Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
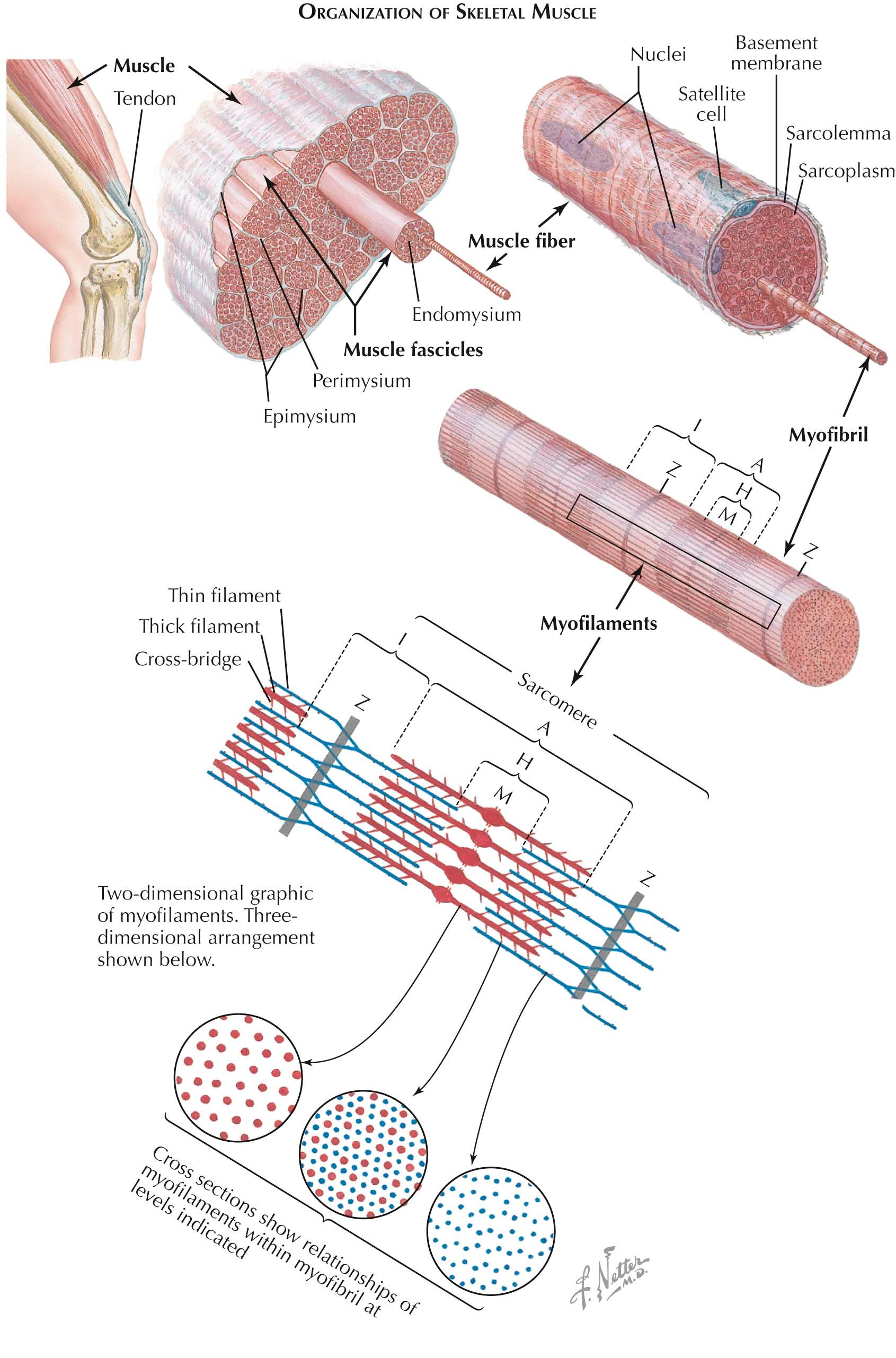
The basic function of skeletal muscle is to move various parts of the body via muscle contraction. Muscle structure is specifically related to its function. Muscles are composed of numerous multinucleated muscle cells called muscle fibers, or myofibers. Muscle fibers insert into tendons at their ends, at what on the microscopic level is referred to as the myotendinous junction. The tendons attach to bones at the origin and insertion points for each particular muscle. Whole muscles are encased in a connective tissue covering, the epimysium. Each muscle is subdivided into smaller bundles of muscle fibers called fascicles. The perimysium is the connective tissue within the muscle that separates one fascicle from another. Within a fascicle, individual muscle fibers are separated by yet another thin layer of connective tissue, the endomysium.
Each muscle fiber is also surrounded by a basal lamina, or basement membrane. Lying under the basement membrane are specialized cells referred to as satellite cells. Satellite cells are derived from embryonic cells called myoblasts. They are likely important in muscle fiber regeneration and are thought to fuse with the muscle fiber during this process. The muscle fiber itself is surrounded by its cell membrane, the sarcolemma. It forms the membrane under which the multiple muscle fiber nuclei reside. Within the boundaries of the sarcolemma, the contractile myofibrils are contained. They are surrounded by the cytoplasm of the muscle fiber, called the sarcoplasm.
Just as a muscle contraction is dependent upon numerous muscle fiber contractions, muscle fiber contraction is dependent upon the action of the numerous muscle fiber subunits, the myofibrils that run longitudinally along the length of the muscle fiber. They are collections of thin and thick filaments—actin and myosin. The contractile unit of the muscle is the sarcomere. Each sarcomere is bound on either end by the Z disk, a proteinaceous structure that is oriented across the myofibril perpendicular to the filaments, and when the fibril is viewed longitudinally, it is apparent as the Z band. Z bands are seen with regular periodicity along the myofibril, defining the several sarcomeres lined up at their ends. The thin filaments, actin, anchor into the Z disk and do not extend along the length of the sarcomere but reside only at the ends.
In contrast, the thick filaments, myosin, are situated at the middle of the sarcomere at the area seen as the A band. A slight enlargement at the middle of the thick filaments, in the midline of the sarcomere, leads to the appearance of the M band, or M line. The thick filaments overlap the ends of the thin filaments not anchored into the Z disk. The thick filaments, however, also do not run the length of sarcomere.
Thus there are two additional areas seen within the sarcomere. On either side of the Z band, there are only thin filaments, and this region straddling the Z disk is the I band. Likewise, in the middle of the sarcomere at rest, there are only thick filaments, which appear as the H zone. Therefore, traveling from the Z disks to the midsarcomere, one sees the Z band, the I band, the A band, the H zone, and the M band.
When seen in cross section, the thick filaments are regularly dispersed throughout the myofibril. Hexagonally arranged around them are the thin filaments. Therefore each thin filament is equally near to three thick filaments. The thick filaments have outwardly oriented heads that are directed toward the thin filaments and that run along the length of the thick filaments, with the exception of the midline. At rest, the heads of the thick filaments are tilted slightly toward their nearest Z disk. During contraction, these thick filaments bind neighboring thin filaments, pulling these toward the sarcomeric midline. The alternating thick and thin filaments develop the contractile force as these thick filaments pull the thin filaments past it. Therefore, when a sarcomere contracts, the Z disks are drawn toward each other, shortening the sarcomere. When all the sarcomeres in a muscle shorten, the muscle contracts.
