Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Patients with severe cardiovascular disease and those undergoing surgery associated with rapid hemodynamic changes should be adequately monitored at all times.
Standard monitoring for cardiac surgery patients includes invasive blood pressure, electrocardiography, central venous pressure, urine output, temperature, capnometry, pulse oximetry, and intermittent blood gas analysis.
Additional monitoring is based on specific patient, surgical, and environmental factors.
The Society of Cardiovascular Anesthesiologists and the American Society of Echocardiography have published recommendations for intraoperative transesophageal echocardiography (TEE). TEE is recommended for all patients undergoing cardiac surgery, unless contraindications to probe insertion apply.
Ultrasound-guided vascular access is now routinely practiced in many institutions.
The use of pulmonary artery catheters (PACs) has been steadily declining. Guidelines for PAC use have been published. Many practitioners still use PACs to guide treatment in patients with low cardiac output or pulmonary arterial hypertension.
The use of additional highly invasive monitoring techniques, such as coronary sinus pressures and cerebrospinal fluid pressures, are restricted to very specific indications.
The availability of monitoring devices is increasing continually. These devices range from those that are completely noninvasive to those that are highly invasive, such as the pulmonary artery catheter (PAC). Limitations to less invasive monitoring technologies often apply, and interventions based on information gained from noninvasive monitoring carry intrinsic risks. To make the best use of any monitoring technology, the potential benefits to be gained from the information must outweigh the potential complications. This risk-benefit ratio is highly variable and must be evaluated for each clinical scenario individually. Although outcome changes are difficult to prove, the assumption that appropriate hemodynamic monitoring should reduce the incidence of major cardiovascular complications is reasonable. This is based on the presumption that the data obtained from these monitors are interpreted correctly and that therapeutic interventions known to improve outcomes are implemented in a timely fashion.
Standard monitoring for all patients undergoing surgery has been defined by the American Society of Anesthesiologists (ASA) practice guidelines. In patients undergoing cardiac or major noncardiac surgery with expected large fluid shifts or hemodynamic instability, invasive blood pressure (BP) monitoring is nearly universally employed, which also enables frequent arterial blood sampling. Transesophageal echocardiography (TEE), a less invasive technology, provides extensive hemodynamic data and other diagnostic information. The Society of Cardiovascular Anesthesiologists and the American Society of Echocardiography have published recommendations for intraoperative TEE use. Unless contraindications to probe insertion apply, TEE is now recommended for all patients undergoing cardiac surgery. Box 10.1 summarizes monitoring typically used in cardiac surgeries.
(Invasive) blood pressure
Electrocardiogram
Pulse oximetry
Capnometry
Temperature
Central venous pressure
Transesophageal echocardiography
Urine output
Intermittent arterial blood sampling for blood gas and laboratory analyses
Neuromonitoring (cerebral oximetry, processed electroencephalography)
The next tier of monitoring is typically more invasive, including PACs with thermodilution cardiac output (CO). The interpretation of these complex data requires an astute clinician who is aware of the patient's overall condition and the limitations of the monitors. Additionally, with the expansion of less invasive surgical techniques, the anesthesiologist is getting more involved in guiding cardiopulmonary bypass (CPB) cannulation and adequacy of cardioprotection techniques. This includes retrograde cardioplegia cannula positioning in the coronary sinus (CS) and pressure monitoring. Advanced monitoring is summarized in Box 10.2 .
Retrograde cardioplegia pressure
Pulmonary artery catheter
Cardiac output measurements
Left atrial pressure
Cerebrospinal fluid (intrathecal) pressure
Anesthesia for cardiac and major noncardiac surgeries is frequently complicated by rapid and sudden changes in BP. Sudden losses of large amounts of blood, direct compression of the heart, impaired venous return attributable to retraction and cannulation of the vena cavae and aorta, arrhythmias, and manipulations that may impair right ventricular outflow and pulmonary venous return all contribute to hemodynamic instability. Therefore a safe and reliable method of measuring acute changes in BP is indispensable. Direct intraarterial monitoring remains the gold standard, providing a continuous, beat-to-beat indication of the arterial pressure and waveform and allowing frequent sampling of arterial blood for laboratory analyses.
The magnitude of BP is directly related to CO and systemic vascular resistance (SVR). This is conceptually similar to Ohm's law of electricity (voltage = current × resistance), in which BP is analogous to voltage, CO to current flow, and SVR to resistance. An increase in BP may reflect an increase in CO or SVR, or both.
