Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mucosal immunity refers to immune responses that occur at mucosal sites. The demands on the mucosal immune system are quite distinct from their systemic counterparts. At mucosal sites, the “outside world” is typically separated from the inner world by a single layer of epithelium. The mucosal immune system exists at a number of sites, including the intestinal tract, respiratory tract (especially the upper respiratory tract), urogenital tract, mammary glands, eyes, and ears. Each of these sites encounters a distinctive array of environmental stimuli and has evolved its own set of cell populations. Nevertheless, these different compartments can interact and share some cell populations and together form a common mucosal immune system. This chapter focuses on the intestinal mucosal immune system.
The intestinal mucosa forms the largest compartment of the mucosal immune system and is unique in several aspects. Relative to other mucosal sites, the intestine contains billions to trillions of microorganisms, mainly bacteria. These organisms and their products, along with ingested food, represent an enormous antigenic load that must be tolerated to maintain mucosal homeostasis. This unusual environment and the demands associated with it have resulted in the development of a distinct immune system composed of inductive lymphoid follicles named gut-associated lymphoid tissue (GALT) and effector cells distributed in the epithelium (intra-epithelial lymphocytes, IELs) and in the lamina propria (LP) as mononuclear cells (LPMCs). Collectively, these cells comprise the largest number of cells in the immune system.
The specific characteristics and peculiarities of the mucosal immune cells reflect the unique milieu in which the cells function. To maintain mucosal homeostasis in the intestinal mucosa, one of the most important tasks of the immune system is to differentiate potentially harmful antigens such as pathogenic bacteria and toxins from products that may benefit the body such as molecules derived from food or commensal bacteria. To achieve homeostasis, unusual cell types, immunoglobulins (Igs), and secreted mediators function in a coordinated fashion. In contrast to the systemic immune system, whose focus is to act quickly within seconds of encountering a foreign antigen, the mucosal immune system is poised to respond but is predominantly tolerant, rejecting harmful antigens but allowing beneficial/harmless ones to persist without evoking harmful immune responses such as allergic reactions or inflammation.
Billions of activated plasma cells, memory T cells, memory B cells, macrophages, and dendritic cells reside in the LP, but significant active inflammation is not present. This phenomenon has been called controlled or physiologic inflammation ( Fig. 2.1 ). Importantly, entry of immune cells into the LP and cell activation is antigen driven. Thus germ-free mice have few immune cells in their LP, but within hours to days after colonization with normal intestinal commensals, a massive influx and activation of cells occurs. A similar process begins in human neonates, whose intestine is colonized at birth, initiating the development of the mucosal immune system and stimulating innate and adaptive systemic immunity. The microbiota expands and changes in infancy, resulting in trillions of gut bacteria that are in communication with host cells in every GI organ and have profound effects on the intestinal vascular, nervous, and immune systems. Humans have co-evolved with their microbiota and have developed multiple mechanisms of response, including intestinal epithelial cells and innate, adaptive, and regulatory immune components.
An abnormal composition of gut microbiota has been implicated in multiple metabolic and immune diseases, including IBD. Many, if not most, of the gene variants conferring susceptibility to IBD are linked to cells and functions involved in each of the components that regulate host-microbiota interactions. The microbiota itself is the subject of the next chapter. Despite the persistence of the antigenic drive of the intestinal microbiota, intestinal lymphocyte effector cells fail to develop into aggressive inflammation-producing cells. Bacteria or their products play a role in this persistent state of suspended activation contributing to the control of inflammation in the intestinal mucosa.
Immunoglobulin A, an immunoregulatory antibody. Secretory IgA (SIgA) is the hallmark of mucosal immune responses ( Fig. 2.2 ). IgG is the most abundant isotype in the systemic immune system, but IgA is the most abundant antibody in mucosal secretions. Given the numbers of IgA + plasma cells and the 3-5 grams of IgA produced daily in humans, IgA is the most abundant antibody in the body.