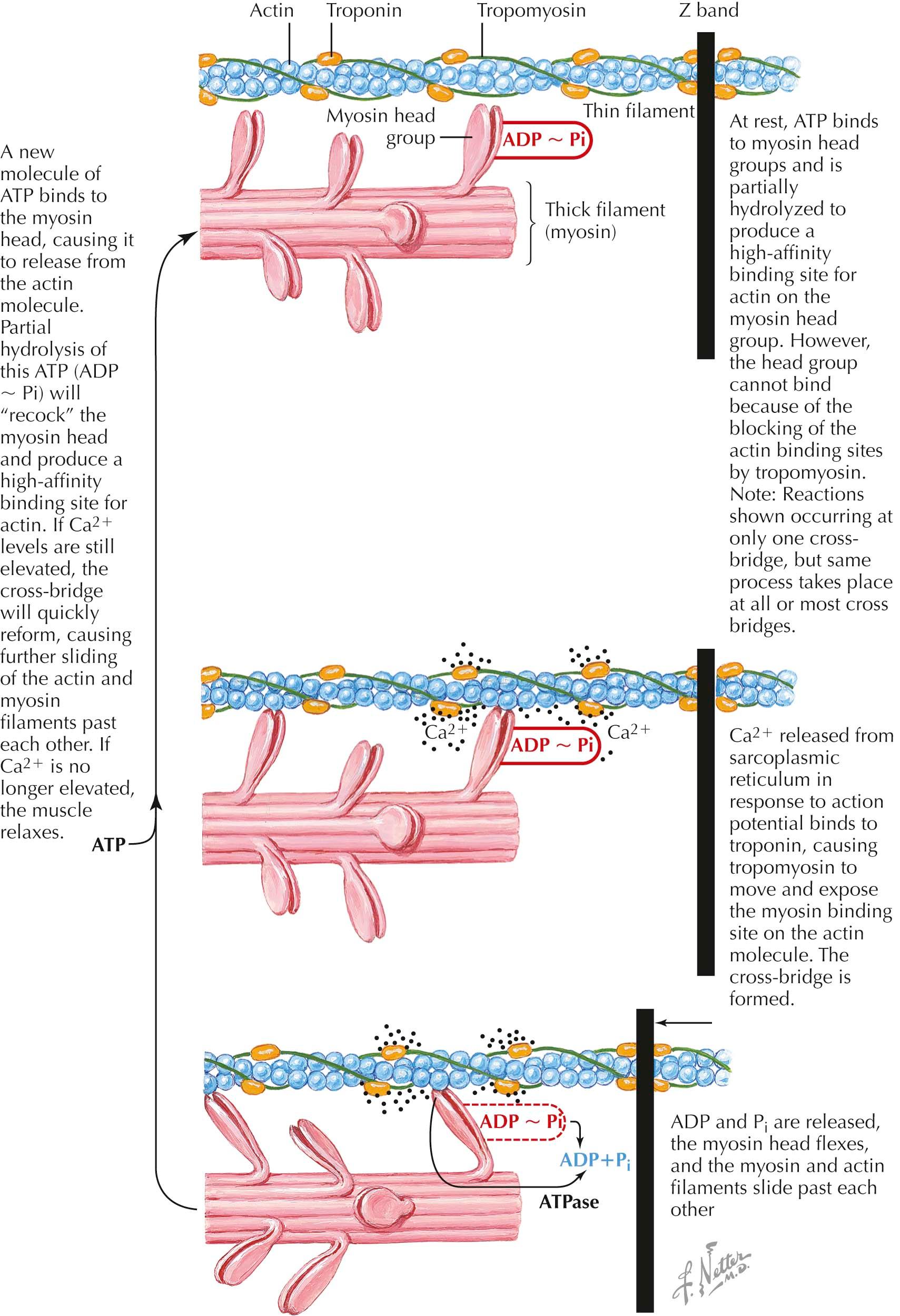
The sarcomere is the fundamental contractile element of skeletal muscle. Multiple sarcomeres align end-to-end along a muscle fiber, are defined by the Z disk at each end of the sarcomere, and give skeletal muscle its striated appearance. Thin filaments have a polymeric filamentous actin core and anchor to the Z disk. They are not continuous throughout the sarcomere, and only one end of each actin filament is associated with the Z disk. Multiple molecules of globular actin self-associate to form filamentous actin . Globular actin harbors a myosin binding site that is blocked at rest by tropomyosin molecules running the length of the thin filament.
Troponin molecules also run the course of the thin filament but occur as complexes bound at regular intervals. Three components comprise troponin: TnI, TnT, and TnC. TnI is an inhibitory molecule that binds actin itself and the other two troponin elements. TnT binds tropomyosin . TnC binds the calcium ion (Ca 2+ ) , which then leads to a conformational change, ultimately rotating tropomyosin off and unblocking the myosin binding site on actin. Thick filaments are also a polymer but comprising numerous myosin molecules. Myosin is a hexamer of two heavy chains and two pairs of light chains. The heavy chain tails also self-associate to form the backbone of the thick filament. The heads of the heavy chains form the myosin heads, which, after self-assembly, protrude in all directions from the thick filament backbone toward the thin filaments that surround the thick filament in a hexagonal arrangement. One segment of the myosin head binds actin and hydrolyzes adenosine triphosphate (ATP), ultimately leading to a conformational change and “power stroke,” thus flexing the myosin head and sliding the bound actin filament toward the middle of the sarcomere.
The cross-bridge cycle describes the steps by which myosin, actin, ATP, and Ca 2+ interact to lead to sarcomere shortening and muscle contraction on an elemental level. At rest, ATP is bound to the myosin head and is partially hydrolyzed to adenosine diphosphate (ADP) and phosphate (P i ), the myosin head is “cocked” and available to form a high-affinity bond with actin, but the myosin binding site on actin is obstructed by tropomyosin. After depolarization of the muscle membrane and generation of a muscle fiber action potential, large amounts of Ca 2+ are released from the sarcoplasmic reticulum. Ca 2+ binds TnC, causing tropomyosin to “unblock” the myosin binding site on actin. The myosin head then binds actin, forming the “cross-bridge.” ADP and P i are released from the myosin head. The myosin head flexes on its backbone (the “power stroke”), pulling the bound actin toward the middle of the sarcomere. ATP again binds to the myosin head, which dissociates from actin. ATP is hydrolyzed again to ADP and P i , the myosin head “recocks,” and the actin binding site on the myosin head is again produced. If Ca 2+ continues to be available, the sequence of events repeats, and actin is pulled further toward the middle of the sarcomere. When this happens multiple times over multiple “cross-bridges” between multiple myosin-actin molecules in multiple thick and thin filaments, the sarcomere shortens. As this occurs along several sarcomeres in the muscle fiber, the muscle fiber shortens. When multiple muscle fibers shorten within a muscle, the muscle contracts. If Ca 2+ is no longer available, however, myosin binding sites on actin become blocked by tropomyosin, myosin cannot bind actin, and the muscle relaxes.
