Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Tuberculosis (TB) is an important public health problem. According to the World Health Organization (WHO), approximately 10 million persons developed TB during 2018, and approximately one-fourth of the world’s population, more than 2 billion persons, are infected with Mycobacterium tuberculosis , the causative agent of TB. Worldwide, TB is the leading cause of death among persons living with human immunodeficiency virus (HIV) infection and the most common cause of death overall attributable to a single infectious agent. Nonetheless, TB incidence rates and prevalence rates of M. tuberculosis infection vary widely, with the majority of both occurring among persons living in a relatively small number of countries, often in resource limited settings. Drug-susceptible TB is curable through established ≤9 months treatment regimens and preventable by providing specific treatment to persons with M. tuberculosis infection. However, the emergence of multidrug-resistant (MDR) TB threatens progress toward curing cases, preventing morbidity for persons, and preserving the effectiveness of first-line treatment regimens for societies. Morbidity, both treatment- and disease-associated, is much more common among patients with drug-resistant TB. Although effective treatment for the majority of forms of drug-resistant TB exists, access to necessary medications is often limited or nonexistent in resource-limited settings where the incidence of drug-resistant TB is highest. To neutralize the dual threats of TB and drug resistance, new and stronger initiatives for improving diagnostic capacity, finding better therapeutics, and developing stronger medical and public health systems are needed.
A 55-year-old woman with a history of well-controlled diabetes mellitus type 2 presented with unexplained weight loss, fatigue, and a productive cough. This patient had been born in a country with a high incidence of TB and had moved to the United States approximately 5 years before presenting to care with these symptoms. The patient reported that her cough had progressively worsened throughout the previous weeks. Clinical examination revealed that her temperature was 39.7°C, blood pressure 114/72 mm Hg, pulse 80 beats per minute, and respiratory rate 18 breaths per minute; she weighed 40.9 kg, approximately 9 kg less than at her previous health care visit 6 months prior. Her finger-stick glucose reading was 160 mg/dL.
This patient had received a diagnosis of tuberculosis (TB) 10 years earlier, which had been treated with a regimen of two medications for 6 months; thereafter her symptoms resolved. Although she reported no known recent contact with patients who had active TB disease, she did return to her country of birth for approximately 1 month each year. A chest radiograph revealed a right-upper-lobe infiltrate. Sputum was collected in the clinic and then again during the next 2 days while she was at home. The three samples were acid-fast bacillus (AFB) smear-negative, smear-positive, and smear-positive, respectively. The patient was started on the first-line TB treatment regimen, which comprised isoniazid, rifampin, pyrazinamide, and ethambutol.
During further interviews, the patient verified that she had been having symptoms consistent with TB for approximately 2 months before her most recent visit to her physician and reported that she had recently lived with extended family to care for an ailing relative. She continued to work throughout this time at her administrative job. She sat in a cubicle alone but had four coworkers in close proximity.
After approximately 6 weeks of therapy, her condition was very similar to that upon her initial presentation. She reported feeling slightly better after initially taking her medication, but her condition had worsened during the past week. She continued to have fevers and reported a decreased appetite. The patient’s family and work contacts were all identified and tested for TB infection; several had positive test results, demonstrating evidence of infection from TB exposure. Some of the contacts were started on treatment for latent TB infection (LTBI) with rifampin.
The patient was initially referred to the public health department to initiate directly observed therapy and case management; however, she wanted to maintain continuity of care and have her private physician communicate with the TB clinic. Upon further review of the patient’s medical chart, her sputum samples remained smear-positive for AFB; one of the sputum samples was then sent for nucleic acid amplification testing, which also included molecular evaluation for resistance. Results from this test demonstrated evidence of rifampin resistance. Culture isolates were sent for additional molecular detection of drug resistance and growth-based drug susceptibility testing by agar proportion. Both methods demonstrated resistance to both isoniazid and rifampin. After confirming that additional resistance was neither present nor acquired, the patient’s treatment regimen was expanded to an all-oral regimen with five second-line anti-TB medications. The treatment course was continued for 12 to 18 months after culture conversion. At that point the patient’s direct contacts had to be reevaluated and restarted on treatment for MDR LTBI with fluoroquinolone-based therapy.
COMMENT: It is important to have an index of suspicion for drug-resistant TB based on risk factors. In this case, a previous TB diagnosis, inadequate TB treatment (i.e., with fewer than three medications), and a lack of response to initial treatment for this episode increased clinical suspicion for MDR TB. For patients with such risk factors, treating clinicians should request molecular testing for drug resistance, culture, and phenotypic drug-susceptibility testing (DST) during the initial TB diagnostic evaluation. These results should guide the selection of an effective therapeutic regimen that minimizes medication-related toxicity. Consultation with an expert in the management of drug-resistant TB is always advisable when MDR TB is being managed. Prompt diagnosis not only benefits the patient with MDR TB but can also guide management of the patient’s contacts. Because neither isoniazid nor rifampin will prevent progression to TB disease among persons with LTBI resulting from MDR organisms, the drug-susceptibility profile should also guide the choice of LTBI treatment regimen.
