Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although all cells are capable of some sort of movement, the dominant function of several cell types is to generate force through contraction . In these specialised contractile cells, movement is generated by interaction of the proteins actin and myosin (contractile proteins). Certain forms of contractile cell function as single-cell contractile units:
Myoepithelial cells are an important component of certain secretory glands (see Ch. 5 ), where they function to expel secretions from glandular acini.
Pericytes are smooth muscle-like cells that surround blood vessels (see Ch. 8 ).
Myofibroblasts are cells that have a contractile role in addition to being able to secrete collagen. This type of cell is generally inconspicuous in normal tissues but becomes important following tissue damage during the process of healing and repair, leading to formation of a scar.
Other forms of contractile cells function by forming multicellular contractile units termed muscles . Such muscle cells can be divided into three types:
Skeletal muscle is responsible for the movement of the skeleton as well as organs such as the globe of the eye and the tongue. Skeletal muscle is often referred to as voluntary muscle since it is capable of voluntary (conscious) control. The arrangement of the contractile proteins gives rise to the appearance of prominent cross-striations in some histological preparations and so the name striated muscle is often applied to skeletal muscle. The highly developed functions of the cytoplasmic organelles of muscle cells has led to the use of a special terminology for some muscle cell components: plasma membrane or plasmalemma = sarcolemma ; cytoplasm = sarcoplasm ; endoplasmic reticulum = sarcoplasmic reticulum .
Smooth muscle is so named because, unlike other forms of muscle, the arrangement of contractile proteins does not give the histological appearance of cross-striations. This type of muscle forms the muscular component of visceral structures such as blood vessels, the gastrointestinal tract, the uterus and the urinary bladder, giving rise to the alternative name of visceral muscle . Since smooth muscle is under inherent autonomic and hormonal control, it is also described as involuntary muscle .
Cardiac muscle has many structural and functional characteristics intermediate between those of skeletal and smooth muscle and provides for the continuous rhythmic contractility of the heart (see Ch. 8 ). Although striated in appearance, cardiac muscle is readily distinguishable from skeletal muscle and should not be referred to by the term ‘striated muscle’.
Muscle cells of all three types are surrounded by an external lamina (see Ch. 4 ). In all muscle cell types, contractile forces developed from the internal contractile proteins are transmitted to the external lamina via link proteins which span the muscle cell membrane. The external lamina binds individual muscle cells into a single functional mass.
Skeletal muscles have a wide variety of morphological forms and modes of action; nevertheless all have the same basic structure. Skeletal muscle is composed of extremely elongated, multinucleate contractile cells, often described as muscle fibres , bound together by collagenous supporting tissue. Individual muscle fibres vary considerably in diameter from 10 to 100 μm and may extend throughout the whole length of a muscle and, in some sites, may be many centimeters in length.
Skeletal muscle contraction is controlled by large motor nerves, individual nerve fibres branching within the muscle to supply a group of muscle fibres, collectively described as a motor unit . Excitation of any one motor nerve results in simultaneous contraction of all the muscle fibres of the corresponding motor unit. The structure of neuromuscular junctions is described in Fig. 7.12 . The vitality of skeletal muscle fibres is dependent on maintenance of their nerve supply which, if damaged, results in atrophy of the fibres (see below). Skeletal muscle contains highly specialised stretch receptors known as neuromuscular spindles which are shown in Fig. 7.23 .