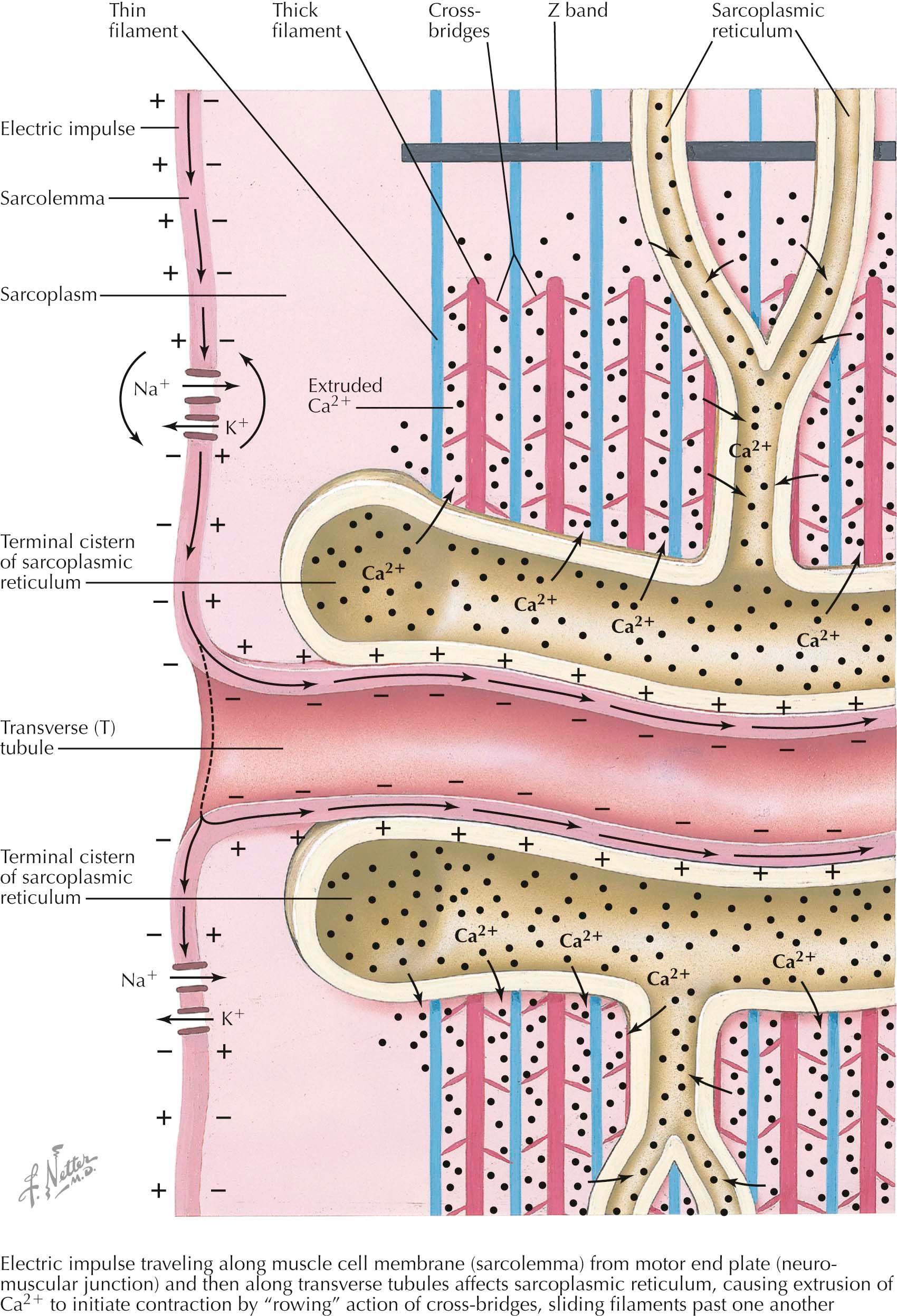
The muscle membrane system includes external ( sarcolemma, transverse (T) tubules ) and internal ( sarcoplasmic reticulum ) components. Although separate, the T tubules and sarcoplasmic reticulum are related in function. The membrane system is specially adapted to propagate the muscle membrane action potential and couple it to calcium ion (Ca 2+ ) release into the sarcoplasm, leading to excitation-contraction coupling. These components meet at junctional triads where a T tubule is flanked by two terminal cisternae of the sarcoplasmic reticulum. Triads recur at predictable intervals that mimic the cross striations of the sarcomeres. In mammals, the triads are situated at the junction of the A band and the I band. This periodicity and proximity to the myofibrils signifies the intricate role the triads have in control of muscle contraction. They are intimately involved in Ca 2+ control—sequestration, release, and reuptake.
T tubules are specialized invaginations of the sarcolemma, with which it is continuous at multiple points. T tubules run transverse to the muscle fibers themselves and the sarcolemma and encircle individual muscle fibrils. T tubules form deep invaginations into the sarcolemma. This provides not only more rapid dispersal of muscle membrane depolarization along the muscle fiber, but it allows for propagation along both the surface of the sarcolemma and into the muscle fiber, where it can interact with deep sarcoplasmic reticulum. T tubules interact directly with components of the sarcoplasmic reticulum, leading to Ca 2+ release into the sarcoplasm. They also harbor voltage-gated Ca 2+ channels, L-type Ca 2+ channels. These Ca 2+ channels do not contribute to depolarization or the muscle fiber action potential but, rather, act as voltage sensors of muscle membrane depolarization. The sarcoplasmic reticulum forms a network of tubules surrounding muscle fibrils inside the sarcolemma.
Junctional sarcoplasmic reticulum (the terminal cisternae ) stores Ca 2+ and is the site of Ca 2+ release in response to a muscle fiber action potential. Junctional sarcoplasmic reticulum contains high amounts of calsequestrin , which binds to Ca 2+ and accounts for the high Ca 2+ storage of the sarcoplasmic reticulum. The sarcoplasmic reticulum is so efficient in this role that the muscle fiber loses very little Ca 2+ even after repeated muscle contractions. Junctional sarcoplasmic reticulum is also the site of the Ca 2+ channel responsible for releasing large stores of calcium into the sarcoplasm, the ryanodine receptor , so named for its binding to the plant alkaloid ryanodine.
Sarcoplasmic reticulum voltage-gated Ca 2+ channels sense the depolarization of the T tubule, interact directly with the ryanodine receptor and induce it to release Ca 2+ from the sarcoplasmic reticulum. Free sarcoplasmic reticulum constitutes the remainder of the intrafiber sarcoplasmic reticulum membrane system. It is not associated with the T tubule system and functions in Ca 2+ reuptake into the sarcoplasmic reticulum. It has a large number of Ca 2+ adenosine triphosphatase (ATPase) pumps on its surface that function in the energy-dependent reuptake of sarcoplasmic Ca 2+ and therefore plays a role in muscle relaxation rather than excitation and contraction.