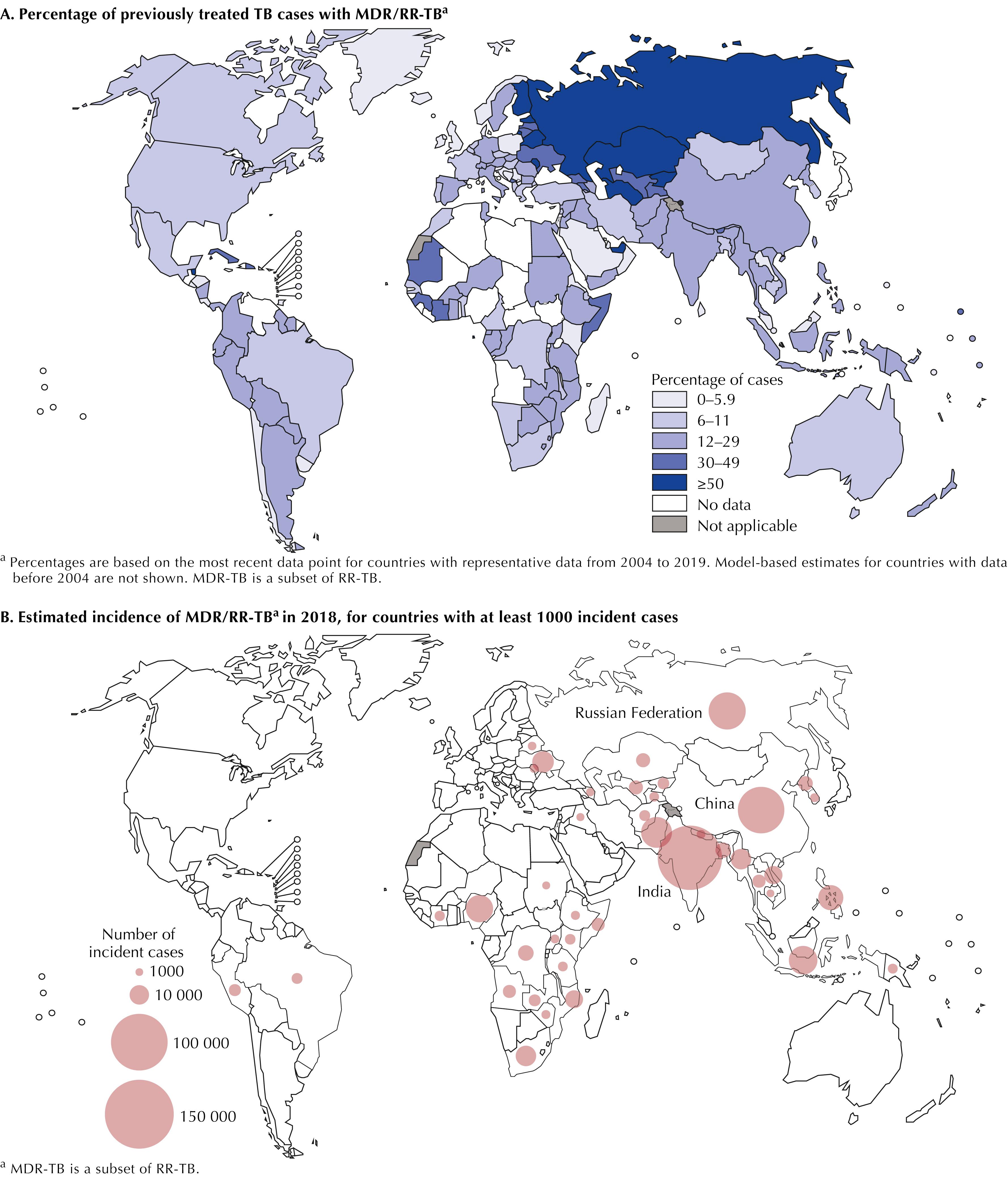
Globally, TB is a common infectious disease and the most common cause of death from a single infectious agent. TB caused an estimated 1.5 million deaths during 2018; while most TB deaths occurred among people without HIV, TB was the most common cause of death among persons living with HIV infection. Of the estimated 10 million TB cases diagnosed during 2018, approximately 500,000, or 5%, were MDR, meaning that they were resistant to isoniazid and rifampin, the two most important medications in first-line treatment regimens. The incidence of drug-resistant TB varies widely; the World Health Organization (WHO) estimates that 90% of MDR disease occurs among persons living in 30 countries with a high MDR TB burden ( Fig. 89.1 ) Comparatively, drug resistance is relatively rare in the United States. In recent years, of the approximately 10,000 TB cases reported annually, 8% to 10% were isoniazid-resistant; 1% to 2% were MDR; and fewer than 10 cases (<1%) were extensively drug-resistant (XDR), meaning that they were resistant to isoniazid, rifampin, fluoroquinolone, and an injectable agent (i.e., capreomycin, amikacin, or kanamycin).
