Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the last 70 years, neuromuscular blocking drugs have become an established part of anaesthetic practice. They were first administered during abdominal surgery in 1942, when Griffith and Johnson in Montreal used Intocostrin, a biologically standardised mixture of the alkaloids of the Indian rubber plant chondrodendron tomentosum to facilitate muscle relaxation during cyclopropane anaesthesia. Previously, only inhalational agents had been used during general anaesthesia, making surgical access for some procedures difficult. To achieve significant muscle relaxation, it was necessary to deepen anaesthesia, which often had adverse cardiac and respiratory effects.
At first, neuromuscular blocking agents were used only occasionally, in small doses, as an adjuvant to aid in the management of a difficult case. A tracheal tube was not always used, the lungs were not ventilated artificially and residual block was not routinely reversed; all these approaches caused significant morbidity and mortality, as demonstrated in the retrospective study by . By 1946, however, it was appreciated that using drugs such as curare in larger doses allowed the depth of anaesthesia to be lightened, and it was suggested that incremental doses should also be used during prolonged surgery, rather than deepening anaesthesia – an entirely new concept at that time. The use of routine tracheal intubation and artificial ventilation then evolved.
In 1946, Gray and Halton in Liverpool reported their experience of using the pure alkaloid tubocurarine in more than 1000 patients receiving various anaesthetic agents. Over the following 6 years, they developed a concise description of the necessary ingredients of any anaesthetic technique; narcosis, analgesia and muscle relaxation were essential – the triad of balanced anaesthesia. A fourth ingredient, controlled apnoea, was added at a later stage to emphasise the need for fully controlled ventilation, reducing the amount of NMBA required.
This concept is the basis of the use of neuromuscular blocking agents in modern anaesthetic practice. In particular, it has allowed seriously ill patients undergoing complex surgery to be anaesthetised safely and to be cared for postoperatively in the intensive therapy unit.
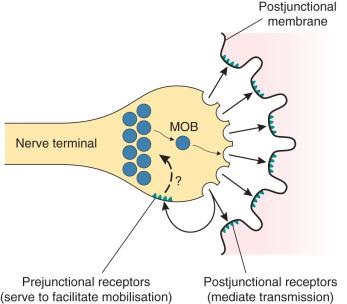
Acetylcholine , the neurotransmitter at the neuromuscular junction, is released from presynaptic nerve endings on passage of a nerve impulse (an action potential) down the axon to the nerve terminal. The neurotransmitter is synthesised from choline and acetylcoenzyme A by the enzyme choline acetyltransferase and stored in vesicles in the nerve terminal. The action potential depolarises the nerve terminal to release the neurotransmitter; entry of Ca 2+ ions into the nerve terminal is a necessary part of this process, promoting further acetylcholine release. On the arrival of an action potential, the storage vesicles are transferred to the active zones on the edge of the axonal membrane, where they fuse with the terminal wall to release the acetylcholine ( Fig. 8.1 ). Three proteins, synaptobrevin, syntaxin and synaptosome-associated protein SNAP-25, are involved in this process. These proteins along with vesicle membrane-associated synaptotagmins cause the docking, fusion and release (exocytosis) of acetylcholine from the vesicles. There are about 1000 active sites at each nerve ending, and any one nerve action potential leads to the release of 200–300 vesicles. In addition, small quanta of acetylcholine, equivalent to the contents of one vesicle, are released at the neuromuscular junction spontaneously, causing miniature end-plate potentials (MEPPs) on the postsynaptic membrane, but these are insufficient to generate a muscle action potential.

The active sites of release are aligned directly opposite the acetylcholine receptors on the junctional folds of the postsynaptic membrane, lying on the muscle surface. The junctional cleft, the gap between the nerve terminal and the muscle membrane, has a width of only 60 nm. It contains the enzyme acetylcholinesterase, which is responsible for the ultimate breakdown of acetylcholine. This enzyme is also present in higher concentrations in the junctional folds in the postsynaptic membrane (see Fig. 8.1 ). The choline produced by the breakdown of acetylcholine is taken up across the nerve membrane to be reused in the synthesis of the transmitter.