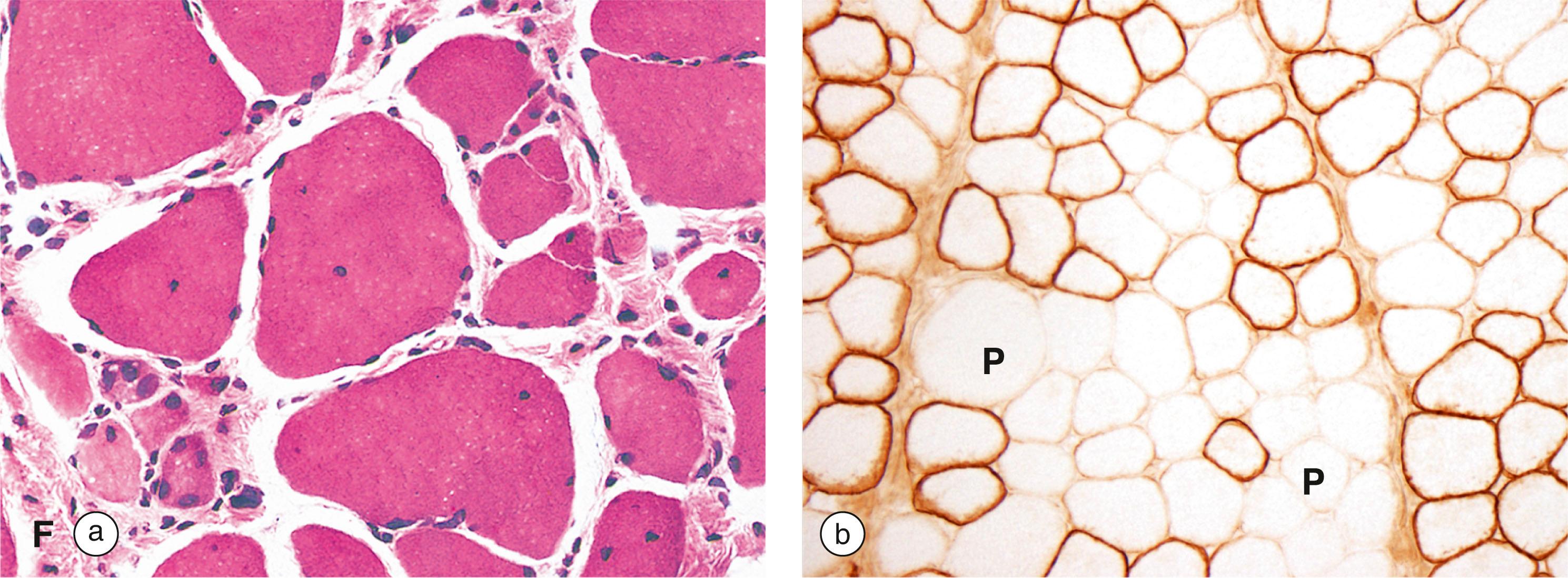
Atrophy is a decrease in the size of an organ or tissue, usually due to disease or changes in functional requirements . When the nerve supply of skeletal muscle is damaged, the individual muscle fibres innervated by that nerve become smaller and thinner, giving the impression of ‘wasting away’ of the affected muscle.
A more common example of this process of atrophy is seen when muscles are not used for a period of time (e.g. when a limb is immobilised in plaster to allow healing of a broken bone). The muscles in that area become visibly smaller and, when the plaster is removed, muscle strength is reduced. So long as the nerves supplying the muscle remain intact, exercise can reverse this process and function of the broken limb can be regained.
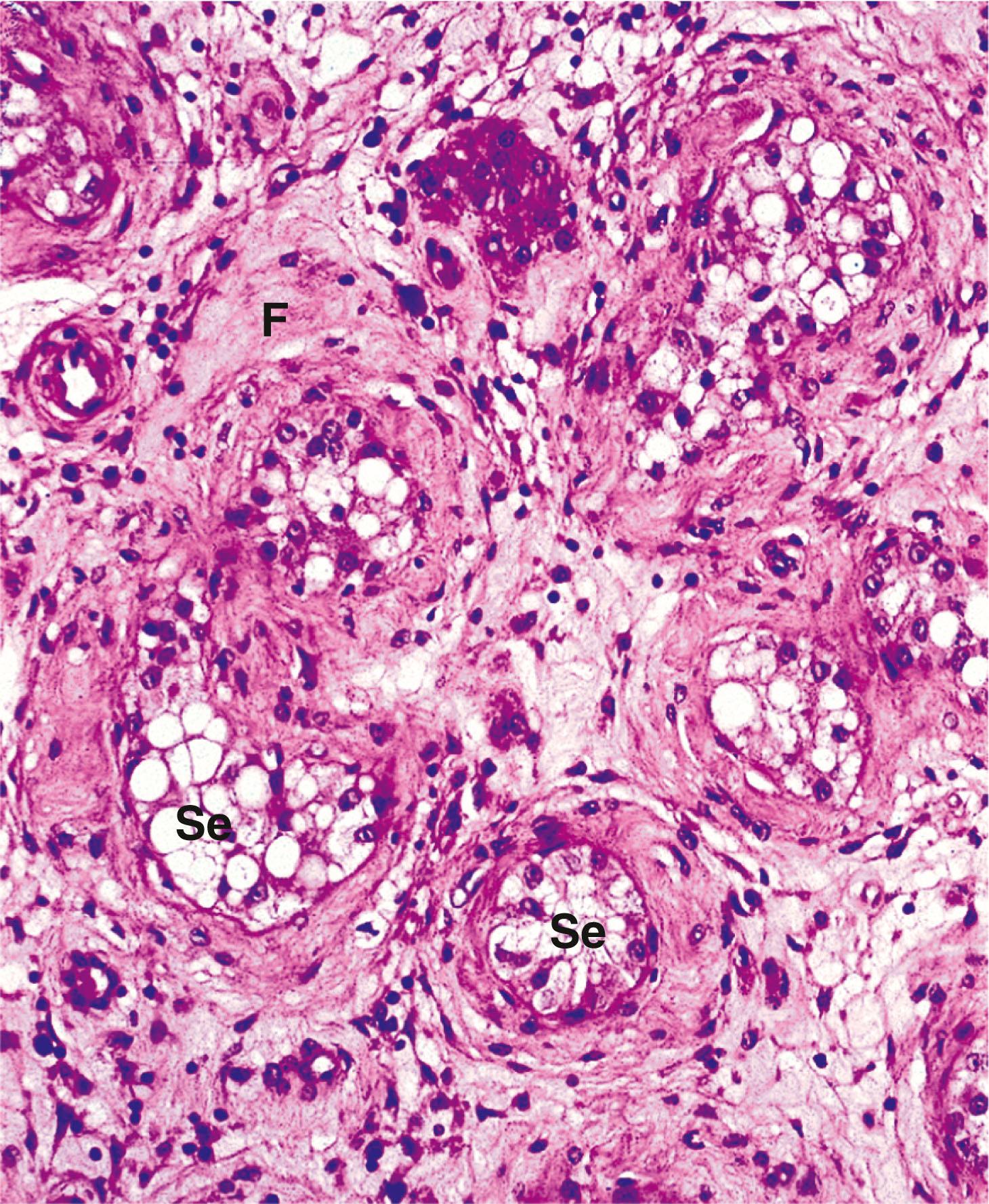
The opposite process occurs when muscles are exercised over long periods. Muscle bulk in athletes is typically increased. This reflects an increase in the size of individual muscle fibres due to synthesis of more contractile proteins within each cell. The process is known as hypertrophy . There is no increase in the number of muscle fibres, as these cells do not normally divide. In contrast, increased bulk of smooth muscle can occur due to a combination of hypertrophy (increase in cell size) and hyperplasia ( an increase in the number of cells), since smooth muscle cells retain the ability to divide.
B blood vessel C capillary E epimysium En endomysium F fascicle FE fetal erythrocytes N nucleus P perimysium
A A band C capillary G glycogen granules H H band I I band M M line Mt mitochondrion My myofibril N nucleus S smooth endoplasmic reticulum Z Z line
Contraction of skeletal muscle is under voluntary control and is initiated via action potentials in alpha motor neurones. Action potentials are transmitted across the neuromuscular junction via release of acetylcholine which binds to nicotinic receptors , resulting in depolarisation of the muscle fibre. Ca 2+ forms the critical link between the excitation of the muscle fibre and the production of force through the cross-bridge cycle. In the resting state, the cross-bridge binding sites on the actin molecules are masked by another filamentous protein called tropomyosin which is bound to the troponin complex.