TB is caused by one of a group of related acid-fast bacilli called the M. tuberculosis complex. Although M. tuberculosis and M. bovis are the most common causes of human disease, the bacille Calmette-Guérin strains of M. bovis, M. africanum, M. caprae, M. microti, M. canetti, and M. pinnipedii are other disease-causing organisms in the M. tuberculosis complex. These mycobacteria are typically transmitted from person to person through airborne droplet nuclei 1 to 5 μm in diameter. Contaminated particles are produced when a person with pulmonary or laryngeal TB coughs, speaks, or sings. Transmission occurs when another person inhales these contaminated droplet nuclei.
The majority of persons who inhale M. tuberculosis –containing droplet nuclei do not develop TB disease; macrophages either kill the mycobacteria and eliminate them from the body entirely or sequester them into granulomas in the lungs or elsewhere in the body; this condition of pathogen–immune system stalemate is often referred to as latent TB infection (LTBI). However, among the minority of persons who do not eliminate or sequester the bacilli after initial exposure, the mycobacteria cause primary TB disease in the lungs or travel through the pulmonary lymphatic system and disseminate through the bloodstream to infect almost any organ (e.g., the lymph nodes, larynx, brain [TB meningitis], spine [Pott disease], other bones and joints, the abdomen, or the genitourinary system). In less common circumstances, the site of infection can be outside of the lungs (e.g., gastrointestinal after the ingestion of contaminated food or cutaneous after direct exposure to infected tissues) and cause disease confined to the site of infection or similarly spread through lymphatic and hematogenous spread to other body sites, including the lungs themselves (when the lungs are seeded with bacilli through hematogenous spread, this sometimes results in the radiographic finding of a “miliary pattern”).
In addition to developing disease after primary infection, disease can also occur months or years after initial infection and development of LTBI, an occurrence often referred to as reactivation of or progression to TB disease . The majority of TB disease cases diagnosed in the United States are attributed to reactivation disease. As in the case of disease following primary infection, the lungs are by far the most common site of reactivation disease. However, disease can occur at almost any organ structure.
The risk for developing TB disease, either primary or reactivation, is higher among young children (i.e., those <5 years of age), persons with immunocompromising conditions (e.g., HIV infection, certain hematologic conditions, or other specific malignancies), persons taking certain immunosuppressing medications (e.g., multiple cancer chemotherapeutic agents, antirejection medications for organ transplantation, tumor necrosis factor-α antagonists, and >15 mg of prednisone or equivalent daily for >4 weeks), persons with certain medical conditions (e.g., diabetes mellitus or silicosis), persons who have had certain surgical procedures (e.g., gastrectomy or jejunoileostomy), and persons who are malnourished (i.e., ≥10% below ideal body weight).
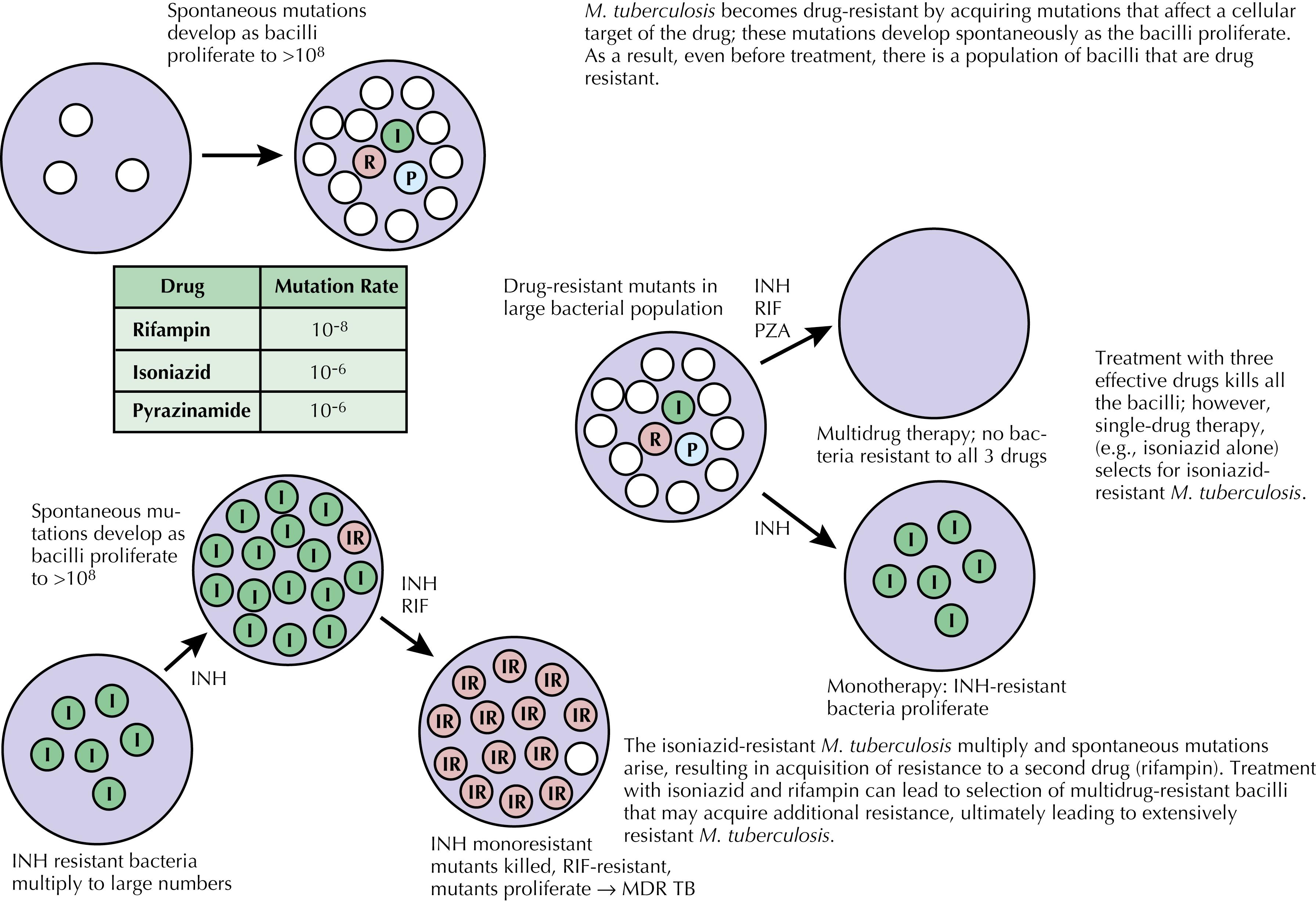