Several differing nicotinic acetylcholine receptors are located at the neuromuscular junction. These are classified initially as either muscle-type or neuronal acetylcholine receptors, with further subclassification on the basis of the subunits that form each receptor ( Table 8.1 ). The muscle-type nicotinic acetylcholine receptors on the postsynaptic membrane are organised in discrete clusters on the shoulders of the junctional folds (see Fig. 8.1 ). Each cluster is about 100 nm in diameter and contains a few hundred receptors. Each receptor consists of five subunits, two of which, the α (molecular weight (MW) = 40,000 Da), are identical. The other three slightly larger subunits are the β, δ and ε. In fetal muscle, the ε is replaced by a γ subunit. Each subunit of the receptor is a glycosated protein coded by a different gene. The receptors are arranged as a cylinder which spans the membrane, with a central, normally closed, channel – the ionophore ( Fig. 8.2 ). Each of the α subunits carries a single acetylcholine binding region on its extracellular surface. They also bind neuromuscular blocking drugs.
| Name | Location | Type | Role in neuromuscular transmission |
|---|---|---|---|
| α 3 β 2 | Presynaptic | Neuronal | Involved in regulation of acetylcholine release, especially during higher rates of stimulation. Inhibition by non-depolarising NMBAs results in fade of the TOF response. Very low affinity for suxamethonium. |
| α 2 βδε | Postsynaptic | Muscle | Main site of action for NMBAs. |
| α 2 βδγ | Postsynaptic | Muscle | Present in denervation states and other neuromuscular disorders. Slower onset of neuromuscular blockade and increased duration of paralysis with NMBAs. |
| α 7 | Postsynaptic | Neuronal | Expressed mainly in denervation states and other neuromuscular disorders. Possible role in inflammation regulation. |

Activation of the receptor requires both α sites to be occupied, producing a structural change in the receptor complex that opens the central channel running between the receptors for a very short period, about 1 ms (see Fig. 8.2 ). This allows movement of cations such as Na + , K + , Ca 2+ and Mg 2+ along their concentration gradients. The main change is influx of Na + ions, the end-plate current, followed by efflux of K + ions. The summation of this current through a large number of receptor channels lowers the transmembrane potential of the end-plate region sufficiently to depolarise it and generate a muscle action potential sufficient to allow muscle contraction.
At rest, the transmembrane potential is about −90 mV (inside negative). Under normal physiological conditions, a depolarisation of about 40 mV occurs, lowering the potential from −90 to −50 mV. When the end-plate potential reaches this critical threshold, it triggers an all-or-nothing action potential that passes around the sarcolemma to activate muscle contraction.
Each acetylcholine molecule is involved in opening one ion channel only before it is broken down rapidly by acetylcholinesterase; it does not interact with any of the other receptors. There is a large safety factor in the transmission process in respect of both the amount of acetylcholine released and the number of postsynaptic receptors. Much more acetylcholine is released than is necessary to trigger the action potential. The end-plate region is depolarised for only a very short period (a few milliseconds) before it rapidly repolarises and is ready to transmit another impulse.
Acetylcholine receptors are also present on the presynaptic area of the nerve terminal. These are of a slightly different structure to the postsynaptic nicotinic receptors (α 3 β 2 ; see Table 8.1 ). It is thought that a positive feedback mechanism exists for the further release of acetylcholine such that some of the released molecules of acetylcholine stimulate these presynaptic receptors, producing further mobilisation of the neurotransmitter to the readily releasable sites, ready for the arrival of the next nerve stimulus ( Fig. 8.3 ). Acetylcholine activates sodium channels on the prejunctional nerve membrane, which in turn activate voltage-dependent calcium channels (P-type fast channels) on the motor neuron, causing an influx of Ca 2+ into the nerve cytoplasm to promote further acetylcholine release.
