. Both are usually systemic at the time of the diagnosis, although occasionally a plasmacytoma may exist as an isolated collection of plasma cells without systemic involvement. Both also have unclear boundaries from each other and from other related malignancies. For example, plasma cell leukemia is usually considered to be in the myeloma family rather than the leukemia family, despite its name, and pathologists may argue over whether a patient has lymphoma with a plasmacytic component or myeloma. Many of the chemotherapeutic agents that work for one of these diseases will have some efficacy for others as well.
Despite the similarities between myeloma and leukemia, each of them is a spectrum of diseases having distinct presentations, treatments, and prognoses. The use of imaging differs significantly between these two broad categories of malignancy. In myeloma, imaging is mainly used as a part of the staging process, to monitor response to treatment, and to look for evidence of disease progression. However, in leukemia there is little role for imaging in diagnosis and staging, and imaging is usually reserved for the diagnosis of treatment-related complications.
Multiple myeloma is a malignant disorder that results from a clonal proliferation of marrow plasma cells that usually results in the production of monoclonal immunoglobulin in the serum or urine. Its the second most common hematologic malignancy in the United States, with approximately 32,110 patients diagnosed and 12,960 dying annually in the United States. The disease is more frequent in males than in females (1.3:1). Multiple myeloma incidence in the African American population is twofold to threefold higher than the incidence in the White population. The exact reason for this disparity is still unknown, but may be attributed to the higher prevalence of monoclonal gammopathy of undetermined significance (MGUS) in Blacks. The disease most commonly occurs during the seventh and eighth decades of life; only 2% of patients are younger than 40 years at the time of diagnosis.
There is no undisputable risk factor for myeloma, but ionizing radiation or work in the paper, pulp, and leather tanning industries, as well as other chemical exposures, have been implicated. There are numerous reports of myeloma in people with chronic exposure to low-dose ionizing radiation, including radiology technicians/physicians before routine shielding and radium watch dial painters; perhaps the best evidence was among atomic bomb survivors at Hiroshima in an initial report, but the follow-up of this report failed to confirm those initial findings. Similarly, benzene has been implicated, but a thorough retrospective analysis of available reports eliminated this factor as well. Hereditary factors have not previously been thought to play a prominent role as risk factors for multiple myeloma, but recent reports suggest that, at least among certain populations, the incidence of MGUS/myeloma may have a familial/genetic association.
Myeloma begins with the clonal expansion of a malignant plasma cell that is usually positive for CD38, CD138, and monoclonal cytoplasmic immunoglobulin, with a clonal light chain of either kappa or lambda type. These cells typically have an eccentric nucleus within a large cytoplasm and often have an area of central clearing that corresponds to intracytoplasmic immunoglobulin. Cells of plasmacytic origin are usually negative for other markers of the B-cell lineage such as CD19 and CD20. Recent studies suggest the existence of different categories of myeloma that may separate the disease into different levels of outcome. A heterogeneous array of cytogenetic abnormalities has been described in patients. Deletions of chromosomes 13 or 17p, certain translocations involving chromosome 14, on which the immunoglobulin heavy chain locus resides (t(4;14) is present in approximately 11% of patients, and t(14;16) is present in approximately 3% of patients), and chromosome 1 abnormalities have predicted shortened survival. In contrast, t(11;14), which is found in approximately 14% of patients, has usually been associated with either an average or an improved prognosis, and is rarely noted to have a subgroup with poor prognosis. The median time to progression from smoldering multiple myeloma (SMM) to multiple myeloma was found to be shorter in patients with t(4;14) than in patients with t(11;14), with a median time of 28 months and 55 months, respectively.
Abnormal clonal proliferation usually results in the production of monoclonal immunoglobulin in the serum and/or urine: immunoglobin (Ig)G is secreted in approximately 60%, IgA in 20%, IgD in 2%, and IgE in less than 1%, whereas biclonal secretion is rare. Secretion of light chain as the sole monoclonal protein is noted in 18%. Previously, 3% of patients were noted to have the nonsecretory disease; this number has declined with the advent of serum free light chain detection (detection of free kappa, free lambda, and the free kappa:free lambda ratio in the serum).