In the United States, TB disease is initially treated with a combination of four first-line medications (i.e., isoniazid, rifampin, pyrazinamide, and ethambutol) to prevent drug resistance. Drug resistance is thought to be a consequence of spontaneous mutations in genes associated with specific molecular targets that results in phenotypic resistance to these drugs either by modifying the drug target, preventing prodrug conversion, or altering efflux mechanisms. Random mutations that confer resistance to any single medication are thought to be rare, occurring with an approximate frequency of 1 in 10 6 to 1 in 10 8 cases ( Table 89.1 ). Combination therapy prevents drug resistance, even for patients with high disease burdens (e.g., with cavitary disease), because random simultaneous mutations conferring resistance to three or four medications is extremely unlikely.
| Agent | Mechanism | Genes Commonly Associated With Resistance |
| Isoniazid | Inhibits mycolic acid synthesis | katG inhA Others |
| Rifampin | Inhibits transcription (DNA-dependent RNA polymerase) | rpoB |
| Pyrazinamide | Unclear but could affect membrane energetics or fatty acid biosynthesis | pncA |
| Ethambutol | Inhibits mycolic acid synthesis | embB |
| Streptomycin | Inhibits protein synthesis | rpsL rrs |
| Amikacin and kanamycin | Inhibits protein synthesis | rrs |
| Ethionamide | Inhibits mycolic acid synthesis | inhA |
| Fluoroquinolones | Inhibits DNA synthesis (DNA-gyrase) | gyrA |
As in the case of drug-susceptible TB, drug-resistant TB can also be transmitted from person to person by contaminated respiratory droplets. Patients who were initially infected with already drug-resistant organisms are referred to as having primary drug resistance . Patients can also develop secondary or acquired drug resistance if the treatment regimen is inadequate because of too few effective medications or nonadherence with a correctly prescribed regimen. If a patient with initially drug-susceptible TB receives only isoniazid, the initially dominant population of isoniazid-susceptible bacteria will be killed, leaving only the isoniazid-resistant mycobacteria to proliferate and become the dominant population. If that patient, now with isoniazid-resistant TB, subsequently receives rifampin alone, the rifampin-susceptible organisms in the remaining population of mycobacteria will be killed, leaving the isoniazid-resistant, rifampin-resistant bacteria to proliferate. By using both drugs simultaneously, this resistance-inducing selection pressure is minimized ( Fig. 89.2 ). Ethambutol is used as part of the standard initial regimen because in most cases the drug susceptibility profile of the patient’s infection is unknown and ethambutol’s bacteriostatic effect can slow the proliferation of unidentified resistant strains. Ethambutol can be discontinued if drug susceptibility studies have demonstrated susceptibility to the first-line drugs; for culture-negative TB that is presumably susceptible to all first-line medications, ethambutol is discontinued after 2 months of treatment. Pyrazinamide is included in the initial 2-month treatment phase because it has been shown to shorten the necessary overall length of treatment from 9 months to 6 months; this might be because pyrazinamide is active against mycobacteria in slightly acidic environments such as the intracellular space of macrophages, which is one of the places where mycobacteria can evade the normal host immune response (see Fig. 89.2 ). Patients can also acquire resistance to second-line TB medications, especially if therapy for drug-resistant TB is inadequate. Acquired drug resistance can also emerge, despite adherence to therapy, if drug levels are subtherapeutic, which can occur in instances of malabsorption or inadequate dosing.