The wave of depolarisation spreads from the postsynaptic membrane along the plasma membrane of the muscle fibres. Deep clefts in the membrane, called T-tubules, allow this wave to penetrate into close proximity with L-type calcium channel receptors, which in turn interact with ryanodine receptors in the sarcoplasmic reticulum to cause the release of Ca 2+ into the myoplasm. The Ca 2+ ions interact with troponin C to counter the inhibitory effect of troponin I and tropomyosin on the sarcomere complex, thus allowing thin actin myofilaments to slide over the thicker myosin microfilaments to generate muscle tension. As Ca 2+ is reabsorbed into the sarcoplasmic reticulum, the actin and myosin become less tightly bound and muscle tension reduces.
In health, postsynaptic acetylcholine receptors are restricted to the neuromuscular junction by a mechanism involving the presence of an active nerve terminal. In many disease states affecting the neuromuscular junction, this control is lost, and acetylcholine receptors of the fetal and α 7 types develop on the adjacent muscle surface. The excessive release of K + ions from diseased or swollen muscle on administration of suxamethonium is probably the result of stimulation of these extrajunctional receptors. They develop in many conditions, including polyneuropathies, severe burns and muscle disorders.
Neuromuscular blocking agents (NMBAs) used regularly by anaesthetists are classified into depolarising (or non-competitive ) and non-depolarising (or competitive ) agents. The individual neuromuscular blocking agents differ in the following aspects:
Potency (effective dose for tracheal intubation in 95% of patients: ED 95 )
Onset time
Duration of action
Metabolism
Mode of elimination
Those with a lower potency, such as rocuronium and suxamethonium, tend to have shorter onset times. The intubating dose recommended for routine use typically equates to twice the ED 95 for each NMBA. Increasing this dose to three or four times the ED 95 shortens the onset further, forming the basis for drug choice during rapid-sequence induction. At these doses the duration of action is markedly increased for the non-depolarising agents, and the therapeutic index also narrows, increasing the likelihood of adverse effects, especially those related to histamine release. Suxamethonium and rocuronium are the drugs of choice for rapid-sequence induction.
The duration of action of suxamethonium is mainly determined by its rapid metabolism in the plasma. In contrast, the elimination half-life of non-depolarising agents far exceeds their duration of clinical effects. Redistribution of these drugs away from the muscle accounts for most of the offset of action. The concept of the ideal neuromuscular blocking agent has been described based on its putative pharmacokinetic and pharmacodynamic properties; some such properties are presented in Box 8.1 .
Non-depolarising
Ultra-short onset of action (<1 min)
Short duration of action and suitable for infusion
Metabolised, independent of renal or hepatic function, to inactive compounds
Complete recovery from block in predictable and short time (with or without reversal agents)
Absence of antimuscarinic or other adverse effects
Does not cross blood–brain barrier or placenta
Stable compound at room temperature; supplied as a solution
Similar effect in all individuals
Inexpensive
The only depolarising NMBA now available in clinical practice is suxamethonium. Decamethonium was used clinically in the UK for many years, but it is now available only for research purposes.
This quaternary ammonium compound is comparable to two molecules of acetylcholine linked together ( Fig. 8.4 ). The two quaternary ammonium radicals, N + (CH 3 ) 3 , have the capacity to cling to each of the α units of the postsynaptic acetylcholine receptor, altering its structural conformation and opening the ion channel, but for a longer period than does a molecule of acetylcholine. Administration of suxamethonium therefore results in an initial depolarisation and muscle contraction, termed fasciculation. As this effect persists, however, further action potentials cannot pass down the ion channels, and the muscle becomes flaccid; repolarisation does not occur.
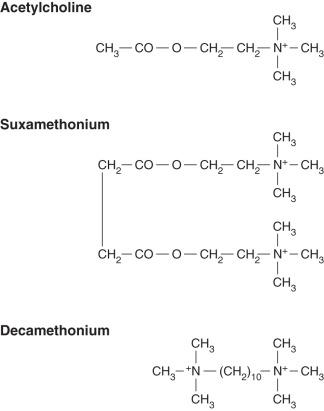
The dose of suxamethonium necessary for tracheal intubation in adults is 1.0–1.5 mg kg –1 . This dose has the most rapid and reliable onset of action of any of the NMBAs presently available, producing profound block within 1 min. Suxamethonium is therefore of particular benefit when it is essential to achieve tracheal intubation rapidly, such as in a patient with a full stomach or an obstetric patient.