A motor nerve action potential propagates down the motor nerve axon to the nerve terminal, causing release of acetylcholine (ACh) into the synaptic cleft of the neuromuscular junction. After binding of acetylcholine to the postsynaptic muscle membrane acetylcholine receptor (AChR), there is generation of an end-plate potential . Suprathreshold end-plate potentials generate muscle fiber action potentials . These propagate along the sarcolemma, depolarizing the muscle membrane sequentially as it travels along the membrane. The action potential continues down the T tubules. Depolarization of the T tubule membrane activates the voltage-gated Ca 2+ channel, thus activating the ryanodine receptor, releasing large amounts of Ca 2+ from the sarcoplasmic reticulum into the sarcoplasm. This leads to cross-bridge formation between thin and thick filaments, activation of the cross-bridge cycle, sliding of the thick and thin filaments past one another, sarcomere shortening , and eventually muscle contraction . Energy-dependent Ca 2+ reuptake into the sarcoplasmic reticulum assists in relaxation and replenishes the supply of Ca 2+ ready for release after the next depolarization.
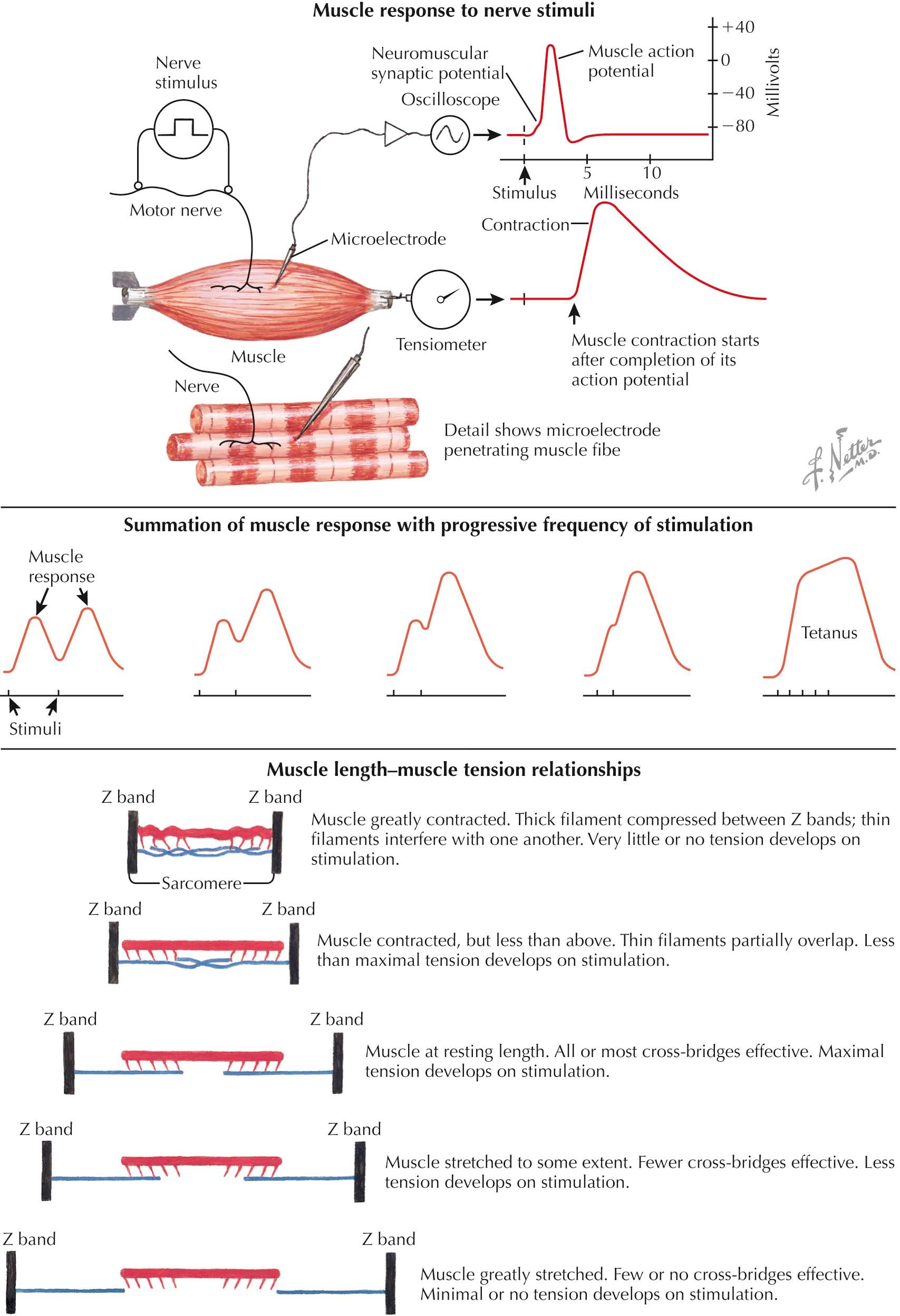
Stimulation applied to a motor nerve produces a motor nerve action potential. This propagates down the motor nerve axon to the nerve terminal. Acetylcholine is released from the nerve terminal, binds the acetylcholine receptor on the postsynaptic sarcolemma, and leads to an end-plate potential. The end-plate potential is an excitatory postsynaptic potential that, in normal conditions, reaches the threshold to generate a muscle fiber action potential that ultimately causes a muscle twitch, or contraction. These potentials are measurable by an intramuscular microelectrode that records the excitatory postsynaptic end-plate potential as a small initial deflection immediately preceding the larger muscle fiber action potential. The small delay from the initial stimulus to the initial deflection represents the time it takes to conduct the action potential down the motor nerve axon, release acetylcholine, bind acetylcholine to the postsynaptic membrane receptor, and generate the end-plate potential. In addition, the muscle fiber action potential continues along the sarcolemma, down the transverse tubules, leading to massive release of Ca 2+ from the sarcoplasmic reticulum into the sarcoplasm, initiating the cross-bridge cycle, and eliciting a muscle twitch. This is measureable by a tensiometer during an isometric contraction (with the muscle held at a constant length so it cannot shorten). The time taken for muscle fiber action potential propagation, calcium release, and initiation of the cross-bridge cycle explains the latent period between the muscle fiber action potential and the measureable muscle contraction.
Measurement of the effects of stimulation frequency is possible under similar conditions of isometric contraction. Given that there is all-or-nothing activation and contraction of fibers within a motor unit after stimulation of a single motor neuron, single stimuli produce identical twitch responses with identical tension created. If a second stimulus is given after full relaxation of the muscle, this second twitch intensity will be the same as the first. If, however, the second stimulus is given before full relaxation (i.e., while the muscle is still at least partially contracted) the tension created by the second stimulus will surpass that created by the first. As the frequency of stimulation is increased, this phenomenon, called summation , is enhanced. At high enough stimulation frequencies, muscle fibers are unable to relax between stimuli, and they produce a prolonged single-peaked contraction, known as tetanus. This is one mechanism by which muscles generate varying degrees of force.
