Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
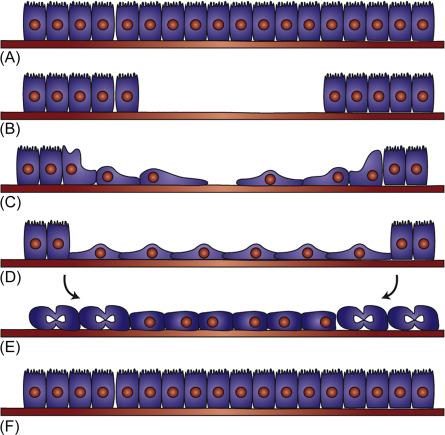
The epithelial lining of the mammalian small intestine and colon is a rapidly renewing population forming a single-cell barrier along the entire length of the lower gastrointestinal (GI) tract. Ulcerated wounds in the epithelium are typically repaired by coordinated molecular, cellular, and tissue processes that serve to reestablish the epithelial sheet continuity and normal cellular phenotypes. Conceptually, the repair process can be broadly segmented in vitro into early and late phases, though these are not guaranteed to be temporally distinct in vivo. The early phase is known as restitution, in which epithelial cells move into the injured or denuded regions, thereby providing an interim seal. Restitution is predominantly characterized by cellular migration and is independent of proliferation. The end result of restitution is that the wound is covered by a sheet of epithelial cells with stretched morphologies. The late phase of repair involves cellular proliferation and tissue morphogenesis, which restore the original density of cells and crypt/villus structures, respectively ( Fig. 29.1 ).
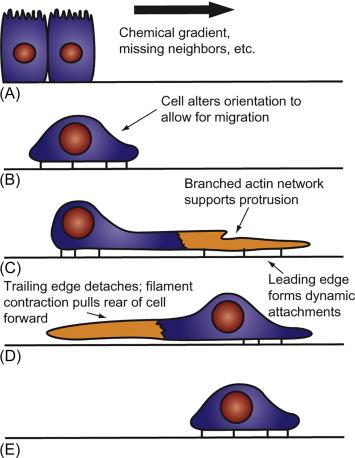
Studies of mechanically generated wounds in layers of intestinal epithelial cells grown in vitro have detailed the cellular changes that underlie restitution. At the most basic level, restitution activates migration pathways that are conserved across nearly all eukaryotic species and utilized in multicellular organisms during embryogenesis, immune responses, and tissue repair (reviewed in Ref. ). Migration behaviors are evoked from a fundamentally distinct polarization of intestinal epithelial cells, as many cytoskeletal proteins, such as villin, normally localized to the apical brush border are relocated to the migration front. Intestinal epithelial cells at the edge of the wound reorient and project lamellipodia, flattened extensions of the cell, in the anticipated direction of migration. These lamellipodia also contain microspikes, or small filopodia, which are rod-like extensions that stick into the wounded area and run perpendicular to the lamellipodial front, or leading edge. The formation of these specialized structures facilitates there the construction and maturation of new focal adhesions, concentrated areas where integrin-like cellular proteins bind to the extracellular matrix, and link to the cellular cytoskeleton. These focal adhesions provide the necessary traction force during cellular locomotion. They also serve as foundations for the newly placed actin fibers in the lamellipodium and filopodia and the remodeling of existing actin stress fibers that run the full length of the cell. During a single cycle of cellular propulsion, myosin motors displace these actin fibers, deforming the cell shape toward the leading edge. The stress fibers disconnect from the focal adhesions at the “rear,” or trailing edge, of the cell, likely due to increased tension. As the cell moves forward, the leading-edge structures and their focal adhesions must be remodeled and relocated so that the propulsive cycle can be repeated ( Fig. 29.2 ).
The sequence of events described above is generalizable to individual cells migrating in space (nonconfluency), but the epithelial cells of the intestine have special properties because they are also able to migrate as a sheet during restitution. Sheet migration has an advantage of maximizing the residual barrier function of the intestinal epithelium during restitution. Studies of sheet migration in cell culture have demonstrated specialized “leader” and “follower” cells following wounding. Leader cells are depolarized and exhibit lamellipodia and other migratory characteristics; they are the first to enter the wounded area and drag follower cells, which maintain their polarization, behind them. The resulting repair pattern resembles discrete “fingers” of cells moving into the wounded area; these fingers highlight the initial spatial distributions of these leader cells. Within these fingers, other cells can later adopt a leader phenotype and contribute to the restitutive process. This pattern of repair thus maintains polarized cell function in most cells at the wound edge by dictating the migratory phenotype to only a specialized set of cells.