The drug is metabolised predominantly in the plasma by the enzyme plasma cholinesterase, at one time termed pseudocholinesterase, at a very rapid rate. Recovery from neuromuscular block may start to occur within 3 min and is complete within 12–15 min. The use of an anticholinesterase such as neostigmine, which would inhibit such enzyme activity, is contraindicated (see later). About 10% of the drug is excreted in the urine; there is very little metabolism in the liver, although some breakdown by non-specific esterases occurs in the plasma.
If plasma cholinesterase is structurally abnormal because of inherited factors, or if its concentration is reduced by acquired factors, then the duration of action of the drug may be altered significantly.
The exact structure of plasma cholinesterase is determined genetically by autosomal genes, and this has been completely defined. Several abnormalities in the amino acid sequence of the normal enzyme, usually designated ![]() , are recognised. The most common is produced by the atypical gene,
, are recognised. The most common is produced by the atypical gene, ![]() , which occurs in about 4% of the Caucasian population. Thus a patient who is a heterozygote for the atypical gene
, which occurs in about 4% of the Caucasian population. Thus a patient who is a heterozygote for the atypical gene ![]() demonstrates a longer effect from a standard dose of suxamethonium (about 30 min). If the individual is a homozygote for the atypical gene
demonstrates a longer effect from a standard dose of suxamethonium (about 30 min). If the individual is a homozygote for the atypical gene ![]() , the duration of action of suxamethonium may exceed 2 h. Other, rarer, abnormalities in the structure of plasma cholinesterase are also recognised, such as the fluoride
, the duration of action of suxamethonium may exceed 2 h. Other, rarer, abnormalities in the structure of plasma cholinesterase are also recognised, such as the fluoride ![]() and silent
and silent ![]() genes. The latter has very little capacity to metabolise suxamethonium, and thus neuromuscular block in the homozygous state
genes. The latter has very little capacity to metabolise suxamethonium, and thus neuromuscular block in the homozygous state ![]() lasts for at least 3 h. In such patients, non-specific esterases gradually clear the drug from plasma.
lasts for at least 3 h. In such patients, non-specific esterases gradually clear the drug from plasma.
It has been suggested that a source of cholinesterase such as fresh frozen plasma should be administered in such cases, or an anticholinesterase such as neostigmine be used to reverse what has usually developed into a dual block (see later). However, it is wiser to:
keep the patient anaesthetised and the lungs ventilated artificially, and
monitor neuromuscular transmission accurately until full recovery from residual neuromuscular block.
This condition is not life-threatening, but the risk of awareness is considerable, especially after the end of surgery, when the anaesthetist, who may not yet have made the diagnosis, is attempting to waken the patient. Anaesthesia must be continued until full recovery from neuromuscular block is demonstrable.
As plasma cholinesterase activity is reduced by the presence of suxamethonium, a plasma sample to measure the patient's cholinesterase activity should not be taken for several days after prolonged block has been experienced, by which time new enzyme has been synthesised. A patient who is found to have reduced enzyme activity and structurally abnormal enzyme should be given a warning card or alarm bracelet, detailing his or her genetic status. Examining the plasma cholinesterase activity and genetic status of the patient's immediate relatives should be considered. Historically, testing for atypical plasma cholinesterases was done using the dibucaine number (see further reading ).
In these instances, the structure of plasma cholinesterase is normal but its activity is reduced. Thus neuromuscular block is prolonged by only minutes rather than hours. Causes of reduced plasma cholinesterase activity include the following:
Liver disease, because of reduced enzyme synthesis.
Carcinomatosis and starvation, also because of reduced enzyme synthesis.
Pregnancy, for two reasons: an increased circulating volume (dilutional effect) and decreased enzyme synthesis.
Anticholinesterases, including those used to reverse residual neuromuscular block after a non-depolarising NMBA (e.g. neostigmine); these drugs inhibit plasma cholinesterase in addition to acetylcholinesterase. The organophosphorus compound ecothiopate, once used topically as a miotic in ophthalmology, is also an anticholinesterase.