Isometric contractions also allow for the measurement of the effect of sarcomere length on the development of force generation, that is, the muscle length-tension relationship. Maximum tensile force results when the greatest number of cross-bridges forms. This occurs when the muscle is at its resting length and all or most of the cross-bridges are available (i.e., there is maximum overlap of myosin heads with actin), and stimulation will produce maximum tension. If the sarcomere is shortened, actin filaments begin to overlap or repulse each other and interfere with cross-bridge formation. As a result, there fewer cross-bridges form, resulting in less tension. Conversely, when the sarcomere is stretched, actin filaments are pulled laterally and myosin heads at the middle of the sarcomere no longer overlap with actin. Therefore fewer cross-bridges can form, also resulting in less tension. At the extreme, there is no actin-myosin overlap, and no tension develops after stimulation.
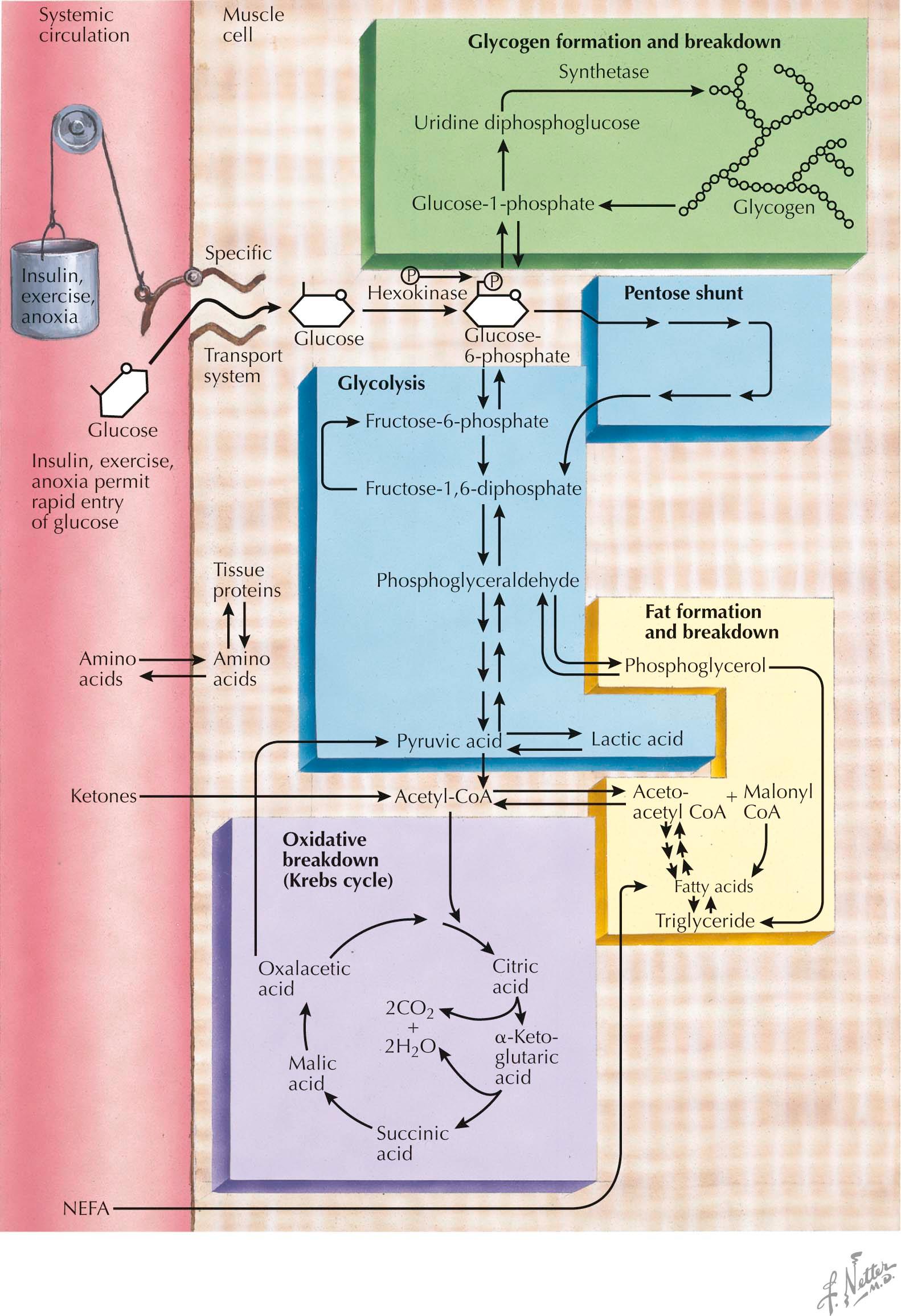
Muscle fiber contraction and relaxation is an energy-dependent process. The source of this energy is adenosine triphosphate (ATP). The muscle fiber requires an ongoing supply of ATP to maintain its function. Most ATP is generated in the muscle via utilization of local glycogen stores, free glucose, and free fatty acids.
Blood glucose enters the muscle fiber through a specialized muscle-specific glucose transporter. It is then phosphorylated to glucose-6-phosphate and undergoes glycolysis . Glucose-6-phosphate is also derived from degradative phosphorylation of muscle glycogen stores. Phosphorylase b kinase activates myophosphorylase that initiates glycogen breakdown , and a debranching enzyme completes the process by which glucose-1-phosphate is produced. This is also converted to glucose-6-phosphate and enters glycolysis.
The rate-limiting step in glycolysis is the conversion of fructose-1-phosphate to fructose-1,6-diphosphate by phosphofructokinase. Ultimately, one molecule of glucose is broken down into two molecules of pyruvate, and three molecules of ATP are generated. Under anaerobic conditions , pyruvate is converted to lactate. Under aerobic conditions, pyruvate is instead converted to acetyl-coenzyme A (acetyl-CoA) and enters the tricarboxylic acid (TCA), or Krebs, cycle. Within the mitochondria, this cycle generates carbon dioxide and water, as well as the reduced forms of nicotinamide and flavin adenine dinucleotide (NADH and FADH 2 , respectively) and guanosine triphosphate (GTP) (which can transfer phosphate to ADP to produce ATP). NADH and FADH 2 then undergo oxidative phosphorylation in the inner mitochondrial membrane, generating additional ATP molecules. Therefore aerobic glycogen and glucose metabolism produces much more ATP than anaerobic glycolysis.
Nonesterified fatty acids ( NEFAs ) enter the muscle fiber from the bloodstream, derived from circulating very-low-density lipoproteins and triglycerides stored in adipocytes. Free fatty acids are first activated to their acyl-coenzyme A (acyl-CoA) thioesters. Short- and medium-chain fatty acyl-CoAs can cross the mitochondrial membranes, where they undergo beta oxidation . Long-chain fatty acyl-CoAs cannot undergo beta oxidation and require esterification with carnitine by carnitine palmitoyltransferase I ( CPT I ). The resultant palmitoylcarnitine is then transferred across the inner mitochondrial membrane and converted back into the long-chain fatty acyl-CoA by CPT II . The long-chain fatty acyl-CoA derivative then can enter the beta-oxidation pathway . Beta oxidation occurs via fatty acid chain length–specific enzymes, producing acetyl-CoA that can then enter the Krebs cycle.