M. tuberculosis infections, regardless of drug-resistance patterns, have similar clinical presentations. Patients with LTBI—those who are infected with M. tuberculosis but do not have clinically evident TB disease—have no symptoms or signs specific to this condition; nonetheless, such patients will often but not always have positive results for an interferon-ɣ release assay (IGRA) and/or a tuberculin skin test (TST), both of which are tests for M. tuberculosis infection. Although LTBI treatment, also called TB preventive therapy , is highly effective for preventing drug-susceptible TB disease, no tests are available that differentiate persons with drug-susceptible LTBI from those with drug-resistant forms. In the United States, the LTBI treatment regimens preferred by the Centers for Disease Control and Prevention (CDC) include rifampin or another rifamycin (e.g., rifapentine) with or without isoniazid, although daily isoniazid monotherapy for 6 or 9 months is also commonly used and is still considered acceptable, but not preferred. Globally, the majority of regimens feature isoniazid. Because of resistance to isoniazid and rifamycins, these drugs are not acceptable for treating persons latently infected with MDR organisms. Current guidance recommends fluoroquinolone-based regimens for preventing TB disease in persons infected with MDR organisms that are presumed to be susceptible to fluoroquinolones.
Clinical presentations of drug-resistant TB disease are also largely indistinguishable from drug-susceptible TB, at least initially. Although TB disease can affect almost any organ structure, pulmonary disease is the most common, affecting more than 70% of patients. Presenting symptoms often include cough that is often prolonged for 2 or more weeks, fever, night sweats, fatigue, anorexia, and unexplained weight loss. Chest pain; dyspnea, especially on exertion; and coughing up blood (hemoptysis) are also common features of pulmonary disease. The presenting symptoms of extrapulmonary disease often include constitutional symptoms (e.g., fever, unexplained weight loss) along with symptoms specific to the affected organ structure (e.g., swollen lymph nodes, abdominal pain, mental status changes in patients with TB meningitis). As many as one-third of patients with extrapulmonary disease also have pulmonary disease.
Because symptomatic presentations of drug-resistant TB are similar to or even indistinguishable from drug-susceptible TB, eliciting specific epidemiologic and clinical risk factors for drug resistance during diagnostic evaluations can facilitate more prompt testing for and diagnoses of drug-resistant TB. These epidemiologic risk factors include:
Exposure to a person with drug-resistant TB
Exposure to or residence in an area or setting where drug resistance is highly prevalent
Clinical risk factors include:
Previous receipt of an inadequate TB treatment regimen
Poor or no improvement despite adherence to a first-line treatment regimen
Diagnosis after treatment for latent infection when signs or symptoms of disease were present
Prolonged use of fluoroquinolones or injectable agents (e.g., aminoglycosides) for prolonged periods before TB was considered, often for nonspecific pulmonary disease
Physical examination should be performed during all diagnostic evaluations for M. tuberculosis infection. Findings of TB disease include cachexia, cervical or regional lymphadenopathy, abnormal findings on auscultation of the lungs related to consolidation or pleural effusions, hepatosplenomegaly, abdominal tenderness related to peritonitis, spinal tenderness related to spinal TB (i.e., Pott disease), meningeal signs, and neurologic deficits.
In the United States, diagnostic evaluations for LTBI and TB disease, regardless of drug-susceptibility patterns, usually include a test for M. tuberculosis infection, either an IGRA or a TST. Because LTBI prevalence and TB disease incidence are relatively low in the United States, clinical guidelines for this setting recommend testing only persons who have specific epidemiologic risk factors for LTBI or TB disease, such as exposure to a person with infectious TB or previous residence in countries or settings where TB disease is common; also to be tested are those persons having signs or symptoms consistent with disease. These tests can be helpful for identifying asymptomatic persons with LTBI who can benefit from treatment to prevent progression to TB disease in this low-incidence setting. Although tests for M. tuberculosis infection can also assist with TB disease diagnoses, negative results do not exclude TB disease. Because these tests depend on the immune reaction to mycobacterial antigens, they have lower sensitivity for LTBI and TB disease among certain persons with strong risk factors for progression to disease (e.g., persons taking immunocompromising medications, those with other immunocompromising medical conditions, children <5 years of age, and those living with HIV infection). Tests can also be negative in the setting of overwhelming mycobacterial sepsis caused by immunosuppression. In resource-limited settings, tests for M. tuberculosis infection are often unavailable or only rarely used in settings where the prevalence of LTBI and incidence of TB disease are high.
When available, chest radiography should also be performed during all diagnostic evaluations for M. tuberculosis infection and TB disease. Although nonspecific, single or multiple areas of upper-lobe consolidation, upper-lobe fibronodular opacities, cavitation, mediastinal or hilar adenopathy, and pleural effusions are all radiographic signs consistent with TB disease. Computed tomography (CT) can be helpful in evaluating extrapulmonary TB and further characterizing pleural abnormalities, pulmonary infiltrates, cavitation, and lymphadenopathy among patients with pulmonary disease. Magnetic resonance imaging (MRI) with contrast can also be helpful in diagnostic evaluations for pulmonary and extrapulmonary disease; ring-enhancing lesions in the brain, lymph nodes, and other tissues are consistent with but not specific for TB disease affecting those organ structures. Although helpful for further characterizing disease, availability of CT, MRI, and even chest radiography can be unpredictable in resource-limited settings where the disease burden is often high.