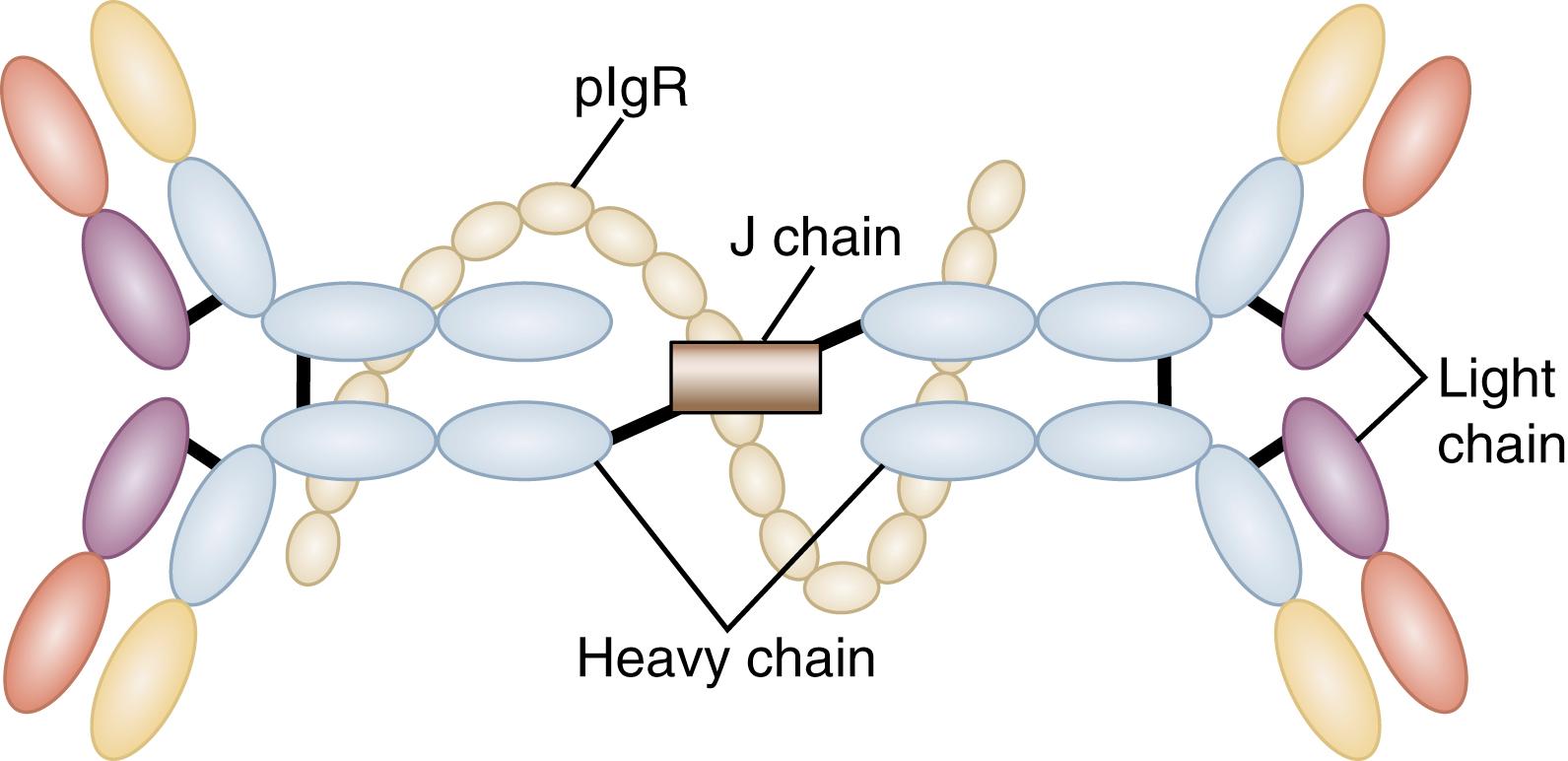
SIgA is a dimeric form of IgA produced by plasma cells in the LP and transported into the gut lumen through the intestinal epithelium by a specialized pathway ( Fig. 2.3 ). Two IgA molecules (homodimers) are bound together by J chain (produced by plasma cells). Subsequently, the homodimer binds to a highly specialized glycoprotein, the polymeric Ig receptor (pIgR), previously called secretory component, a 55-kd glycoprotein produced by epithelial cells. The pIgR is expressed on the basolateral membrane of the intestinal epithelial cell (IEC) and binds only to dimeric IgA or IgM (also polymerized with J chain). Once bound to the pIgR on the IEC, SIgA is actively transported within vesicles to the apical membrane of the IEC. The vesicle fuses with the apical membrane, and the pIgR/IgA complex is released into the intestinal lumen. Within the lumen, pIgR serves to protect the SIgA dimer from degradation by luminal proteases and gastric acid.