Energy utilization in muscle is activity dependent; that is, the specific energy source is dependent upon the level and intensity of activity, type of activity, duration of activity, conditioning, and diet. At rest, the predominant energy source is fatty acids , particularly long-chain fatty acids. During low level, low-intensity exercise, the muscle primarily utilizes glucose and fatty acids. With increasing intensity, glucose utilization is increased, and muscle glycogen becomes a principle energy source. With maximal, isometric exercise, anaerobic glycolysis is the primary source. Also, during long-duration low-level exercise, lipid metabolism becomes the main source of energy.
A classic example of a defect of carbohydrate metabolism is McArdle syndrome, or myophosphorylase deficiency . Here the deficiency in activity of myophosphorylase leads to the inability to initiate glycogen breakdown . This leads to accumulation of glycogen in the muscle. Because there is no ability to utilize glycogen as an energy source, there is a reduction in the production of pyruvate. As a result, less acetyl-CoA is available to go through the Krebs cycle, and less NADH and FADH 2 undergo oxidative phosphorylation. Patients develop exercise intolerance with myalgia, fatigability, and exertional weakness, and potentially rhabdomyolysis . This is more common with isometric or sustained moderate-intensity exercise . Patients typically experience a “second wind,” whereby a brief rest or reduction in activity leads to improved exercise tolerance. This correlates to increased availability of blood glucose and free fatty acids.
The most common disorder of lipid metabolism is CPT II deficiency . CPT II deficiency causes an inability to convert long-chain palmitoylcarnitines back into long-chain fatty acyl-CoAs on the inner mitochondrial membrane. Therefore the long-chain fatty acyl-CoAs cannot enter the beta-oxidation pathway, and cannot produce acetyl-CoA to enter the Krebs cycle. Patients develop exercise intolerance and exertional rhabdomyolysis after prolonged exercise, rather than brief, intense exercise, and experience no “second-wind” phenomenon.
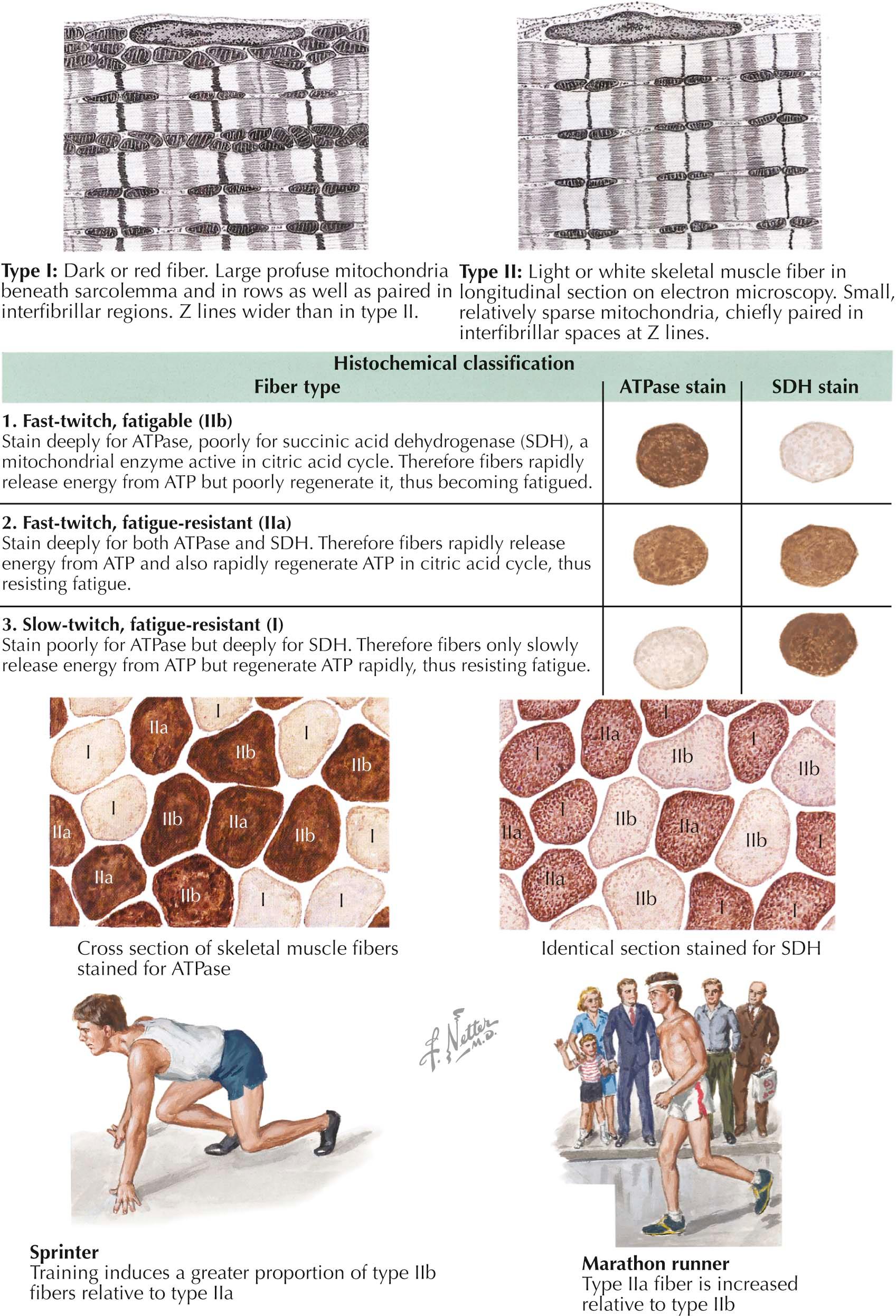
Muscle fiber types differ regarding twitch speed, fatigability, and preferential use of glycolytic versus oxidative pathways for energy production. They also differ in muscle color, being white or red. Fiber typing involves differentiating between type I and type II fibers. In addition, type II fibers are further subdivided into type IIa and type IIb fibers.
Type I fibers are adept at utilizing the aerobic oxidative pathway for long periods of time. As shown by electron microscopy, they contain large numbers of mitochondria , which confer a red color to the muscle itself. They react darkly for oxidative enzymes, such as reduced nicotinamide adenosine dinucleotide (NADH) dehydrogenase, succinate dehydrogenase, and cytochrome c oxidase. They have low glycolytic activity, and react weakly for the glycolytic enzyme myophosphorylase. Accordingly, type I fibers have high lipid but low glycogen content. The myofibrillar adenosine triphosphatase (ATPase) in type I fibers is acid stable and alkaline labile; thus they react darkly for ATPase activity after acid preincubation but do not react for ATPase activity after alkaline preincubation. These are slow-twitch fibers, generating low maximum tension. They are, however, fatigue resistant and can maintain activation for longer periods of time. Due to this quality, they are referred to as slow-twitch, fatigue-resistant fibers. They are relatively small compared with type II fibers. Type I fibers activate before type II fibers at low levels of muscle contraction, and their motor unit potentials are those that electromyography assesses .