When Ca 2+ binds to troponin, this causes a change in the configuration of tropomyosin, unmasking the active sites on the actin filaments and allowing the cross-bridge cycle to commence. When Ca 2+ levels fall once more due to reuptake of ions into the sarcoplasmic reticulum, the tropomyosin once more blocks the binding sites on the actin molecules and contraction ceases. Extending the boat analogy used earlier, the myosin boat can only row through the sea of actin molecules whilst the layer of tropomyosin ice remains thawed.
Myasthenia gravis occurs due to failure of neuromuscular transmission. Skeletal muscle contraction is entirely dependent upon transmission of the action potential across the neuromuscular junction (see Ch. 7 ). The action potential in the alpha motor neurone triggers release of acetylcholine into the synaptic cleft at the muscle end plate. Normally, acetylcholine then binds to post-synaptic nicotinic receptors to initiate depolarisation of the muscle fibre. In myasthenia gravis, these post-synaptic receptors are damaged by an autoantibody , an immunoglobulin erroneously produced by the patient’s immune system that targets the patient’s own tissue. As a result, nerve impulses are not transmitted effectively to the muscle, and the patient experiences weakness and rapid muscle fatigue upon voluntary movement.
A A band I I band M mitochondrion SR sarcoplasmic reticulum T T tubule system TC terminal cisternae Td tubular triad
Ar artery C capillary ICS intercellular space L external lamina M mitochondrion SR sarcoplasmic reticulum T T tubule system
In muscular dystrophy, there is weakness and wasting of muscles due to a defect in one of the proteins involved in muscle function. Gene mutations affecting proteins of the membrane linkage complex are an important cause of the group of muscular dystrophies. Other genes coding for contractile and other structural proteins may also cause muscular dystrophy.