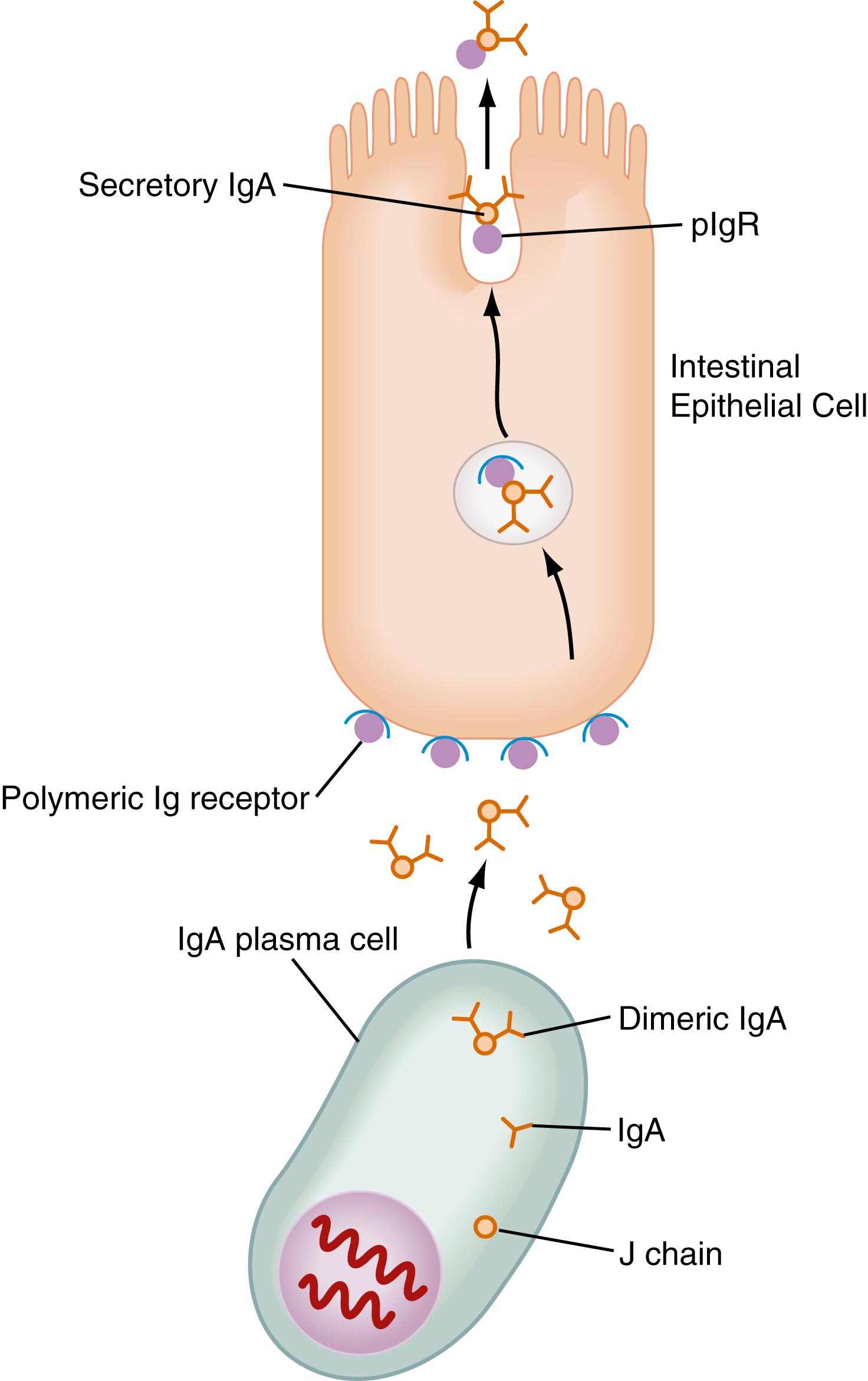
SIgA binds to mucus, enhancing its ability to bind and trap microbial products. In addition to its unique form, SIgA is also unusual in that it is anti-inflammatory in nature. It does not bind classical complement components but rather binds luminal bacterial antigens, preventing their uptake by epithelium and promoting their agglutination and subsequent removal. This process, referred to as “immune exclusion,” includes agglutination, entrapment, and clearance of antigen due to specific interaction with the secreted antibody, as opposed to nonspecific mechanisms of exclusion exerted by the epithelium (e.g., mucus production, proteolytic digestion, defensin secretion). SIgA also may exert specific protective immunity against certain pathogens via more direct mechanisms such as the suppression of bacterial virulence , as well as non-antigen specific binding to bacterial glycan residues on free or bound pIgR, or the SIgA complex. M cells in Peyer patches (discussed later) selectively bind SIgA and SIgA immune complexes, and uptake of antigen-IgA complexes via M cells is a potential mechanism to dampen local inflammatory responses.
IgM is another antibody capable of binding the pIgR. Like IgA, IgM uses J chain produced by plasma cells to form polymers—in the case of IgM, a pentamer. pIgR binds to the Fc portion of the antibody formed during polymerization. The ability of IgM to bind pIgR may be important in patients with IgA deficiency, where secretory IgM (SIgM) compensates for the absence of IgA in the lumen.
SIgA is the major antibody isotype produced in the mucosa, but IgG is present in the mucosa as well. The neonatal Fc receptor (FcR N ) expressed by IECs can serve as a bidirectional transporter of IgG and is important in control of certain neonatal infections and IgG metabolism. In patients with IBD, marked increases in IgG within the LP and lumen have been detected.