Other drugs which are metabolised by plasma cholinesterase and which therefore decrease its availability, including etomidate, ester local anaesthetics, anticancer drugs such as methotrexate, monoamine oxidase inhibitors and esmolol.
Hypothyroidism.
Cardiopulmonary bypass, plasmapheresis.
Renal disease.
Although suxamethonium is a very useful drug for achieving tracheal intubation rapidly, it has several undesirable effects which may limit its use.
Muscle pains occur especially in the patient who is ambulant soon after surgery, such as the day-case patient. The pains, thought possibly to be caused by the initial fasciculations, are more common in young, healthy patients with a large muscle mass. They occur in unusual sites, such as the diaphragm and between the scapulae, and are not relieved easily by conventional analgesics. The incidence and severity may be reduced by the use of a small dose of a non-depolarising NMBA given immediately before administration of suxamethonium (e.g. atracurium 0.05 mg kg –1 ). However, this technique, termed precurarisation or pretreatment, reduces the potency of suxamethonium, necessitating administration of a larger dose to produce the same effect. Between 1 and 3 min have been recommended between administering the non-depolarising NMBA and the subsequent suxamethonium, which can lead to a period of partial curarisation in an awake patient. Many other drugs have been used in an attempt to reduce the muscle pains, including lidocaine, calcium, magnesium and repeated doses of thiopental, but none is completely reliable.
Increased intraocular pressure is thought to be caused partly by the initial contraction of the external ocular muscles and contracture of the internal ocular muscles after administration of suxamethonium. It is not reduced by precurarisation. The effect lasts for as long as the neuromuscular block, and concern has been expressed that it may be sufficient to cause expulsion of the vitreal contents in the patient with an open eye injury. This is unlikely. Protection of the airway from gastric contents must take priority in the patient with a full stomach in addition to an eye injury, as inhalation of gastric contents may threaten life (see Chapter 38 ). It is also possible that suxamethonium may increase intracranial pressure, although this is less certain.
In the presence of a normal lower oesophageal sphincter, the increase in intragastric pressure produced by suxamethonium should be insufficient to produce regurgitation of gastric contents. However, in the patient with incompetence of this sphincter from, for example, hiatus hernia, regurgitation may occur.
It has long been recognised that administration of suxamethonium during halothane anaesthesia increases serum potassium concentration by 0.5 mmol l –1 . This is thought to be caused by muscle fasciculation. It is probable that the effect is less marked with the newer potent inhalational agents isoflurane, sevoflurane and desflurane. A similar increase occurs in patients with renal failure, but as these patients may already have an elevated serum potassium concentration, such an increase may precipitate cardiac irregularities and even cardiac arrest.
In some conditions in which the muscle cells are swollen or damaged, or in which there is proliferation of extrajunctional receptors (see Table 8.1 ), this release of potassium may be exaggerated. This is most marked in the burned patient, in whom K + concentrations up to 13 mmol l –1 have been reported. Suxamethonium should be avoided in this condition. In diseases of the muscle cell or its nerve supply, hyperkalaemia after suxamethonium may also be exaggerated. These include the muscular dystrophies, dystrophia myotonica and paraplegia. Hyperkalaemia has been reported to cause death in such patients. Suxamethonium may also precipitate prolonged contracture of the masseter muscles in patients with these disorders, making tracheal intubation impossible. The drug should be avoided in any patient with a neuromuscular disorder, including the patient with malignant hyperthermia, in whom the drug is a recognised trigger factor.
Hyperkalaemia after suxamethonium has also been reported, albeit rarely, in patients with widespread intra-abdominal infection, severe trauma and closed head injury.
Suxamethonium has muscarinic, in addition to nicotinic, effects, as does acetylcholine. The direct vagal effect (muscarinic) produces sinus bradycardia, especially in patients with high vagal tone, such as children and the physically fit. It is also more common in the patient who has not received an anticholinergic agent (such as glycopyrronium bromide) or who is given repeated increments of suxamethonium. It is advisable to use an anticholinergic routinely if it is planned to administer more than one dose of suxamethonium. Nodal or ventricular escape beats may develop in extreme circumstances.