Laboratory services are vital for diagnosing TB disease, including sputum smear microscopy as a key part of diagnostic evaluations for pulmonary TB. In pulmonary TB, acid-fast bacilli are often observed after smear and culture of sputa from affected patients. Although only approximately 50% of patients with diagnosed TB disease in the United States have positive sputum smear results, those results are usually available within 24 hours. Therefore, when positive, these results can assist with rapid diagnoses and measuring response to therapy. Current guidance recommends performing nucleic acid amplification tests (NAATs) on at least one specimen from patients undergoing a diagnostic evaluation for tuberculosis; NAATs on sputum are 70% sensitive for diagnosing pulmonary TB. However, because initial sputum smear—even with the added sensitivity of NAATs—is relatively insensitive for diagnosing tuberculosis, empiric therapy for TB should be strongly considered when clinical suspicion for pulmonary disease is high, sputum smear results are negative, and alternative diagnoses have been excluded. NAATs are much more specific for TB disease compared with sputum smear, which may detect nontuberculous mycobacteria. Sputum culture, which can require as many as 6 to 8 weeks, should be performed when available. In the United States, more than 70% of patients with diagnosed TB disease will have positive cultures; identification of growth in culture should be performed to confirm infection with one of the organisms in the M. tuberculosis complex after positive culture results. Culture is particularly important for diagnosing drug-resistant pulmonary TB because growth-based DST can be performed on the organisms isolated from culture. Smear and culture of other fluids (e.g., cerebrospinal fluid and peritoneal fluid) often facilitates diagnosis of extrapulmonary TB disease. Unfortunately, as with radiography, laboratory capacity for performing culture is often limited or nonexistent in resource-limited settings.
After culture of M. tuberculosis isolates, drug resistance is determined through DST using antituberculosis drugs in liquid or solid media. DST is the standard of practice for cases of culture-confirmed TB disease in the United States. Therefore testing for resistance to first-line medications in the United States is often performed in commercially available automated liquid culture systems to facilitate more prompt diagnoses of drug-resistant disease. Solid media is more commonly used to test for resistance to second-line medications. Capacity for this kind of testing, often referred to as phenotypic (or growth-based) DST , is often limited or nonexistent in resource-limited settings. Molecular methods for determining drug-resistance patterns are becoming more prevalent; although specific procedures differ among laboratories, these methods typically rely on nucleic acid amplification to evaluate the mycobacterial DNA for mutations potentially associated with drug resistance. Some molecular methods combine NAATs for identifying M. tuberculosis complex in sputum specimens with tests to evaluate for mutations associated with resistance to specific drugs, especially rifampin. Because M. tuberculosis isolates that are solely resistant to rifampin are extremely rare, rifampin resistance often indicates MDR disease. These molecular methods can assist in promptly diagnosing TB and can offer results within 1 day of laboratory receipt; moreover, multiple platforms have been adapted for use in resource-limited settings. These tests also can enable more prompt development of treatment plans for drug-resistant TB. Although molecular methods for determining drug resistance are extremely valuable and some laboratories are reducing traditional phenotypic DST with primary use of advanced molecular methods such as whole genome sequencing, interpretation of results depends on identifying a mutation that has been characterized with respect to drug resistance. Because not all genetic mechanisms or mutations have been characterized, ongoing capacity for phenotypic DST is needed.
In the absence of phenotypic and molecular testing, drug resistance is usually diagnosed on the basis of the lack of response to first-line therapy (i.e., treatment for drug-susceptible TB). Typically, in drug-susceptible disease, symptoms related to TB disease improve; fewer acid-fast bacilli are visible by sputum smear microscopy; and eventually but not immediately, chest radiograph findings related to TB disease improve. Clinicians should always consider drug resistance when cultures remain positive after 2 months of treatment or patients do not improve after 2 months of first-line TB treatment, particularly in the context of epidemiologic or clinical risk factors for drug resistance.
Although drug resistance, including multidrug resistance, should be considered in patients with specific risk factors and those who are not improving with first-line treatments, nonadherence to medications and subtherapeutic drug levels should also be considered in the differential diagnosis for drug-resistant TB. Because symptoms of TB disease can be nonspecific, differential diagnosis for pulmonary TB, regardless of drug-resistance profile, also includes community-acquired pneumonia caused by viral or bacterial pathogens. Furthermore, fungal pathogens and nontuberculous mycobacteria can have similar clinical presentations. Neoplastic processes (e.g., lung cancer) and autoimmune diseases should also be considered as possibilities. Sarcoidosis—another disease with similar clinical features—deserves special mention because, like pulmonary TB, it is a granulomatous process. Although sarcoidosis is classically associated with noncaseating granulomas and TB disease is associated with caseating granulomas, TB disease should be excluded by a thorough diagnostic evaluation before a sarcoidosis diagnosis is made. Extrapulmonary disease has a broad differential diagnosis that depends on the organ structure involved and the clinical presentation.