IgE production may play an important role in the intestinal response to helminths and in food allergy (see Chapter 10 ). CD23 (low-affinity IgE Fc receptor) has been reported to be expressed by gut epithelial cells, and one model has suggested that it may facilitate antigen uptake and resultant mast cell degranulation in food allergy. In this condition, IgE transcytosis and mast cell degranulation may be associated with fluid and electrolyte loss into the lumen, an event intimately associated with an allergic reaction in the lung and gut.
The cells, structures, and mediators that separate the intestinal lumen from the LP function as a physical barrier, which constantly interacts with its ever-changing luminal environment. The intestinal barrier changes not only on a day-to-day basis but also through the years. Many barrier mechanisms are not fully developed at birth, and evidence in animal studies supports increased antigen transport in neonates compared to adults.
A mucus coat, produced by goblet cells, lines the intestinal tract and is composed of a mixture of glycoproteins (mucins) heavily glycosylated with O -linked oligosaccharides and N -glycan chains linked to a protein backbone. At least 21 different mucin genes, encoding secreted and membrane bound mucins, each with a distinct carbohydrate and amino acid composition, have been identified in the human genome. The major colonic mucins are MUC1, MUC2, MUC3A, MUC3B, MUC4, MUC13, and MUC17. MUC2 is a secreted mucin and serves as the primary component of intestinal mucus, whereas the other mucins are membrane bound. The membrane-bound mucins participate in processes such as cell signaling, adhesion, growth, and immune modulation. Mucus protects the intestinal epithelium by several mechanisms, including its stickiness and competitive binding of its glycoprotein receptors to decrease microorganism penetration. In the colon there are two mucus layers, a loose outer layer containing bacteria and a dense inner layer devoid of bacteria . The mucus stream also moves luminal contents away from epithelial cells. Further, intestinal infection and inflammation are associated with disruption or dysfunction of the mucus barrier, which may be accompanied by altered innate and adaptive host immune responses to the microbiota.