Immunoglobulin E (IgE)–mediated allergic reactions to suxamethonium are rare but may occur, especially after repeated exposure to the drug. They are more common after suxamethonium than any other neuromuscular blocking agent. A blood test is available to measure plasma suxamethonium IgE concentrations to aid in the diagnosis of allergic anaphylaxis to this agent. No such test is available commercially for the other NMBAs.
If neuromuscular block is monitored (see later), several differences between depolarising and non-depolarising block may be defined. In the presence of a small dose of suxamethonium:
A decreased response to a single, low-voltage (1 Hz) twitch stimulus applied to a peripheral nerve is detected. Tetanic stimulation (e.g. at 50 Hz) produces a small but sustained response.
If four twitch stimuli are applied at 2 Hz over 2 s (train-of-four stimulus), followed by a 10-s interval before the next train-of-four, no decrease in the height of successive stimuli is noted ( Fig. 8.5 ).
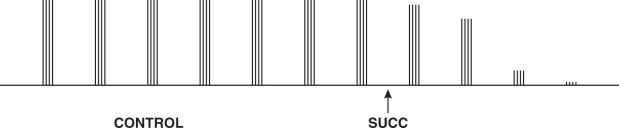
The application of a 5-s burst of tetanic stimulation after the application of single twitch stimuli, followed 3 s later by a further run of twitch stimuli, produces no potentiation of the twitch height; there is no post-tetanic potentiation (sometimes termed facilitation ) .
Neuromuscular block is potentiated by the administration of an anticholinesterase such as neostigmine.
If repeated doses of suxamethonium are given, the characteristics of this depolarising block alter; signs typical of a non-depolarising block develop (see later). Initially, such changes are demonstrable only at fast rates of stimulation, but with further increments of suxamethonium, they may occur at slower rates. This phenomenon is termed dual block or phase 2 block.
Muscle fasciculation is typical of a depolarising block.
Unlike suxamethonium, these drugs do not alter the structural conformity of the postsynaptic acetylcholine receptor and therefore do not produce an initial contraction. Instead, they compete with the neurotransmitter at this site, binding reversibly to one or two of the α receptors whenever these are not occupied by acetylcholine. The end-plate potential produced in the presence of a non-depolarising agent is therefore smaller; it does not reach the threshold necessary to initiate a propagating action potential to activate the sarcolemma and produce an initial muscle contraction. More than 75% of the postsynaptic receptors have to be blocked in this way before there is failure of muscle contraction – a large safety factor. However, in large doses, non-depolarising NMBAs impair neuromuscular transmission sufficiently to produce profound neuromuscular block.
Metabolism of NMBAs does not occur at the neuromuscular junction. By the end of surgery, the end-plate concentration of the NMBA is decreasing as the drug diffuses down a concentration gradient back into the plasma, from which it is cleared. Thus more receptors are stimulated by the neurotransmitter, allowing recovery from block. An anticholinesterase given at this time increases the half-life of acetylcholine at the neuromuscular junction, facilitating recovery.
Non-depolarising neuromuscular blocking agents are highly ionised, water-soluble drugs which are distributed mainly in plasma and extracellular fluid. Thus they have a relatively small volume of distribution. They are of two main types of chemical structure: either benzylisoquinolinium compounds, such as atracurium, mivacurium, cisatracurium, tubocurarine and alcuronium; or aminosteroid compounds, such as rocuronium, vecuronium, pancuronium and pipecuronium. All these drugs possess at least one quaternary ammonium group, N + (CH 3 ) 3 , to bind to an α subunit on the postsynaptic receptor. Some benzylisoquinolinium compounds consist of quaternary ammonium groups joined by a thin chain of methyl groups. They are therefore more liable to breakdown in the plasma than are the aminosteroids. They are also more likely to release histamine.