Some people have a mutation in the gene for dystrophin that results in inefficient linking of contractile forces to the support tissues in muscle . The muscle does not function correctly and fibres undergo progressive damage with repeated contraction, ultimately leading to death of muscle cells.
Skeletal muscle is specialised for relatively forceful contractions of short duration under voluntary control. In contrast, smooth muscle is specialised for continuous contractions of relatively low force, producing diffuse movements through contraction of the whole muscle mass, rather than contraction of individual motor units. Contractility is an inherent property of smooth muscle, occurring independently of innervation, often in a rhythmic or wave-like fashion. Superimposed on this inherent contractility are the influences of the autonomic nervous system, hormones and local metabolites which modulate contractility to accommodate changing functional demands. For example, the smooth muscle of the intestinal wall undergoes continuous rhythmic contraction ( peristalsis ), propelling the luminal contents distally. This activity is enhanced by parasympathetic stimulation and influenced by a variety of hormones which are released in response to changes in the nature and volume of the gut contents. The structure of autonomic neuromuscular junctions is described in Ch. 7 .
The cells of smooth muscle are relatively small, with only a single nucleus. The fibres are bound together in irregular branching fasciculi, the arrangement varying considerably from one organ to another according to functional requirements.
A aerobic fibre An anaerobic fibre I intermediate fibre
Smooth muscle does not show the longitudinally organised system of contractile proteins that is seen in striated muscle, but has an arrangement where bundles of contractile proteins criss-cross the cell, being inserted into anchoring points ( focal densities ) within the cytoplasm, as well as anchoring to the cell membrane as focal adhesion densities . Tension generated by contraction is transmitted through anchoring densities in the cell membrane to the surrounding external lamina, thus allowing a mass of smooth muscle cells to function as one unit. The intermediate filaments of smooth muscle, desmin , are also inserted into the focal densities ( Fig. 6.20 ).
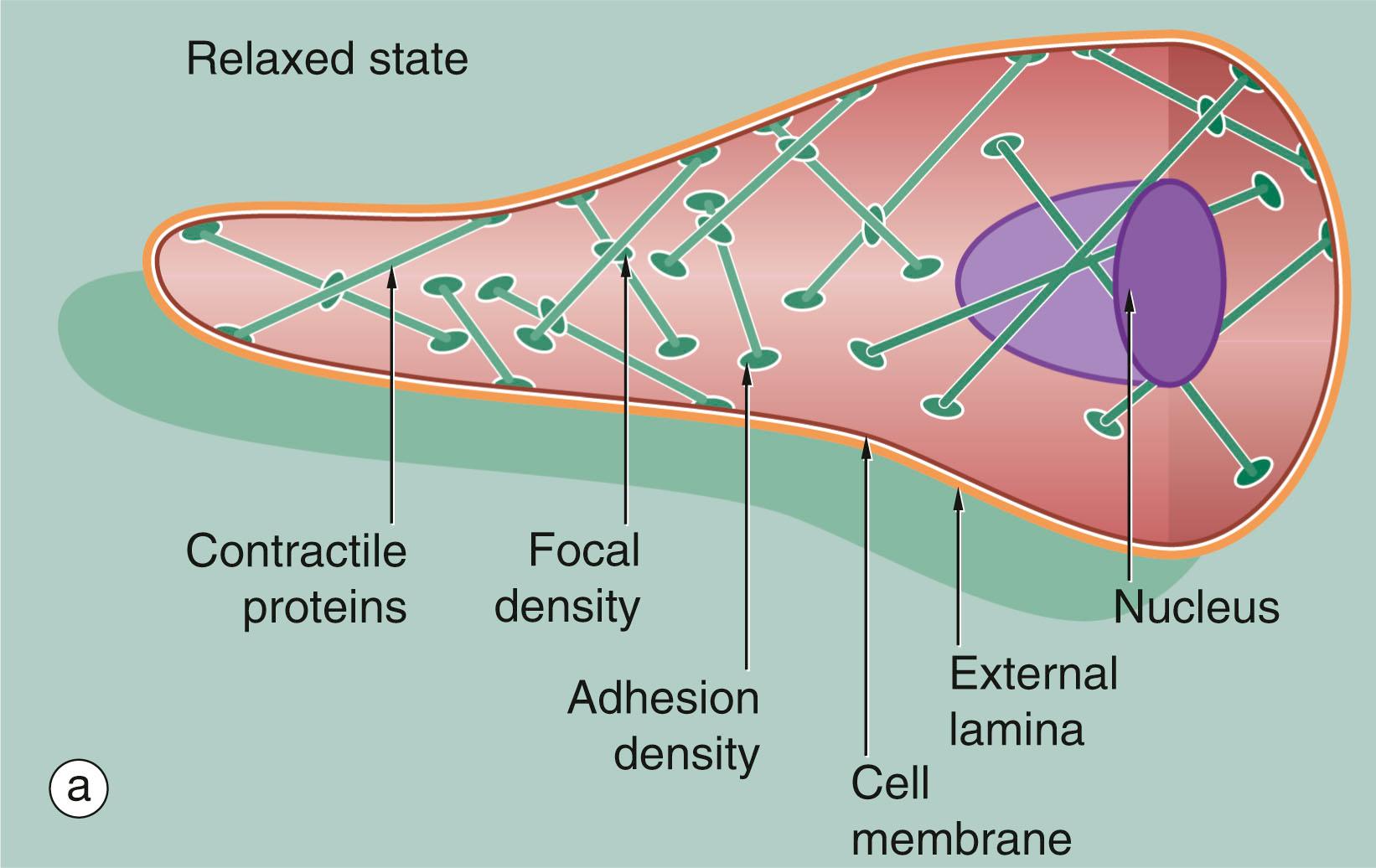
The contraction mechanism of smooth muscle differs from that for striated muscle. Because the contractile proteins are arranged in a criss-cross lattice inserted around the cell membrane, contraction results in shortening of the cell, which assumes a globular shape in contrast to its elongated shape in the relaxed state (see Fig. 6.20 ).
The mechanism of smooth muscle contraction is as follows:
Thin filaments of actin are associated with tropomyosin .
Thick filaments composed of myosin only bind to actin if one chain is phosphorylated.