Below the mucus layer, the epithelium acts as a physical barrier that normally prevents antigen penetration through epithelial cells (the transcellular route) and through intercellular spaces (the paracellular route), the latter regulated by tight junction (TJ) complexes (e.g., zona occludens) and the subjunctional space. Tight junctions have the greater role in preventing macromolecular diffusion across the epithelium, because these junctions exclude almost all molecules present in the lumen. The barrier formed by the TJ is a dynamic structure that may be modified by various cytokines and growth factors. The cytokines IFN-γ, TNF-α, IL-1β, IL-4, IL-6, and IL-13 increase intestinal TJ permeability, whereas IL-10, IL-17, and TGF-β decrease intestinal TJ permeability, a feature that may limit intestinal inflammation such as that in IBD.
Several key features of the mucosal immune system facilitate the maintenance of homeostasis and clearance of pathogens. The mucosal immune system is comprised of inductive and effector compartments. Peyer patches and other lymphoid follicles comprise the inductive compartment and are known as GALT. Lymphoid cells in the epithelium (IELs) and the LP (LPLs) are innate and antigen-experienced memory cells and comprise the effector compartment. Cell populations and the immune response in the epithelium, subepithelial region, LP, Peyer patches, and mesenteric lymph nodes (MLNs) may differ substantially. Cells residing in these compartments differ not only topographically but also phenotypically and functionally.