Mean arterial pressure (MAP) is probably the most useful parameter when assessing overall end-organ perfusion. MAP is measured directly by integrating the arterial waveform tracing over time or using the formula: MAP = (SBP + [2 × DBP]) ÷ 3 (where SBP is systolic blood pressure and DBP is diastolic blood pressure). Perfusion of the heart differs from most other organs, with coronary perfusion of the left ventricle mostly occurring during diastole. Coronary blood flow to the normal right ventricle (RV) is maintained during systole and diastole.
Factors that influence the site of arterial cannulation include the location of surgery, the possible compromise of arterial flow attributable to patient positioning or surgical manipulations, CPB cannulation and perfusion techniques, and any history of ischemia or prior surgery on the limb to be cannulated. Monitoring arterial BP at two or more sites may be warranted in complex cases with complex perfusion techniques.
Temporary central aortic pressure monitoring can be achieved by using a needle (attached to pressure tubing) that is placed in the aorta or by pressure tubing connected to the aortic CPB cannula or the anterograde cardioplegia cannula. Central aortic monitoring is usually only necessary for several minutes until the problem resolves; in rare cases, a femoral arterial cannula is placed from the surgical field.
The radial artery is the most commonly used artery for continuous BP monitoring because it is easy to cannulate, readily accessible during surgery, and the collateral circulation is usually adequate and easy to check. The ulnar artery provides most of the blood flow to the hand in approximately 90% of patients. The radial and ulnar arteries are connected by a palmar arch, which provides collateral flow to the hand in the event of radial artery occlusion. Some clinicians perform the Allen test before radial artery cannulation to assess the adequacy of collateral circulation to the hand; however, the predictive value of the Allen test has been challenged.
The brachial artery lies medial to the bicipital tendon in the antecubital fossa in close proximity to the median nerve. Brachial artery pressure tracings resemble those in the femoral artery with less systolic augmentation than radial artery tracings. Brachial arterial pressures were found to reflect central aortic pressures more accurately than radial arterial pressures before and after CPB. A few series of patients with perioperative brachial arterial monitoring have documented the relative safety of this technique.
The femoral artery may be cannulated for monitoring purposes and typically provides a more reliable central arterial pressure after discontinuation of CPB. In patients undergoing thoracic aortic surgery, distal aortic perfusion (using partial CPB, left-sided heart bypass, or a heparinized shunt) may be performed during aortic cross-clamping to preserve spinal cord and visceral organ blood flow. In these situations, measuring the distal aortic pressure at the femoral artery or a branch vessel is useful (ie, dorsalis pedis or posterior tibial artery) to optimize the distal perfusion pressure. Consulting the surgeon before cannulating the femoral vessels is necessary, because these vessels may be used for extracorporeal perfusion or placement of an intraaortic balloon pump during the surgical procedure.
The indications for invasive arterial monitoring are provided in Box 10.3 .
Major surgical procedures involving large fluid shifts or blood loss
Surgery requiring cardiopulmonary bypass
Surgery of the aorta
Patients with pulmonary disease requiring frequent arterial blood gases
Patients with recent myocardial infarctions, unstable angina, or severe coronary artery disease
Patients with decreased left ventricular function (congestive heart failure) or significant valvular heart disease
Patients in hypovolemic, cardiogenic, or septic shock, or with multiple organ failure
Procedures involving the use of prolonged deliberate hypotension or deliberate hypothermia
Massive trauma cases
Patients with right-sided heart failure, chronic obstructive pulmonary disease, pulmonary hypertension, or pulmonary embolism
Patients requiring inotropes or intraaortic balloon counterpulsation
Patients with electrolyte or metabolic disturbances requiring frequent blood samples
Inability to measure arterial pressure noninvasively (eg, extreme morbid obesity)
Proper technique is helpful in obtaining a high degree of success in arterial catheterization. The wrist is often placed in a dorsiflexed position on an armboard over a pack of gauze and immobilized in a supinated position. Overextension of the wrist should be avoided, since this flattens and decreases the cross-sectional area of the radial artery and may cause median nerve damage by stretching the nerve over the wrist. When the artery is entered, the angle between the needle and skin is reduced to 10 degrees, the needle is advanced another 1 to 2 mm to ensure that the tip of the catheter also lies within the lumen of the vessel, and the outer catheter is then threaded off the needle. If blood ceases flowing while the needle is being advanced, then the needle has penetrated the back wall of the vessel.