Calcium ions in the cytosol of smooth muscle cells cause contraction, as in striated muscle, but the control of Ca 2+ ion movements is different. In relaxed smooth muscle, free Ca 2+ ions are normally sequestered in sarcoplasmic reticulum throughout the cell. On membrane excitation, free Ca 2+ ions are released into the cytoplasm and bind to a protein called calmodulin (a calcium-binding protein). The calcium–calmodulin complex then activates an enzyme called myosin light-chain kinase , which phosphorylates myosin and permits it to bind to actin. Actin and myosin subsequently interact by filament sliding to produce contraction in a similar way to that for skeletal muscle.
Contraction of smooth muscle can be modulated by surface receptors activating internal second messenger systems. Expression of different receptors allows smooth muscle in different sites to respond to several different hormones.
Compared with skeletal muscle, smooth muscle is able to maintain a high force of contraction for very little ATP usage.
Most smooth muscle is present in the walls of hollow viscera (e.g. gut, ureter, Fallopian tube) where it is arranged in sheets with cells aligned circumferentially or longitudinally, with contraction resulting in reduction of the lumen diameter.
In these so-called unitary smooth muscles , cells tend to generate their own low level of rhythmic contraction, which may also be stimulated by stretch and is transmitted from cell to cell via the gap junctions. Such smooth muscle is richly innervated by the autonomic nervous system (see Ch. 7 ), which increases or decreases levels of spontaneous contraction rather than actually initiating it. Physiologically, this is termed tonic smooth muscle and is characterised by slow contraction, no action potentials and a low content of fast myosin.
A second arrangement of smooth muscle is typified by that of the iris of the eye. Here, rather than simply modulating spontaneous activity, autonomic innervation precisely controls contraction, resulting in opening and closing of the pupil. Similar neurally controlled or multi-unit smooth muscle is found in the vas deferens and in some large arteries. Physiologically, this is termed phasic smooth muscle , and it is characterised by rapid contraction associated with an action potential.
Benign tumours of smooth muscle are termed leiomyomas . These are typically found in the uterus where they are more commonly known as fibroids but they can be found at other sites such as the skin or gastrointestinal tract. Malignant smooth muscle tumours are known as leiomyosarcomas . These tumours retain the appearance of smooth muscle but also show malignant features such as necrosis, atypia and/or increased mitotic activity.
C caveola CM circular smooth muscle layer D focal density ER endoplasmic reticulum F fibroblast Fi filaments G gap junction J attachment junction LM longitudinal smooth muscle layer M mitochondrion N nucleus PG parasympathetic ganglion S supporting tissue T tubular structure
Cardiac muscle or myocardium exhibits many structural and functional characteristics intermediate between those of skeletal and visceral muscle. Like the former, its contractions are strong and utilise a great deal of energy; like the latter, the contractions are continuous and initiated by inherent mechanisms, although they are modulated by external autonomic and hormonal stimuli.