The follicle-associated epithelium (FAE) is a specialized epithelium overlying the Peyer patch and isolated lymphoid follicles. The M (microfold) cells in the FAE, in contrast to the adjacent absorptive epithelium, have few microvilli, a limited mucin overlayer, a thin elongated cytoplasm, and a shape that forms a pocket surrounding subepithelial lymphocytes, macrophages, T cells, B cells, and dendritic cells (DCs) ( Fig. 2.4 ). M cells are highly specialized for phagocytosis and transcytosis and are capable of taking up large particulate antigens from the lumen and transporting them intact into the subepithelial space. These cells contain few lysosomes, and thus little or no processing of antigen occurs. The apical surface of M cells expresses several unique lectin-like molecules that help promote binding to specific pathogens such as poliovirus. Antigens that bind to the M cell and are transported to the underlying Peyer patches generally elicit a positive (SIgA) response. Thus M cells appear to be critical for the initial positive aspects of mucosal immunity. However, certain pathogens or their toxins may exploit M cells and use M cell transcytosis to penetrate the intestinal mucosa.
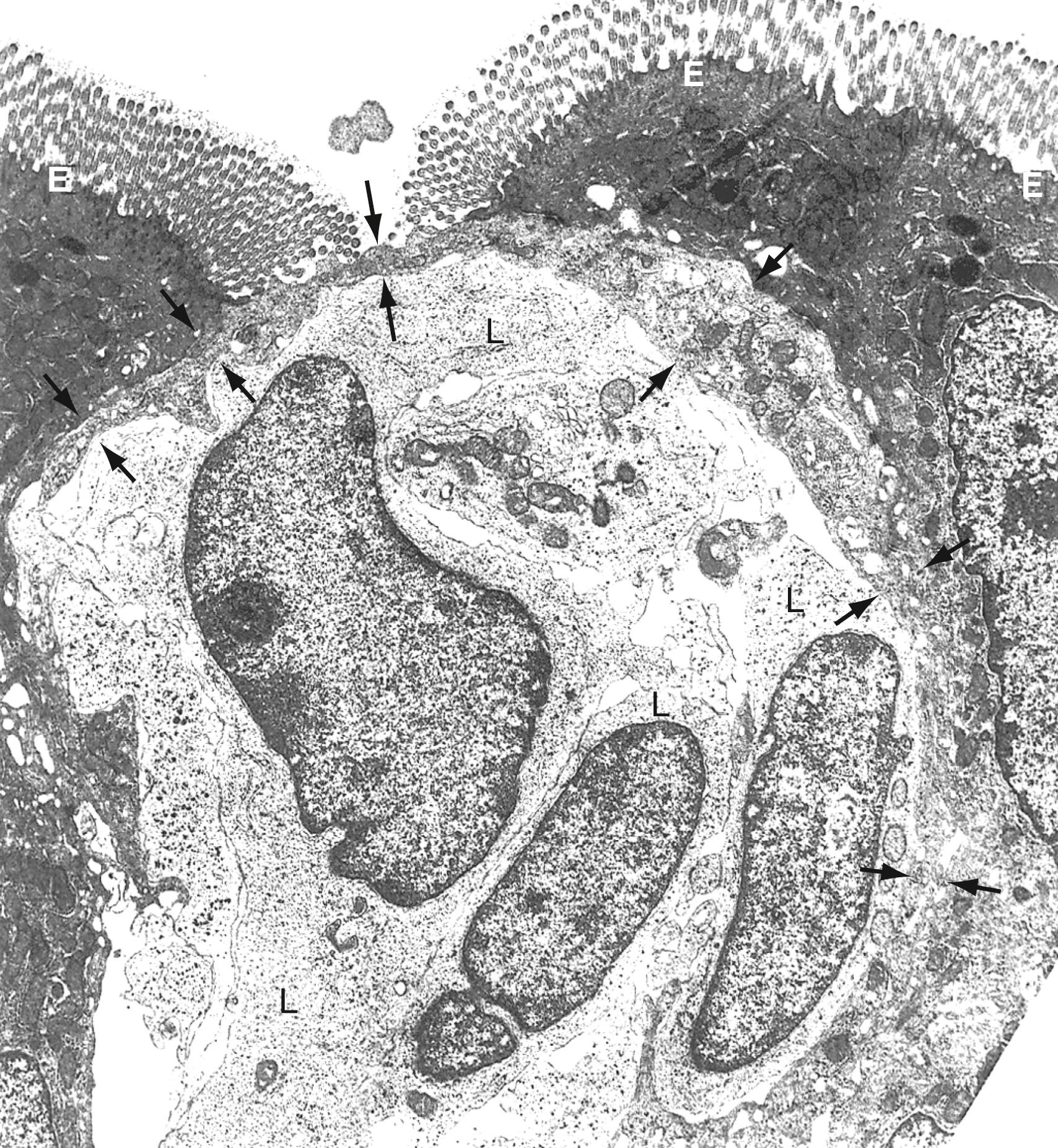
The M cell is a conduit to Peyer patches and lymphoid follicles. Antigens transcytosed across the M cell and into the sub-epithelial pocket are taken up by macrophages and DCs and carried into the Peyer patch. Once antigens enter the Peyer patch, TGF-β-secreting T cells promote B cell isotype switching to IgA. Induction of M cell differentiation is dependent on direct contact between the epithelium and Peyer patch lymphocytes, mediated, at least in part, by the expression of NOTCH receptors and ligands. In the absence of Peyer patch B cells, M cells are not present, as M cells have not been identified in B cell–deficient animals, which lack Peyer patches. The Peyer patches have T cell-dependent areas and B cell-dependent/germinal centers typical of lymph nodes, but only efferent lymphatics.