Cells are usually:
CD38- and CD138-positive
CD19- and CD20-negative
Either kappa or lambda light chain–positive
Shortened survival with:
Chromosome 13 deletion by karyotype
Chromosome 17p deletion by karyotype
Chromosome 14 translocations t(4;14), t(14;16), or t(14;20)
Chromosome 1 abnormalities
Average or improved prognosis with:
Chromosome 14 translocation t(11;14)
Immunoglobin (Ig)G in 60%
IgA in 20%
IgD in 2%
IgE in less than 1%
Light chain only in 18%
Multiple myeloma may present with a number of characteristic clinical features and complications such as those that result from bone marrow infiltration (anemia), subsequent bone destruction (lytic bone lesions, fractures, bone pain, and hypercalcemia), complications of circulating monoclonal proteins, or reduced uninvolved immunoglobulins and/or light chains (hyperviscosity, increased infections, and renal failure). Patients with multiple myeloma most commonly present with fatigue and bone pain, with anemia, which contributes to fatigue, occurring in approximately 75% of patients.
Bone disease occurs in nearly 70% of newly diagnosed patients with multiple myeloma and results, in part, from RANKL overexpression and OPG inhibition resulting in unbridled activation of osteoclasts. Other cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α also have a role in lytic bone disease. Progressive bone destruction results in hypercalcemia in approximately 20% of patients with newly diagnosed myeloma.
Marrow infiltration with plasma cells results in normocytic, normochromic anemia in the majority of patients with previously untreated myeloma. Recently, upregulation of hepcidin mRNA in myeloma patients has been noted and may play a causative role in this complication. In patients with high levels of circulating monoclonal serum protein, stacking of red blood cells on the peripheral blood smear, known as rouleaux formation, may occur.
Nearly half of the patients will develop renal failure at some time during the course of their disease. Cast nephropathy, resulting from accumulation of light chains in the distal tubule where they can combine with Tamm–Horsfall urinary glycoprotein to form obstructing casts, is the most common cause of this complication. However, hypercalcemia, dehydration, hyperuricemia, and concomitant conditions such as amyloidosis and light or heavy chain deposition diseases may also be contributing factors.
Frequent bacterial infections may also be noted in patients with myeloma because of suppression of uninvolved immunoglobulins, decreased antibody response, and impaired opsonization, among other abnormalities reflective of the impaired immune response in patients with multiple myeloma.
Although the diagnosis of myeloma by a fixed criteria may be difficult, the International Myeloma Working Group (IMWG) proposed has revised diagnostic criteria for MGUS, SMM, and multiple myeloma. The term multiple myeloma refers to multiple myeloma requiring therapy, based on measurement of serum monoclonal protein levels, assessment of the bone marrow, and presence or absence of myeloma-defining events (MDEs). MDEs include “CRAB” (hypercalcemia, renal failure, anemia, bone lesions) features and the presence of one or more of the following malignancy biomarkers: a clonal bone marrow plasma cell (BMPC) percentage of greater than or equal to 60%, an involved:uninvolved serum free light chain ratio of greater than or equal to 100, or the presence of two or more focal lesions on magnetic resonance imaging (MRI). CRAB features are used to determine whether there has been end-organ damage, which would classify a patient as having a symptomatic disease that would require initiation of treatment. These features include serum calcium levels of greater than 1 mg/dL higher than the upper limit of normal levels (or serum calcium levels of >11 mg/dL), Renal insufficiency (creatinine clearance of <40 mL/min or serum creatinine levels of >2 mg/dL), Anemia (hemoglobin levels of >2 g/dL below the lower limit of normal levels or <10 g/dL), Bone lesions (presence of one or more bone lytic lesions detected by conventional radiology, computed tomography (CT) imaging or positron-emission tomography (PET)/CT, or osteoporosis with compression fractures), or other complications (hyperviscosity, amyloidosis, and light chain deposition diseases).