The need for coordinated wound healing by intestinal epithelium gives rise to a complementary restitutive mechanism known as “purse-string” wound closure. This mechanism was first discovered in embryonic epidermal tissue, but has since been shown in intestinal epithelial cell culture. Instead of invoking classical migration pathways, cells at the wound edge generate a shared arc of actin fibers. This arc may be linked between cells at adherens junctions. The cells then undergo a coordinated contraction of the arc, akin to closing a purse by pulling on a string, that rapidly pulls the cells together into the wounded area. In purse-string restitution, epithelial cells maintain their polarity. Thus, they can participate in wound repair without undergoing an energy-intensive transdifferentiation/dedifferentiation procedure.
Cell migration and purse-string wound closure are not mutually exclusive. The size and shape of the wound may determine which mechanism is predominantly used. Purse-string closure is only observed in wounds with convex edges (e.g., round wounds). Larger wounds are healed by migration, while smaller wounds may be healed by either migration or purse-string closure. In straight-edge wounds, repair fingers created by migrating leader cells may result in locally round edges that can be healed by the purse string mechanism. However, because of relative difficulties in optimizing culture, wounding, and visualization systems to analyze molecular mechanisms of purse-string healing, most molecular studies of restitution focus on the more-reproducible cell migration phenomenon.
Recent advances in intestinal wound models and imaging techniques in mice have defined some of the changes in intestinal tissue architecture during epithelial repair. Normal colon contains a repeating array of thin crypts that are axially oriented, with progenitor cells located at the crypt base and differentiated cells at the luminal surface. After ulceration, surviving crypts at the wound edge shorten and open laterally—these lateral extensions provide a path for epithelial cells to migrate into the wound. These migratory cells are referred to as wound-associated epithelial (WAE) cells and can be identified by their expression of claudin-4. As restitution progresses, the crypt lateral openings extend and deepen; surface reconstructions of the epithelium show a network of “canals” or “channels” that represent lengthy, lateral invaginations of crypt structures. Resumption of proliferation characterizes the late phase of ulcer healing. During this phase, new crypts are seeded along the lateral extent of the wound canals, and WAE cells are replaced by homeostatic absorptive and secretory cell lineages.
Restitution in vivo is assisted by the activities of immune, mesenchymal, and microbial cells, which directly activate restitutive signaling pathways in epithelial cells. These are described in more detail later in this chapter. An important indirect mechanism assisting restitution is the contraction of tissue structures after injury, which reduces the effective wound area. Colons with severe experimental colitis are shorter. Villus structures in the small intestine are also contracted after injury. The contraction phenomenon is tetrodotoxin-sensitive, suggesting that it is partially mediated by neuronal activity.
Due to the highly interconnected nature of signaling networks inside of cells, hundreds to thousands of proteins and smaller biomolecules impact restitution. For simplicity, however, a discussion of the intracellular mechanisms of restitution can be centered on two structures: actomyosin (actin- and myosin-containing) filaments and focal adhesions. Biomolecules involved in the regulation of formation and breakdown of these structures have demonstrated effects on cell migration, the central cellular process underlying epithelial restitution.
Formation of lamellipodial and filopodial structures requires higher-order assembly of actin filaments (reviewed in Refs. ). These filaments are formed against the edge of the cell, leading to deformation and protrusion of the plasma membrane. In lamellipodia, actin filaments are arranged in a meshwork, with filaments oriented ~ 70 degrees to one another. In filopodia, actin filaments are bundled straight together by fascin proteins. Several scaffolding and enzymatic proteins are critical to the assembly actin filaments. The ARP2/3 complex is responsible for nucleating F- (filamentous-) actin branches from the surface of existing filaments. ARP2/3 complexes are bound to WASP/WAVE-family proteins, which are nucleation-promoting factors that dissociate from ARP2/3 after nucleation. The formation of branched actin networks is especially important for lamellipodia. A second class of F-actin-nucleating machinery centers on the formin (also known as diaphanous) complex. Unlike ARP2/3, formin proteins can nucleate unbranched actin. Formin proteins follow the growing (or “barbed”) end of the filament, protecting it from premature capping. During the filament elongation process, formin proteins attract unpolymerized, or G- (globular-) actin, by binding to profilin proteins carrying the G-actin. To enhance directional propagation of actin filaments, cofilin protein frees actin monomers from the filamenťs “pointed” end, thereby enabling recycling of these monomers to the growing end.