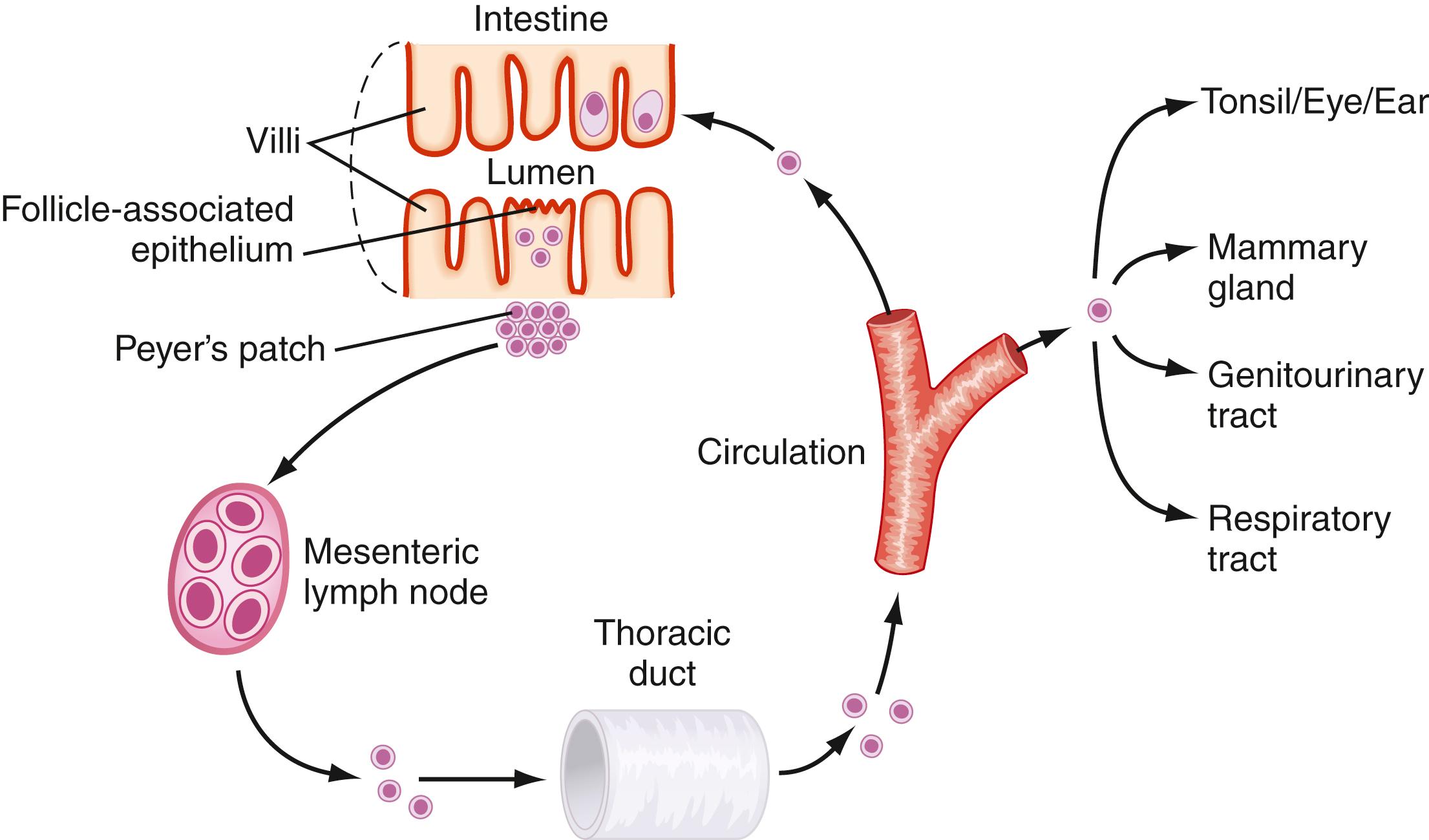
After activation in the Peyer patch, lymphocytes are induced to express specific integrins (α4β7) that provide a homing signal for mucosal sites where the endothelial ligand is MadCAM-1. Lymphocytes exit the Peyer patch, traffic to the MLN and then the thoracic duct, into the main intestinal lymphatic drainage system, which empties into the circulation ( Fig. 2.5 ). There, mucosally activated cells with their mucosal “addressins” circulate in the bloodstream to exit in high endothelial venules in various mucosal sites. Cells bearing α4β7 molecules exit in the LP, where they undergo terminal differentiation. Chemokines and their receptors (discussed later) as well as adhesion molecules and ligands help direct this trafficking pattern.
Intestinal epithelium is composed of a single layer of columnar cells. These IECs (enterocytes) are derived from the stem cell zone at the base of crypts, from which they migrate up the villus and differentiate into absorptive epithelium or secretory cells, including goblet cells, enteroendocrine cells, Paneth cells, and tuft cells. In addition to their function as a physical barrier, IECs contribute to both innate and adaptive immunity in the gut mucosa and play a key role in maintaining intestinal homeostasis.