It is important to distinguish multiple myeloma from other related plasma cell dyscrasias such as MGUS, SMM, and solitary bone plasmacytoma (SBP) or extramedullary plasmacytoma (EMP). The diagnosis of MGUS relies on the presence of less than 10% clonal BMPC infiltration and serum monoclonal protein levels of less than 3 g/dL in a patient who has no evidence of end-organ/tissue damage, including anemia, hypercalcemia, renal failure, or lytic bone lesions attributable to myeloma and no evidence of a B-cell proliferative disorder. The diagnosis of SMM requires the presence of 10% to 60% clonal bone marrow plasma cell infiltration and/or serum monoclonal protein levels of greater than or equal to 3 g/dL, plus the absence of MDEs or amyloidosis. SBP or EMP is diagnosed when a single lesion of biopsy-proven clonal plasma cells is detected without evidence of another disease, including negative MRI of the spine and pelvis (except for the primary solitary lesion), and absence of end-organ damage that can be attributed to myeloma with no or minimal (<10% clonal plasma cells in the bone marrow) bone marrow plasmacytosis; it is particularly important to identify these patients, because they have about a 10% progression rate to multiple myeloma within 3 years, and around 35% to 65% of these patients may be curable when given radiation therapy with a curative intent.
<10% clonal marrow plasma cells
And serum monoclonal protein level of less than 3.0 g/dL
Without hypercalcemia, renal failure, anemia, bone lesions (CRAB) features or any of the complications (attributable to myeloma) listed below
Single lesion of biopsy-proven clonal plasma cells without other evidence of myeloma, including negative magnetic resonance imaging (MRI) of the spine and pelvis, and negative or minimal bone marrow plasmacytosis (clonal plasma cells <10%)
Between 10% and 60% clonal bone marrow plasma cell infiltration
And/or serum monoclonal protein level of 3 g/dL or greater
Absence of myeloma-defining events (MDEs) or amyloidosis
Clonal bone marrow plasma cell percentage of 10% (or more) or biopsy-proven bony or extramedullary plasmacytoma
And the presence of one or more of the MDEs listed
CRAB features
A clonal bone marrow plasma cell percentage of 60% or more
An involved:uninvolved serum free light chain ratio of 100 or more
Two or more focal lesions on MRI
Elevated serum calcium
Renal insufficiency
Bone lesions
Serum hyperviscosity
Amyloidosis
More than two bacterial infections in 12 months
Numerous factors have been suggested to determine the prognosis of patients with myeloma. In the 1970s, Durie and Salmon proposed a staging system based on the degree of anemia, level of paraprotein, presence or absence of hypercalcemia, bone lytic lesions, and renal insufficiency that subsequently became the standard staging system for myeloma for several decades ( Table 29.1 ). Although this system was predictive of the degree of myeloma tumor mass, several components were imprecise, such as the extent of bone lesions and the inclusion of factors such as anemia, hypercalcemia, and renal function, which may be affected by factors other than myeloma infiltration. Subsequently, multiple new prognostic factors emerged, including plasma cell hypodiploidy, C-reactive protein, lactate dehydrogenase, labeling index, cytogenetic abnormalities, and β-2 microglobulin (β2M).
| STAGE | CRITERIA | MYELOMA CELL MASS (× 10 12 /M 2 ) | |
|---|---|---|---|
| I |
|
<0.6 (low) | |
| II | Not fitting stage I or III | 0.6–1.2 (intermediate) | |
| III |
|
>1.2 (high) | |
| SUBCLASSIFICATION | CRITERION | ||
| A | Normal renal function (serum creatinine level <2.0 mg/dL) | ||
| B | Abnormal renal function (serum creatinine level ≥2.0 mg/dL) | ||
In 2005, Greipp and coworkers proposed the International Staging System (ISS) that separated patients into three stages based on the level of serum albumin and β 2 M. The data from 10,750 patients were analyzed by univariate and multivariate analysis; the system was designed using half of the data set and validated in the other half of the population ( Table 29.2 ). This system effectively identified a group of patients with a relatively short median survival of 29 months (β 2 M ≥5.5 mg/L, stage III), another with longer median survival of 62 months (β 2 M <3.5 mg/L and albumin >3.5 g/dL, stage I), and a group with intermediate survival (stage II). Although some patients in the data set may have received thalidomide, the impact on survival of novel agents including bortezomib, lenalidomide, and multiple combinations utilizing both of these drugs will likely lead to improved median survivals for one or more stages defined by the ISS. The Greek Myeloma Study Group also confirmed the validity of the ISS in the era of novel agents. The ISS has subsequently become a standard staging system for patients with myeloma.