Type IIa fibers are fast-twitch fibers with both glycolytic and oxidative metabolic activity. They also contain a large number of mitochondria and react somewhat darkly for oxidative enzymes. Unlike Type I fibers, however, they have high glycogen content and stain darkly for glycolytic enzymes. Type IIa myofibrillar ATPase is acid labile and alkaline stable; thus these fibers stain darkly for ATPase activity after alkaline preincubation but not after acid preincubation. As fast-twitch fibers, they are able to generate an intermediate level of maximum tension quickly, but they also fatigue slowly. They are therefore referred to as fast-twitch, fatigue-resistant fibers. They are larger than type I fibers and activate later as sustained contractions are required.
Type IIb fibers lie on the opposite end of the spectrum from type I fibers. They harbor few mitochondria and have a white appearance. As a result, they react faintly for oxidative enzymes. They have high glycolytic activity, however, and react darkly for glycolytic enzymes. They have high glycogen and low lipid content. Their myofibrillar ATPase is acid labile and alkaline stable, similar to type IIa fibers, but they show intermediate reactivity after preincubation at more moderate acidity levels (pH 4.6), unlike type IIa fibers, which have no reactivity under similar conditions. These are fast-twitch fibers but fatigue easily. They are accordingly referred to as fast-twitch, fatigable fibers. They reach high maximal force very quickly but only for short periods of time.
Muscle fiber–type predominance is reflective of certain types of activity typical for a particular muscle. Short bursts of anaerobic activity (such as sprinting) are especially suited to type IIb fast-twitch, fatigable fibers. Longer, aerobic exercise, however, is more suited to type IIa and type I fatigue-resistant fibers. There is evidence to suggest that consistent activity mimicking motor nerve activity of neurons that innervate fatigue-resistant fibers can slow contraction and increase fatigue resistance, suggesting that muscle fiber types can change in response to activity type.
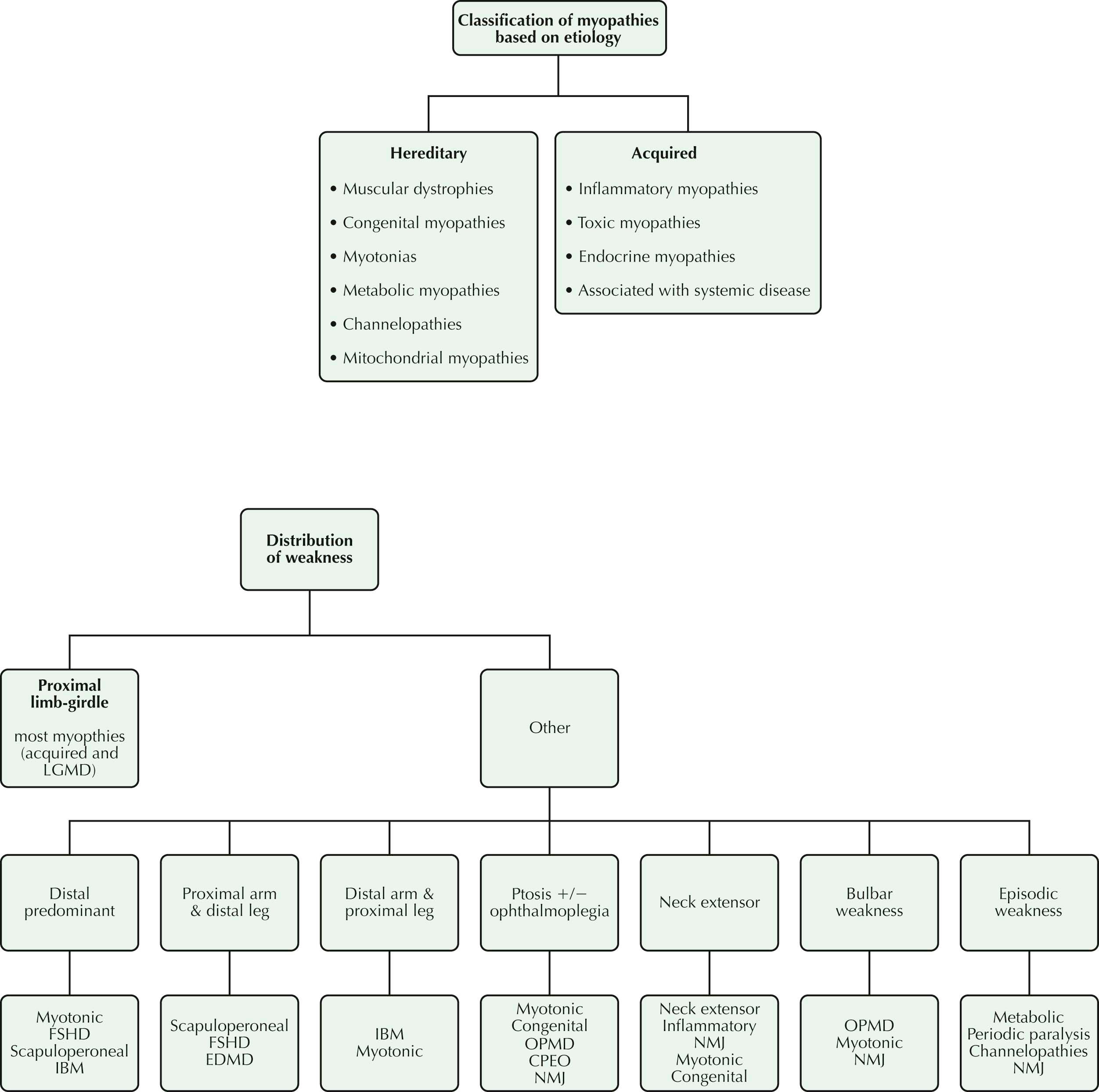
In approaching a patient with weakness, the first task of the clinician is “lesion localization”: to determine by comprehensive clinical evaluation which one of the four organs of the motor unit—motor neuron, peripheral nerve, neuromuscular junction (NMJ), and muscle—is the site of the “lesion.” Once localized to the muscle, a thorough history often helps clinicians determine whether the disorder of muscle (the myopathy) is inherited or acquired. By characterizing the distribution of muscle weakness, the physical examination may then provide additional diagnostic clues that help to identify correctly specific muscle disorders. Finally, the results of laboratory studies help to confirm and refine the diagnosis.
The history provides clinical clues. In most myopathies, weakness is proximal, affecting movements of the hips, thighs, shoulders, and upper arms. Typically, these patients have difficulty climbing stairs, arising from low chairs, and carrying out tasks above their head, such as placing a book on a high shelf or brushing their hair. Less commonly, weakness is distal (affecting movements of the feet/toes and hands/fingers), manifested by tripping over uneven terrain because of footdrop or difficulty opening jars. Much less commonly, weakness affects the cranial muscles so that patients report ocular symptoms (lid drooping, or ptosis), difficulty swallowing (dysphagia), effortful speech (dysarthria), and sometimes inability to keep the neck straight up, leading to a head-drop.