Alternatively, the artery can be transfixed by the passage of the catheter-over-needle assembly “through-and-through” the artery. The needle is then completely withdrawn. As the catheter is slowly withdrawn, pulsatile blood flow emerges from the catheter when its tip is within the lumen of the artery. At this point the catheter can either be advanced in the lumen of the artery or a guidewire advanced into the lumen first, followed by advancing the catheter over the wire (modified Seldinger technique). Compared with a direct cannulation method, using the Seldinger technique increases the success rate of arterial catheter placement.
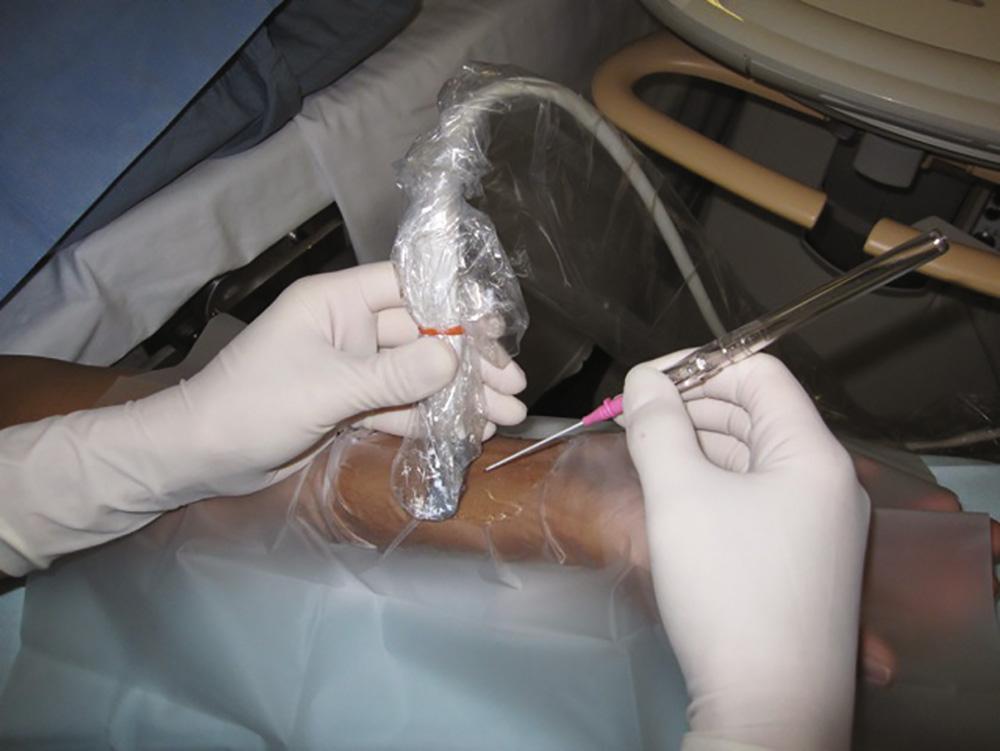
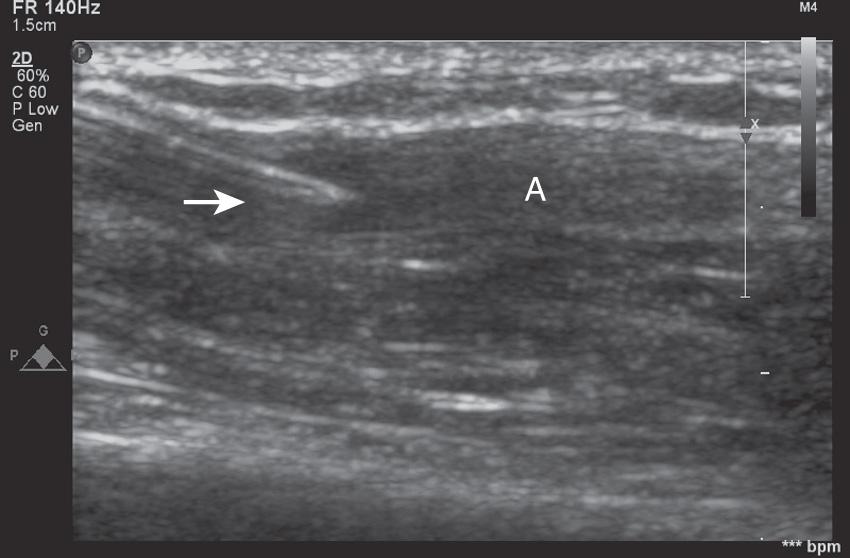
An ultrasound-guided (UG) technique is probably most useful in patients with severe peripheral vasculopathy, as well as in infants and small children. The use of ultrasound in guiding arterial catheter placement is easy to learn when proper training in this technique is provided. There is, however, a significant learning curve. Fig. 10.1 shows a proper full-sterile set up for UG arterial cannulation. Fig. 10.2 demonstrates the “triangulation” technique typically applied with UG arterial cannulation. The ultrasound imaging plane and the needle plane can be viewed as the two sides of a triangle that should meet and intersect at the depth of the structure (eg, radial artery) for which cannulation is attempted. The experienced operator will choose the distance (needle insertion site vs imaging plane) and insertion angle, depending on the depth of the target vessel. After perforating the