In the United States and other areas where the rates of antituberculosis drug resistance are low to moderate, standard treatment of fully drug-susceptible TB consists of an initial daily regimen of isoniazid, rifampin, pyrazinamide, and ethambutol. Therefore patients with diagnosed TB are often started on this four-drug regimen while specimens are undergoing laboratory testing to confirm drug susceptibilities. After 8 weeks of therapy, pyrazinamide and ethambutol are discontinued and isoniazid and rifampin are continued, thus completing 6 to 9 months (26 to 38 weeks) of therapy.
Patients who have drug-resistant TB are often initiated on these inadequate treatment regimens while specimens are being examined in the laboratory. Because of the implications for both the patient and public health, providers should have an increased index of suspicion for drug resistance among patients with epidemiologic or clinical risk factors such as residence in areas with high rates of drug-resistant TB or previous treatment for TB (especially with less than three medications). Treatment for drug-resistant TB is more complex and requires a balance of choosing a regimen that maximizes efficacy while minimizing potential adverse events. Because resistance testing has not been as available in resource-limited countries, global trials of MDR-TB treatment have recently focused on evaluating entire regimens administered to patients suspected of having drug-resistant TB. In the United States, laboratory capacity for quickly identifying resistance and drug availability has allowed providers to design individualized regimens based on a patients’ specific susceptibility pattern.
If drug resistance is suspected, diagnostic laboratory studies can be expedited, expanded regimens can be initiated, and patients can be promptly isolated to prevent further transmission.
Substantial changes have occurred in the approach to treatment of drug-resistant TB. Previously, providers had to create a five-drug regimen often including toxic medications that were difficult for patients to tolerate; these sometimes led to irreversible adverse effects ( Table 89.2 ). New medications introduced during the last 5 years have substantially improved providers’ ability to treat patients without the same risk. Both US-based and WHO guidelines now emphasize using all oral regimens for treating drug-resistant TB, and these have been associated with better treatment outcomes.
| System | Effect | Typical Causative Agent | Management Option | |
| First Line | Second Line | |||
| Gastrointestinal | Gastritis, nausea, vomiting | Pyrazinamide Rifampin Isoniazid |
Ethionamide Para-aminosalicylic Fluoroquinolone Linezolid |
Consuming a small predose snack or meal |
| Proton pump inhibitor | ||||
| If severe, discontinuation | ||||
| Liver injury | Pyrazinamide Isoniazid Rifampin |
Ethionamide Bedaquiline |
Interrupt therapy if transaminases >3 times upper limit of normal with symptoms or >5 times upper limit of normal without symptoms Follow resolution with challenge a |
|
| Central nervous system | Paresthesia | Isoniazid | Ethionamide | Increase pyridoxine dose |
| Asthenia | Isoniazid | Any drug | Reassurance Time dose for later in the day |
|
| Depression | — | Cycloserine | Antidepressant medication Discontinuation if severe |
|
| Sleep disturbance, agitation, tremulousness | — | Fluoroquinolone | Discontinuation if severe | |
| Development or worsening of movement disorder | Isoniazid | — | Discontinuation if severe | |
| Seizure | Isoniazid | Cycloserine Ethionamide |
High-dose pyridoxine, antiseizure medication, discontinuation | |
| Vision change | Ethambutol | — | Interruption and ophthalmologic examination with observation for recovery | |
| Hearing or vestibular change | — | Streptomycin Amikacin Kanamycin Capreomycin |
Discontinuation Can progress despite interruption and can be irreversible Consider a different injectable agent if treatment options are limited |
|
| Psychosis | Isoniazid | Cycloserine | Discontinuation and consult a psychiatrist | |
| Optic neuropathy | — | Linezolid | Discontinuation | |
| Renal | Uremia | — | Streptomycin | Discontinuation |
| Interstitial nephritis | Rifampin Isoniazid |
— | Discontinuation | |
| Skin | Noninflammatory pruritus | Isoniazid | — | Attempt treatment with antihistamine |
| Urticaria | Any drug | Any drug | Attempt treatment with antihistamine | |
| Acneiform rash | Isoniazid Rifampin |
Cycloserine | Topical therapy Discontinuation if severe |
|
| Maculopapular rash | Any drug | Any drug | Discontinue, consider rechallenge after resolution | |
| Endocrine | Hypothyroidism | — | Ethionamide | Attempt treatment with levothyroxine |
| Hematologic | Myelosuppression | — | Linezolid | Discontinue, consider rechallenge after resolution, if feasible |
| Cardiovascular | Heart rhythm changes (prolonged QT interval) | — | Bedaquiline | Discontinue |
a Patients should be counseled to stop taking medication immediately upon experiencing symptoms (vomiting or jaundice) and seek medical care.