Associated “negative” symptoms of muscle disease include fatigue, exercise intolerance, and muscle atrophy. “Positive” symptoms often point in the direction of a specific myopathy. For example, myalgias tend to be associated with a toxic or infectious myopathy, with certain forms of inflammatory myopathy (associated with connective tissue disease) and with some myotonic myopathies. Cramps suggest metabolic or endocrine myopathies; stiffness or impaired relaxation of muscle points to the possibility of myotonia, as seen in the myotonic disorders; episodic tea-colored urine or myoglobinuria is strongly suggestive of a metabolic myopathy; and complaints of muscle enlargement, deemed muscle hypertrophy by the clinician, is often associated with muscular dystrophy, notably the dystrophinopathies.
Determining onset, duration, and evolution of muscle disease enhances the effectiveness of the diagnostic process. For example, weakness presenting at birth suggests some forms of congenital myopathy and infantile myotonic dystrophy; weakness emerging in childhood is typical of most types of muscular dystrophy, congenital myopathy, metabolic and mitochondrial myopathy, and rarely, inflammatory myopathy; and later-onset weakness, coming on in adulthood, is often seen in inflammatory, toxic and endocrine myopathies, and less commonly, is a first manifestation of muscular dystrophy and metabolic and mitochondrial myopathies.
In the vast majority of myopathies, weakness, once established, is fairly stable or constant over the course of a 24-hour period and is relatively uninfluenced by physiologic state (for example, active or resting, or fasting or postprandial). There are certain muscle diseases, however, such as the periodic paralyses and the metabolic myopathies, that are characterized by episodic weakness of varying intervals and intensity, and with or without concomitant metabolic derangements such as myoglobinuria. Disorders of neuromuscular transmission, particularly myasthenia gravis, have a diurnal variation that is worse in the evening.
The rate of the progression of weakness over time offers a clue to the character of the myopathy. Acute to subacute progression (that is, weakness evolving over weeks to several months) is typically seen in inflammatory myopathies (polymyositis/dermatomyositis). Chronic progression (with weakness developing over many months to years) is typical of most muscular dystrophies and the inclusion body myositis (IBM) form of inflammatory myopathy. Nonprogressive or mildly progressive weakness over the course of decades is characteristic of most forms of congenital myopathy, which explains why, in some rare instances of mild congenital myopathy, diagnosis is not established until adulthood.
Obtaining a detailed family history in any patient with a suspected myopathy is crucial to unlocking an underlying inherited disorder and identifying its pattern of inheritance. Patients are asked to reflect on certain details of their relatives' medical history, such as overall strength, functional capacity, ability to walk and run, whether there was a need for assistive devices or orthoses to walk, need for a wheelchair or scooter, history of cardiac disease, whether the patient's (i.e., proband's) symptoms were shared by males and females or were gender specific. Armed with such information, the clinician may have a heightened index of suspicion for an inherited muscle disease. This places the physician in the position to hypothesize the pattern of inheritance (autosomal dominant, autosomal recessive, X-linked, or mitochondrial) and thereby improve the ability to provide genetic counseling.
Clues to the diagnostic process are often found in exploring the patient's medication list, exercise experience, dietary preferences, and motor function in a cool or cold environment. For example, subacute myopathies in adulthood may have a toxic etiology, caused by a cholesterol-lowering agent (statin), colchicine use for gout, or chronic alcohol use. Corticosteroids prescribed for a wide spectrum of medical disorders may be responsible for a subacute or chronic myopathy. In susceptible individuals with a glycolytic pathway defect, short bursts of intense activity sometimes lead to muscle cramping, weakness, and myoglobinuria. In contrast, patients having lipid oxidation disorders generally require longer periods of low-intensity exercise to predispose to muscle weakness and myoglobinuria. In individuals with a genetic predisposition to periodic paralysis, a high-carbohydrate meal is sometimes the trigger for an attack of severe muscle weakness. Muscle stiffness worsening with cold exposure is typical of paramyotonia congenita.
The physical examination provides additional important clues that help in the diagnostic process. Some myopathies affect t issues other than skeletal muscle per se, because they are multisystem disorders, such as myotonic dystrophy. In this instance, extramuscular involvement may be truly multiorgan and multisystem, including cataracts, arrhythmia, cognitive impairment, and glucose intolerance. In others, concomitant cardiac muscle skeletal muscle involvement occurs as in the dystrophinopathies, Emery-Dreifuss muscular dystrophy (EDMD), and polymyositis/dermatomyositis, and there may be a serious concomitant cardiomyopathy, with a clinical course punctuated by arrhythmia and congestive heart failure. Although the diaphragm is a striated muscle, it is infrequently involved in muscle disease, with notable exceptions, including myotonic dystrophy type I, centronuclear myopathy, nemaline myopathy, and acid maltase deficiency.
Muscle thinning, or hypertrophy, especially enlarged calves, are important clinical signs that often suggest a dystrophinopathy or congenital myopathy. Dysmorphic features may be associated with congenital myopathies; nemaline myopathy is a good example, especially exhibiting an elongated facies, high-arched palate, and foreshortened toes. Skin changes, especially a rash over the face and hands, are typical of dermatomyositis. Musculoskeletal contractures indicate long-standing, usually inherited myopathies: Emery-Dreifuss muscular dystrophy and Bethlem myopathy. Myopathies presenting within the context of pronounced multiorgan involvement suggest sarcoidosis, amyloidosis, endocrinopathies, connective tissue, infectious disorders, and mitochondrial cytopathies.
Distribution of muscle weakness provides a clue to the diagnostic process. In most myopathic disorders, the proximal and limb-girdle muscles bear the brunt of involvement; but there are important exceptions. Distal muscle involvement is quite characteristic of classic myotonic dystrophy type I and dysferlinopathy . Ocular weakness is often an early manifestation of mitochondrial myopathy and oculopharyngeal muscular dystrophy (OPMD).
Laboratory tests often provide diagnostic confirmation in the clinical context of a patient with suspected myopathy. These tests include serum creatine kinase (CK) levels, which are elevated in myopathic disorders marked by muscle fiber necrosis and normal in muscle disorders with little injury to the muscle fiber membrane. Electrodiagnostic studies will show early recruitment of short-duration, low-amplitude motor unit potentials in weak muscles, irrespective of the cause of the myopathy. However, fibrillation potentials and positive sharp waves primarily occur in aggressive myopathies, including inflammatory, toxic, or dystrophic types. These potentials are less commonly seen in most congenital and endocrine myopathies.
Muscle biopsy analysis by light microscopy usually provides information that helps corroborate the classification into inherited and acquired myopathy and often provides further diagnostic specificity (for example, acquired myopathy that is inflammatory with features of dermatomyositis). Immunohistochemical analysis of frozen muscle tissue sections identifies specific muscle proteins when muscular dystrophy is suspected. In cases where muscular dystrophy is suspected but cannot be confirmed by immunohistochemical studies, molecular genetic testing by deoxyribonucleic acid (DNA) analysis of leukocytes can sometimes confirm the diagnosis of a muscular dystrophy by identifying a specific known mutation. In selected cases, when metabolic myopathy is suspected, biochemical analysis of frozen muscle tissue for analysis of the glycolytic, oxidative, or mitochondrial metabolic pathways can be performed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here