The F-actin assembly process is regulated by the activities of small GTPases (reviewed in Ref. ). When bound to GTP, these GTPases interact with downstream effectors of actin polymerization. When bound to GDP, the GTPases are inactive. Guanine nucleotide-exchange factors (GEFs) replace GDP with GTP in the binding pocket of the GTPase; thus, GEFs activate GTPase-mediated downstream signaling. In contrast, GTPase-activating proteins (GAPs) increase the GTP-hydrolyzing activity of the GTPase; thus, GAPs inactivate GTPase-driven signaling. A second class of inhibitor of GTPase signaling consists of the guanine nucleotide-dissociation inhibitors (GDIs), which bind to GTPases and sequester them to the cytosol, thereby interfering with the GTPase-effector interactions normally occurring at the plasma membrane.
Specific GTPases have targeted functions in the protruding structures associated with cell migration. In filopodia, CDC42·GTP activates WASP and formin proteins to nucleate F-actin filaments. In lamellipodia, the RAC1GTPase achieves a similar function (creation of an actin meshwork) through activation of WAVE and formin proteins. The Rho family proteins including RhoA, RhoB, and RhoC are responsible for nucleating actin stress fibers throughout the cell. Thus, the coordinated activities of CDC42, RAC1, and Rho GTPases mediate cellular-wide remodeling of the actin cytoskeleton to promote restitution.
In order to generate the force for locomotion, intestinal epithelial cells primarily utilize myosin II motors attached to the actin filaments (reviewed in Ref. ). Immunohistochemical stains have demonstrated that myosin II is enriched at the lamellipodium and the leading edge. Myosin II is composed to three pairs of subunits: two heavy chains, two regulatory light chains, and two essential light chains that support the heavy chains. Phosphorylation of the regulatory light chains increases the ATPase activity of myosin, thereby facilitating the intramolecular power stroke that displaces the actin filament. There are more than a dozen kinases known to phosphorylate nonmuscle myosin II, including myosin light-chain kinase (MLCK) and Rho-associated, coiled coil-containing kinase (ROCK). In turn, MLCK and ROCK are regulated by upstream cellular activities including Ca 2 + -calmodulin signaling (in the case of MLCK) and RhoA GTPase activity (in the case of ROCK). In addition to phosphorylating myosin directly, ROCK negatively regulates the MLC phosphatase, thereby further favoring the activation of myosin.
The cytoskeletal attachment to the underlying extracellular matrix through focal adhesions enables enough force to be generated to bend the cell during migration. A central protein that contributes to the structure of focal adhesions and regulates their assembly, disassembly, and actin attachment is focal adhesion kinase (FAK) (reviewed in Ref. ). FAK also represents a signaling hub through which information about cell state can be traded with the cell motility machinery. FAK-knockout mice have impaired developmental morphogenesis that has been ascribed to impaired migration, but paradoxically exhibit an excess number of focal adhesions. FAK therefore seems to be important for the remodeling of focal adhesions, which is particularly important for restitution because the focal contacts must be disassembled and reassembled as the cell moves. At focal adhesions, integrins binding to specific matrix ligands are linked to intracellular actomyosin filaments through talin and paxillin proteins. FAK binds to both talin and paxillin. FAK can be phosphorylated at multiple sites by multiple kinases, but it can importantly also be autophosphorylated (e.g., at Tyr397). Phosphorylated FAK can potently interact with SH2-domain containing proteins including Src-family kinases (e.g., Src, Yes, Fyn), PLCγ, p120Ras GAP, PI3K, and SOCS. FAK attains peak catalytic activity when bound to and phosphorylated by Src-family kinases. Active FAK-Src can then phosphorylate p130Cas, which influences RAC1 activation and nucleation of F-actin, and paxillin, which may stimulate remodeling of the focal adhesion. In addition, by activating GEFs (such as p190), ROCK, Cdc42, and WASP proteins, FAK promotes cytoskeletal fluidity and actinomyosin contraction. When activated, FAK can bind GRB2 and coordinate downstream ERK- (MAPK-) signaling, thereby enhancing the effect of growth factor-related cues. Certain phospho-signals on FAK also mark it for detachment from the focal adhesion, ubiquitination, and subsequent degradation. Thus, FAK regulates focal adhesions, actin assembly, and migration through diverse molecular mechanisms.