| STAGE | CRITERIA | SURVIVAL (MONTHS) |
|---|---|---|
| I |
|
62 |
| II |
|
44 |
| III | β 2 M ≥5.5 mg/L | 29 |
Although the Durie–Salmon staging system and ISS effectively separate patients into different survival categories, the impact of chromosomal abnormalities (CAs) on prognosis is significant, and some have even suggested a molecular classification of myeloma. Certain translocations involving chromosome 14 (t(4;14), t(14;16)) and deletion of chromosome 13 have previously predicted a poor prognosis, but treatment with novel agents such as bortezomib appears to partially ameliorate the prognostic impact of these abnormalities; the same data with lenalidomide are premature and need to be validated over time. Deletion of chromosome 17p remains prognostic even in the era of novel agents; other considerations include the poor prognosis of chromosome 1 abnormalities and the possibility of an improved prognosis with t(11;14).
In 2015, Palumbo et al. developed the Revised International Staging System by incorporating CAs and serum lactate dehydrogenase (LDH) levels into the ISS to predict the prognosis and overall survival (OS) in patients with newly diagnosed multiple myeloma. They pooled data from 11 international trials involving 4,445 patients. CAs, detected by interphase fluorescence in situ hybridization after CD138 plasma cell purification, classified patients into two groups: a high-risk group characterized by the presence of high-risk CAs, including deletion of chromosome 17 (17p), translocation t(4;14), and translocation t(14;16), and a standard-risk group characterized by the absence of high-risk CAs. Serum LDH levels were classified as high or normal: high LDH was defined as serum LDH levels above the upper limit of the normal range, whereas normal LDH was defined as serum LDH levels lower than the upper limit of the normal range. Later, they assessed the prognosis and 5-year OS rates in each group ( Table 29.3 ).
| STAGE | CRITERIA | FREQUENCY (% OF PATIENTS) | 5-YEAR SURVIVAL RATE (%) |
|---|---|---|---|
| I |
|
28 | 82 |
| II | Not R-ISS stage I or III | 62 | 62 |
| III |
|
10 | 40 |
Imaging strategies are somewhat different for MGUS, plasmacytoma, and multiple myeloma. MGUS is a benign condition with a small (1%/year) chance of progression to multiple myeloma or occasionally to other malignancies. Imaging in this condition is limited to evaluation of any specific patient complaint that could be attributed to myeloma and to staging to exclude myeloma.
When a patient is suspected of having an isolated plasmacytoma, imaging initially serves two roles. The first is similar to its role in any focal solid tumor—to define the anatomy of the tumor and its relationship to other structures to assist with local treatment planning, which will usually be radiation therapy with a curative intent. The second is to determine whether the seemingly isolated plasmacytoma is the only site of disease, or whether it is merely the tip of the iceberg in a patient who should properly be classified as having multiple myeloma. To that end, one may weigh and choose between the various forms of systemic imaging, to be discussed later. If no other tumor is found elsewhere, and the patient remains classified as having solitary plasmacytoma, then follow-up imaging also serves two purposes. The first is again similar to its role in other solid tumors—to evaluate the site of the primary disease for evidence of healing or, alternatively, recurrence or progression ( Fig. 29.1 ). The second is surveillance for the development of multiple myeloma, again by the use of systemic imaging along with other criteria.