Overarching principles for treating drug-resistant TB include the following:
Consultation should be requested with an expert in TB when suspicion for or confirmation of drug-resistant TB exists. In the United States, TB experts can be located through local health department TB control programs ( https://www.cdc.gov/tb/links/tboffices.htm ), through TB Centers of Excellence for Training, Education, and Medical Consultation supported by the Centers for Disease Control and Prevention (CDC) ( http://www.cdc.gov/tb/education/rtmc/default.htm ) and through international MDR-TB expert groups (e.g., the Global TB Network).
Molecular testing should be obtained for the rapid detection of mutations associated with resistance.
Regimens should include only drugs to which the patient’s isolate has been documented or where a high likelihood of susceptibility exists. Patients should not be treated with medications to which their isolate is resistant.
A regimen of five or more oral medications should be initiated ( Table 89.3 ). After 5 to 7 months with documented clinical response (demonstrated by conversion of sputum cultures to negative and clinical improvement), a continuation phase of four or more oral medications should be continued for a total of 15 to 21 months after culture conversion.
|
| Step | Drug |
|
Levofloxacin Moxifloxacin |
|
Bedaquiline Linezolid |
|
Clofazimine Cycloserine |
|
Amikacin Streptomycin |
|
Pyrazinamide Ethambutol Delamanid c |
|
Ethionamide or prothionamide d Imipenem-cilastin/clavulanate or meropenam/clavulanate e para -aminosalicylic acid f High-dose isoniazid g |
| Drugs no longer recommended for inclusion in MDR-TB regimens: | Capreomycin and kanamycin Amoxicillin or clavulanate when used without a carbapenem Azithromycin and clarithromycin |
a Amikacin and streptomycin should be used only when the patient’s isolate is susceptible to these drugs. Because of their toxicity, these drugs should be reserved for when more effective or less toxic therapies cannot be assembled to achieve a total of at least five effective drugs.
b Patient preferences in terms of the harms and benefits associated with injectables (the use of which is no longer obligatory), the capacity for monitoring for substantial adverse effects, drug-drug interactions, and patient comorbidities should be considered in selecting step 5 agents over injectables. Ethambutol and pyrazinamide had mixed or marginal performance on outcomes assessed in the individual patient data meta-analyses (IPDMA); however, certain experts might prefer these drugs over injectable agents for building a regimen of at least five effective oral drugs.
c Data regarding dosing and safety of delamanid are available for children at least 3 years of age.
d Mutations in the inhA region of M. tuberculosis can confer resistance to ethionamide/prothionamide as well as to isoniazid at low concentrations. In this situation, ethionamide/prothionamide might not be the best choice of a second-line drug unless the isolate has been demonstrated to be susceptible with in vitro testing.
e Divided daily intravenous dosing limits feasibility. Optimal duration of use is not defined.
f Fair or poor tolerability and low performance. However, adverse effects are reported to be less common among children.
g Data not reviewed in the IPDMA. However, high-dose isoniazid can be considered in the presence of mutations with inhA promoter mutations but not with katG mutations, which result in inhibition of catalase activity and the development of high-level resistance (resistance at 1.0 mg/mL on solid media) to isoniazid that cannot be safely and effectively overcome by increasing the dose.
Treatment response should be monitored clinically, radiographically, and bacteriologically, with cultures obtained at least monthly for pulmonary TB. If cultures remain positive after 3 months of treatment, DST should be repeated.
Evidence is mounting for the use of therapeutic drug monitoring where available, particularly in the case of delayed clinical response to treatment and to limit toxicity or adverse events.
Patients should be asked and educated about possible adverse effects at each visit. The toxicities and poor tolerability of drugs used to treat drug-resistant TB are well established, and all adverse effects should be thoroughly investigated and ameliorated (see Table 89.2 ).
Patient-centered case management should be used to help patients understand their diagnosis, understand and participate in their treatment selection, and discuss potential barriers to treatment completion and achieving a cure.
In the event of progressive resistance or intolerance, providers should never add or change less than 2 drugs in a failing regimen.
The American Thoracic Society/CDC/Infectious Diseases Society of America drug-resistant treatment guidelines outline the best strategies for constructing regimens for treating patients with drug-resistant TB.
For isolated isoniazid resistance, treatment options include the following:
Rifampin, pyrazinamide, ethambutol, and fluoroquinolone for 6 months
or
Rifampin, pyrazinamide, and ethambutol for 9 months
A randomized controlled trial demonstrated equivalent outcomes with use of either regimen, but the fluoroquinolone arm had slightly better efficacy and similar safety. For isolated pyrazinamide resistance (or for any course of treatment using only isoniazid and rifampin), therapy should be extended to 9 months.
Resistance to rifampin alone is rare; the treatment approach for when it is present has been evolving over recent years. If susceptibility to isoniazid can be confirmed, treatment would include isoniazid, fluoroquinolone, pyrazinamide, and ethambutol for 6 to 9 months. If either extensive disease is present or concern exists regarding further resistance, a regimen similar to one designed for the treatment of MDR-TB might be used.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here