The absorptive epithelial cells maintain a physical barrier via their tight junctions, which are comprised of claudins, zonal occludens, other occludens, and junctional adhesion molecules. Tight junctions seal the space between epithelial cells and dynamically regulate solute transport. Some DCs can express tight junction proteins and can extend processes between epithelial cells to sample antigens in the lumen. As shown in Figure 2.6 , IECs communicate with immune cells, particularly IELs, and produce various chemokines that regulate cell trafficking into the mucosa, as discussed below.
![Fig. 2.6, A normal intestinal epithelial cell (IEC). The IEC is shown to express classic MHC molecules (classes I and II) that have the potential to present conventional antigen to local T cell populations and a broad array of nonclassic class I molecules (e.g., CD1d, MICA/MICB, and β2m [shown in the figure] and MR-1, ULBP, and HLA-E), which have the potential to present unconventional antigens to unique T cell populations. In addition, alternate pathways of activation appear to be functional in the intestine (e.g., activation via a CD58-CD2 interaction), and classic co-stimulatory molecules are not expressed on IECs, although CD86 may be induced in patients with UC. Other members of the B7 family are expressed, such as PO-L1 (CD274) and ICOS-L (CD275), and may play a role in local T cell activation. β2 microglobin (β2m) associates with MHC class I, CD1d, HLA-E, HLA-G, and FcRn. β2m, β2 microglobulin; gp180, membrane glycoprotein 180 (a CD8 ligand); IEL, intraepithelial lymphocyte; LPL, lamina propria lymphocyte; MHC, major histocompatibility complex; MICA/MICB, MHC class I-related chains A and B; TCR, T cell receptor. Fig. 2.6, A normal intestinal epithelial cell (IEC). The IEC is shown to express classic MHC molecules (classes I and II) that have the potential to present conventional antigen to local T cell populations and a broad array of nonclassic class I molecules (e.g., CD1d, MICA/MICB, and β2m [shown in the figure] and MR-1, ULBP, and HLA-E), which have the potential to present unconventional antigens to unique T cell populations. In addition, alternate pathways of activation appear to be functional in the intestine (e.g., activation via a CD58-CD2 interaction), and classic co-stimulatory molecules are not expressed on IECs, although CD86 may be induced in patients with UC. Other members of the B7 family are expressed, such as PO-L1 (CD274) and ICOS-L (CD275), and may play a role in local T cell activation. β2 microglobin (β2m) associates with MHC class I, CD1d, HLA-E, HLA-G, and FcRn. β2m, β2 microglobulin; gp180, membrane glycoprotein 180 (a CD8 ligand); IEL, intraepithelial lymphocyte; LPL, lamina propria lymphocyte; MHC, major histocompatibility complex; MICA/MICB, MHC class I-related chains A and B; TCR, T cell receptor.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MucosalImmunologyandInflammation/4_3s20B9780323609623000023.jpg)
Paneth cells are a secretory population derived from epithelial stem cells and reside in the base of the crypts in the small intestine. These cells can be readily identified on stains due to the large eosinophilic granules that occupy most of their cytoplasm (see Chapter 98 ). These granules contain high concentrations of antimicrobial peptides, including α-defensins, lysozyme, C-type lectins, and phospholipase-A2. They secrete these antimicrobial peptides into the lumen of the crypt, thus keeping it relatively sterile. Defensins are released upon stimulation by various bacterial ligands, including endotoxin, which induce Paneth cells via Toll-like receptors (TLRs) and nuclear oligomerization domain 2 (NOD2). Paneth cells co-localize with stem cells at the base of crypts and have been shown to provide important factors preserving stem cell function in addition to the antimicrobial peptides. Paneth cell development is regulated by transcription factors in the WNT pathway and the NOD signaling cascade such as the transcription factor MATH-1. A transcriptional repressor, GFI-1, is important in Paneth cell development by suppressing a pro-enteroendocrine cell transcription factor, neurogenin-3 (NEUROG3). Decreased numbers of Paneth cells and of defensins 5 and 6 are associated with ileal Crohn disease. Because of their highly secretory nature with strong synthesis of various molecules, Paneth cells are dependent on the unfolded protein response to maintain intracellular homeostasis. Interference with the unfolded protein response in mice has led to ileitis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here