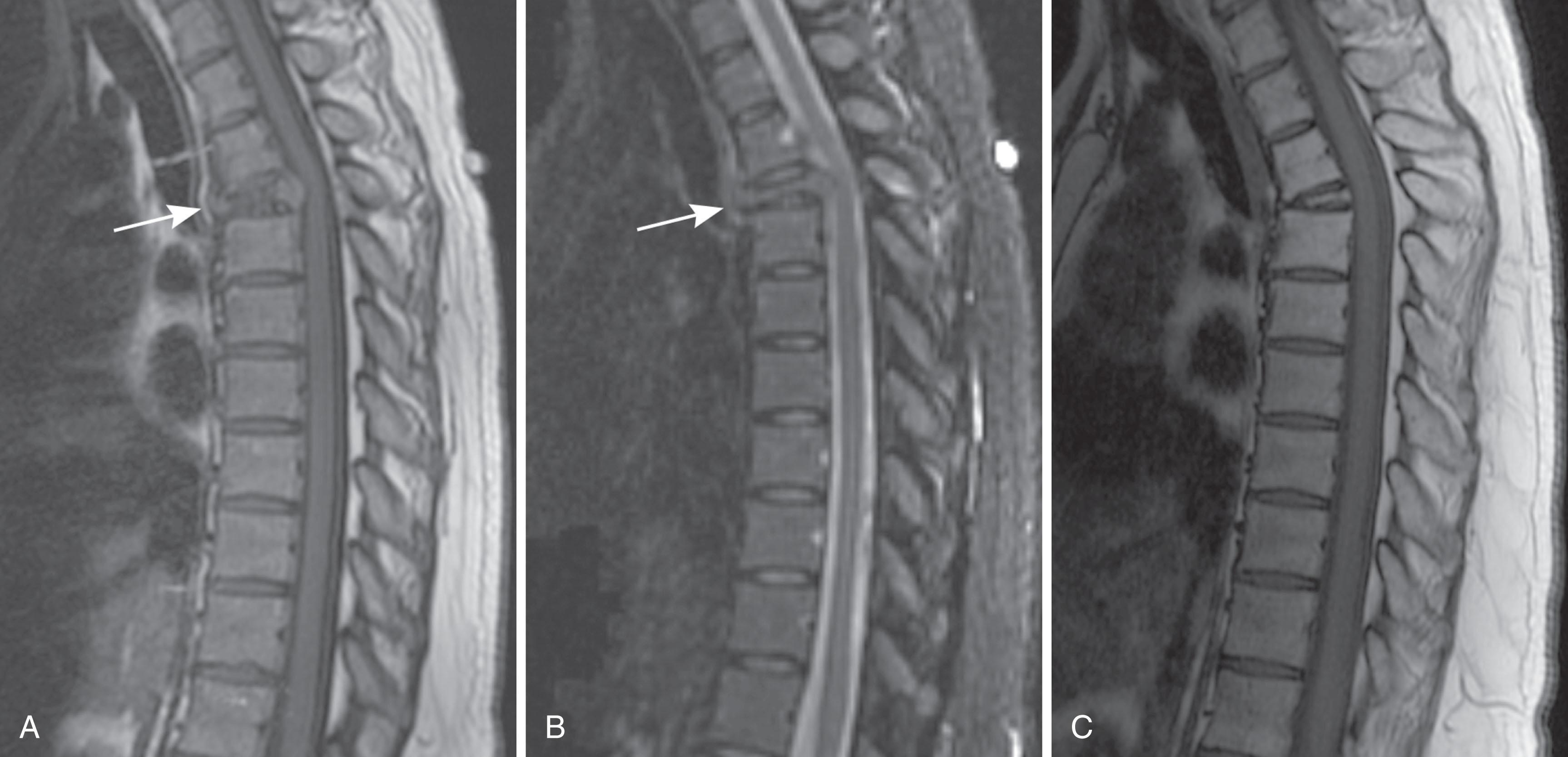
For patients with multiple myeloma, imaging helps at the initial evaluation to distinguish those with smoldering or asymptomatic myeloma, who have no visible bone disease, from those with symptomatic myeloma. Once the patient has been categorized into the symptomatic or smoldering group, imaging is used to assess stability versus progression of the disease.
Identify and characterize focal bone lesions: number, location, and size when practical. (For multiple lesions, it is often impractical to discuss each separately.)
Clearly indicate when a bone lesion is thought to be attributed to disease other than myeloma, such as a geode because of degenerative joint disease.
Discuss specific lesions with a significantly elevated risk of pathological fracture.
Assess subjective bone mineralization—normal, decreased absolutely, decreased allowing for age and gender.
Look for and discuss compression fractures in the spine.
Follow-up examinations: indicate whether the disease is stable, progressive, or shows signs of healing; include resolution of 2-[ 18 F] fluoro-2-deoxy-D-glucose avidity at positron-emission tomography/computed tomography scans or decrease in contrast enhancement at magnetic resonance imaging.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here