There are numerous potential points for modulation of the cellular signaling network in restitution. Growth factor signaling and polyamine signaling “plug” into many of these pathways and potently enhance restitutive behaviors in intestinal epithelial cells. Among the best-described mediators of epithelial cell migration in the growth factor class are the epidermal growth factor (EGF) receptor (R) family ligands EGF and TGF-α, and the antiproliferative peptide TGF-β. Both epidermal growth factor receptor (EGFR) and TGF-β pathways are targeted by microbial and immune signals to promote restitution (described below in Section 29.6 ). Both EGFR and TGF-β can, like FAK, be thought of as signaling “hubs” in restitution. Other growth factors or signaling peptides that promote restitution in the intestinal epithelium include HGF, which enhances both motility and proliferation through its cognate receptor c-Met, various fibroblast growth factor (FGF) family members, VEGF, and GLP-2. Studying the mechanistic basis for growth factor- and polyamine-mediated effects on restitution has helped to define critical intracellular signals that may serve as therapeutic targets to enhance wound healing.
EGF receptor family ligands, including EGF, TGF-α, betacellulin, amphiregulin, neuregulins, and heparin binding EGF-like growth factor (HB-EGF), represent a major growth factor family with profound impact on intestinal epithelial cell restitution and proliferation. These ligands bind to, stimulate dimerization of, and activate members of the ErbB receptor tyrosine kinase family, of which EGFR is the canonical and best-described member. EGFR is activated in epithelial cells immediately after injury. After EGFR ligand binding and autophosphorylation, numerous signaling molecules are rapidly activated. These include adaptor proteins (e.g., Grb2), Src-family kinases, FAK, the PLCγ/PKC cascade, PI 3-kinase, the p38 MAPK cascade, ERK MAP kinases, Akt, NF-κB, and Rac. All of these effectors except ERK MAPK seem to be required for EGF-stimulated migration. The interdependent relationships between these signal messengers have not been fully resolved. Many of them are activated in parallel, but there also might be hierarchical relationships between them. For example, p38 activation is downstream of Src activation but not PI3K or PLC pathways.
An interesting question is how EGFR activity promotes restitution, a proliferation-independent process, even though the EGFR signaling pathway is also strongly associated with proliferation and cell survival. A provocative idea is that although EGF activates a wide variety of signals, each downstream signal (or group of signals) may be linked to a distinct cellular function. Preferential activation of a specific set of signals can dictate the dominant cellular function at a given time. This has been supported in some cell lines but not others. For example, inhibition of p38 kinase after EGF stimulation of leads to an increase in ERK activation, which then increases the proliferative program in the cell, while decreasing the migration rate. We have also observed that EGFR activation regulates a select set of microRNAs, and that different microRNAs control intestinal epithelial migration versus proliferation (unpublished data, C.Y. Liu). Costimulatory growth factors, cytokines, or fatty acids present in the wound bed may bias the downstream signaling cascades toward generating the restitutive phenotype.
A variety of growth factors and cytokines potentiate restitution and many of these do so through convergence on the TGF-β pathway. EGFR activation can cross talk with TGF-β signaling in the context of cell migration. The activation of TGF-β signaling in the wound repair context usually involves upregulated transcription, translation, secretion, and cleavage of TGF-β from intestinal epithelial cells. TGF-β can then activate in an autocrine manner the TGF-βRII and TGF-βRI receptors sequentially. In the canonical pathway, activated receptors signal to Smad proteins to induce transcription of target genes involved in wound healing. When applied to intestinal epithelium, TGF-β strongly increases migration rate but has no effect on cell proliferation, consistent with cellular behaviors in restitution. The exact mechanisms by which TGF-β enhances restitution remain to be elucidated. TGF-β can activate specific MAPK signals. In addition, TGF-β activity can modify the types of integrins expressed at focal adhesions; specific sets of integrins expressed at the cell surface may be optimally adapted for wound healing.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here