Tubocurarine chloride is the only naturally occurring neuromuscular blocking agent. It is derived from the bark of the South American plant chondrodendron tomentosum, which has been used for centuries by South American Indians as an arrow poison. It was the first non-depolarising neuromuscular blocking agent to be used in humans. It has a marked propensity to produce histamine release and thus hypotension, with possibly a compensatory tachycardia. Historically, tubocurarine, alcuronium and gallamine have been used in clinical practice, but they are no longer available in the UK. Alcuronium chloride is a semisynthetic derivative of toxiferin, an alkaloid of calabash curare. Gallamine triethiodide is a trisquaternary amine. It was first used in France in 1948. The only recent use of gallamine in the UK was as a small pretreatment dose (10 mg) before suxamethonium.
Atracurium besilate, introduced into clinical practice in 1982, was developed by Stenlake at Strathclyde University. Quaternary ammonium compounds break down spontaneously at varying temperature and pH, a phenomenon recognised for more than 100 years and known as Hofmann degradation. Atracurium was developed in the search for such an agent which broke down at body temperature and pH. Hofmann degradation may be considered as a safety net in the sick patient with impaired liver or renal function as atracurium is still cleared. Some renal excretion occurs in the healthy patient (10%), as does ester hydrolysis in the plasma; probably only about 45% of the drug is eliminated by Hofmann degradation in the normal patient.
Atracurium (and vecuronium) was developed in an attempt to obtain a non-depolarising agent which had a more rapid onset, was shorter acting and had fewer cardiovascular effects than the older agents. Atracurium 0.5 mg kg –1 does not produce neuromuscular block as rapidly as suxamethonium; the onset time is 2.0–2.5 min ( Table 8.2 ). However, recovery occurs more rapidly from it than after use of the older non-depolarising agents, and atracurium may be reversed easily 20–25 min after administration of a dose of 2 × ED 95 (0.45 mg kg –1 ). The drug does not have any direct cardiovascular effect but may release histamine and may therefore produce a local wheal and flare around the injection site, especially if a small vein is used. This may be accompanied by a slight reduction in arterial pressure. Using 3 × ED 95 is associated with increased histamine release, and therefore rapid sequence with atracurium is not advised. It can produce anaphylaxis, but to a lesser degree than suxamethonium.
| 95% Twitch depression (s) | 20%–25% Recovery (min) | |
|---|---|---|
| Depolarising | ||
| Suxamethonium | 60 | 10 |
| Non-depolarising | ||
| Benzylisoquinoliniums | ||
| Atracurium | 110 | 43 |
| Cisatracurium | 150 | 45 |
| Mivacurium | 170 | 16 |
| Doxacurium | 250 | 83 |
| Aminosteroids | ||
| Rocuronium | 75 | 33 |
| Vecuronium | 180 | 33 |
| Pancuronium | 220 | 75 |
| Pipecuronium | 300 | 95 |
| No longer used | ||
| Tubocurarine | 220 | 80+ |
| Alcuronium | 420 | 70 |
| Gallamine | 300 | 80 |
| Rapacuronium | <75 | 15 |
A metabolite of Hofmann degradation, laudanosine, has epileptogenic properties, although fits have never been reported in humans. The plasma concentrations of laudanosine required to make animals convulse are much higher than those occurring during general anaesthesia, even if large doses of atracurium are given during a prolonged procedure, and there is little cause for concern about this metabolite in clinical practice. In patients with multiple organ failure, who may receive atracurium for several days in ICU, laudanosine concentrations are higher, but no reports of cerebral toxicity have occurred.
Cisatracurium is the most recently introduced benzylisoquinolinium neuromuscular blocking drug. It is of particular interest because it is an example of the development of a specific isomer of a drug to produce a clean substance with the desired clinical actions but with reduced side effects. Cisatracurium is the 1R- cis 1′R- cis isomer of atracurium and one of 10 isomers of the parent compound. It is three to four times more potent than atracurium (ED 95 = 0.05 mg kg –1 ) and has a slightly slower onset and longer duration of action. Its main advantage is that it does not release histamine and therefore is associated with greater cardiovascular stability. It undergoes even more Hofmann degradation than atracurium.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here