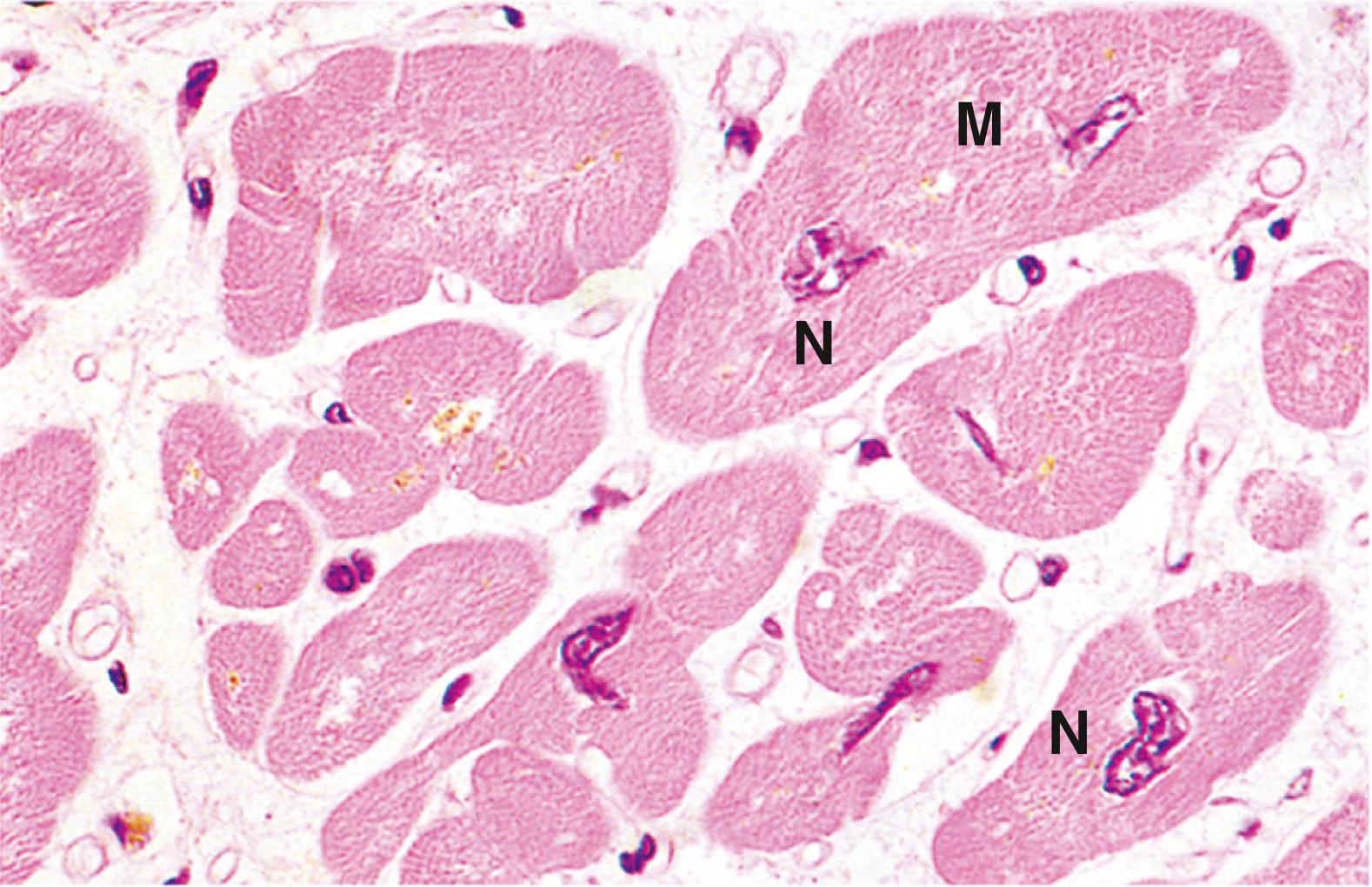
Cardiac muscle fibres are essentially long, cylindrical cells with one or at most two nuclei which are centrally located within the cell. The ends of the fibres are split longitudinally into a small number of branches, the ends of which abut onto similar branches of adjacent cells, giving the impression of a continuous three-dimensional cytoplasmic network; this was formerly described as a syncytium before the discrete intercellular boundaries were recognised.
Between the muscle fibres, delicate collagenous tissue analogous to the endomysium of skeletal muscle supports the extremely rich capillary network necessary to meet the high metabolic demands of strong, continuous activity.
Cardiac muscle fibres have an arrangement of contractile proteins similar to that of skeletal muscle and are consequently striated in a similar manner. However, this is often difficult to see with light microscopy due to the irregular branching shape of the cells and their myofibrils. Cardiac muscle fibres also have a system of T tubules and sarcoplasmic reticulum analogous to that of skeletal muscle. In the case of cardiac muscle, however, there is a slow leak of Ca 2+ ions into the cytoplasm from the sarcoplasmic reticulum after recovery from the preceding contraction; this causes a succession of automatic contractions independent of external stimuli. The rate of this inherent rhythm is then modulated by external autonomic and hormonal stimuli.
Between the ends of adjacent cardiac muscle cells are specialised intercellular junctions called intercalated discs which not only provide points of anchorage for the myofibrils but also permit extremely rapid spread of contractile stimuli from one cell to another. Thus, adjacent fibres are triggered to contract almost simultaneously, thereby acting as a functional syncytium . In addition, a system of highly modified cardiac muscle cells constitutes the pacemaker regions of the heart and ramifies throughout the organ as the Purkinje system , thus coordinating contraction of the myocardium as a whole in each cardiac cycle. This is illustrated and described in more detail in Ch. 8 .
Cardiac muscle cells in certain locations in the heart are responsible for secreting hormones into the bloodstream.
The heart muscle has high metabolic demands as it is constantly contracting to supply the body with blood. The blood supply to the heart itself is through three main coronary arteries which are prone to atheroma (see Wheater’s Pathology , Ch. 8 ), leading to narrowing of the vessels and a reduction in blood flow. If there is a critical failure of blood flow to the myocardium, the cardiac muscle cells may be deprived of oxygen and other nutrients and may die. Death of cardiac muscle cells causes myocardial infarction .
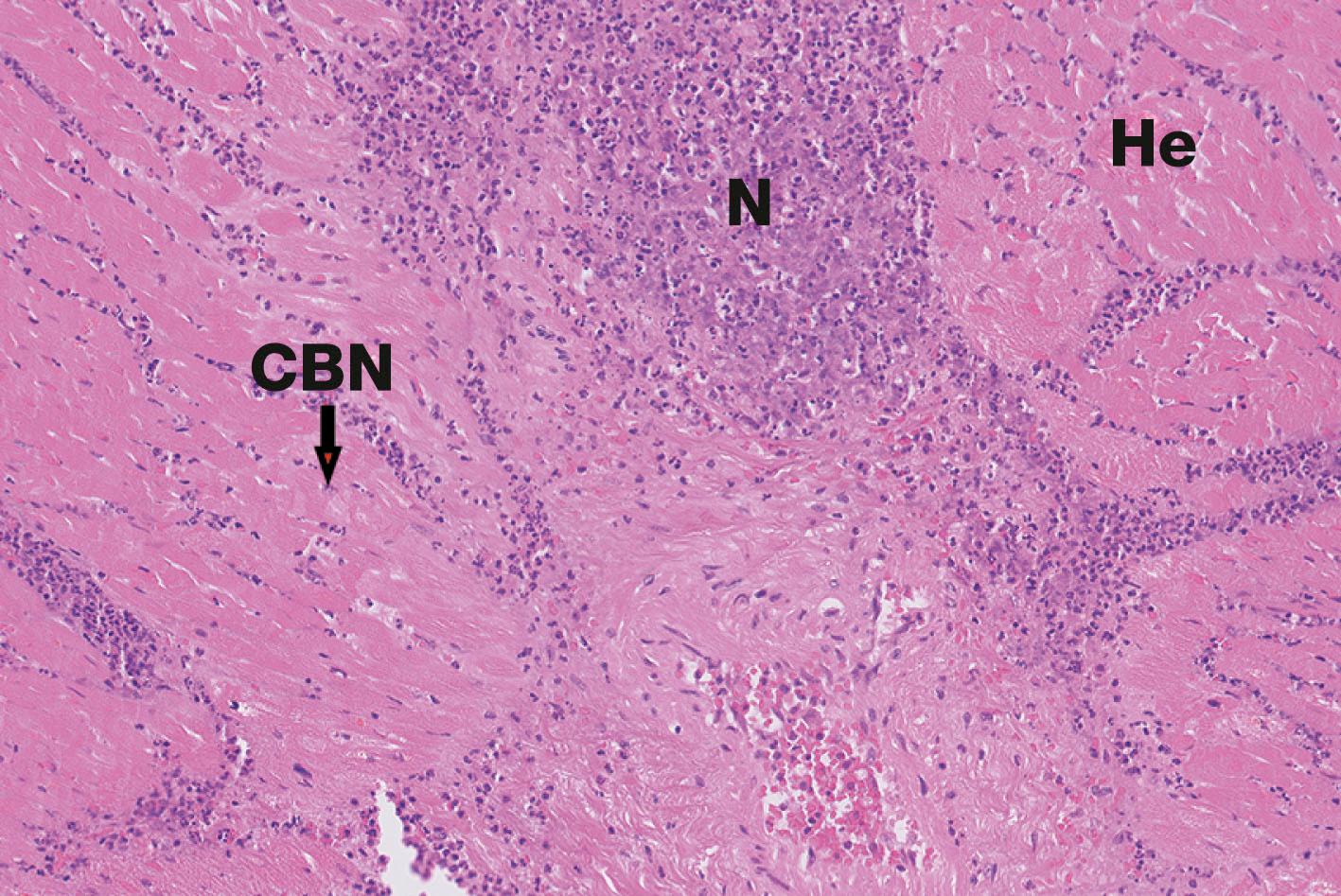
In the normal heart, conduction of depolarisation for contraction runs along the muscle cells themselves. This is interrupted in the case of an area of infarction. A common complication of a myocardial infarct is the development of disturbances of the heart rhythm, for example ventricular fibrillation . This can lead to sudden cardiac death. If the patient survives myocardial infarction, loss of a significant portion of muscle mass from the heart can cause heart failure , where the heart is unable to pump blood at a sufficient rate to meet the metabolic needs of the tissues.
ID intercalated disc N nucleus
Coordinated contraction of cardiac muscle is critical in allowing the heart to function effectively as a pump, supplying blood to the tissues of the body. Rapid spread of excitation through the whole myocardium is achieved via a highly specialised system of modified cardiac muscle cells known as the conducting system . This is discussed more fully in Ch. 8 .
In short, electrical excitation begins in the cells of the sino-atrial node (see Fig. 8.5 ), also known as ‘pacemaker cells’, and then spreads through the atria of the heart, initiating atrial contraction before the ventricles are depolarised, so that blood can first be pumped from the atria to fill the ventricles. The atrio-ventricular node then transmits the cardiac action potential into the ventricles via the bundle of His or atrio-ventricular bundle .
This electrical pathway runs through the interventricular septum of the heart and allows ventricular depolarisation and contraction to spread from the apex of the heart towards the ventricular outflow tracts, facilitating effective expulsion of blood from the ventricles with each heart beat.
Disorders of heart rhythm may arise due to myocardial ischaemia or infarction, as described previously (see textbox), or may be produced by more specific disorders of the conducting system itself. Abnormal heart rhythm or arrhythmia is a fairly common medical problem and its clinical features may range from immediately life-threatening medical emergencies requiring a pacemaker, through to other forms which may give rise to only mild or intermittent symptoms.
C capillary C1–C6 cardiac muscle cells F fibroblast G glycogen granules ID intercalated disc L lipid droplet M mitochondrion N nucleus T T tubule SR sarcoplasmic reticulum
D desmosome E endothelium of capillary EL external lamina F fibroblast FA fascia adherens G glycogen granules ID intercalated disc L leukocyte M mitochondrion N nexus junction S sarcomere filaments SR sarcoplasmic reticulum T T tubule
Heart disease is an important cause of morbidity and mortality worldwide. Its causes vary dramatically across different geographical areas and, in some parts of the world, conditions such as poor nutrition and infections result in serious cardiac disease. In the developed world, coronary artery atheroma is the root cause of the vast majority of cardiac pathology, including disorders such as angina pectoris (crushing chest pain due to ischaemia of the heart muscle), arrhythmias (see textbox ‘The conducting system of the heart’) and cardiac failure .
Cardiac failure occurs when the muscle of the heart is unable to pump effectively and cannot deliver enough blood supply to meet the body’s metabolic needs. This results in a diverse range of symptoms because of the effects on many other organs of the body, particularly the lungs. Symptoms include fatigue, breathlessness (fluid can build up in the lungs due to back pressure caused by ineffective emptying of blood from the heart) and peripheral oedema (swelling of the tissues occurs because of accumulation of tissue fluid in the interstitial space due to back pressure in the venous system). Treatment options are very diverse but aim to relieve these symptoms, as well as attempting to manage the underlying cause of cardiac failure.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here