skin, the ultrasound plane and the needle insertion angle both have to be adjusted further to follow the needle tip when viewed in the transverse (short-axis) approach. Failure to align the ultrasound plane accurately with the needle tip results in viewing the needle shaft instead. Fig. 10.3 shows a typical ultrasound image obtained during short-axis (transverse) cannulation. After puncturing the vessel, the catheter can be advanced into the lumen. A significantly higher success rate can usually be achieved using the through-and-through and modified Seldinger techniques.
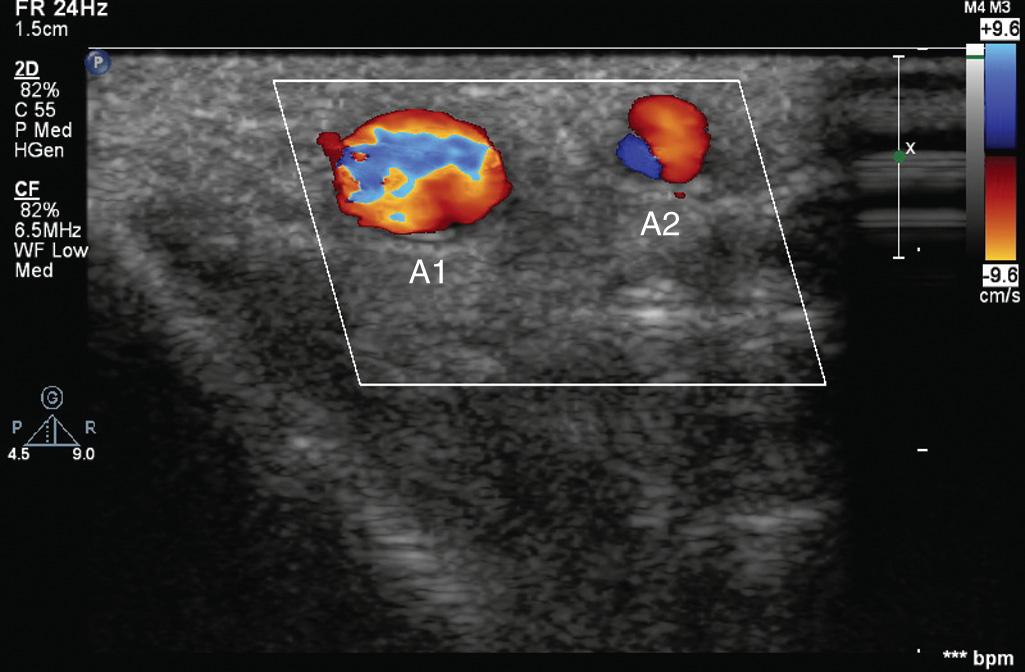
![Fig. 10.2, Demonstration of the “triangulation” technique typically applied with ultrasound-guided (UG) venous and/or arterial cannulation in the transverse imaging approach. The echo imaging plane and the needle plane can be viewed as the two sides of a triangle that should meet and intersect at the depth of the structure (eg, radial artery [red line] ) for which cannulation is attempted. The experienced operator will change the angle (α) between the two planes (ultrasound and needle) and the distance (needle insertion site vs imaging plane), depending on the depth of the structure. To follow the needle tip in the transverse approach (vessel viewed in short axis), the echo plane or needle insertion angle has to be further adjusted from needle entry through the skin to the perforation of the vessel. A greater angle is used (echo plane angled toward the skin [1] ) to visualize the needle tip after it penetrates the skin, and then a more perpendicular angle relative to the skin is applied to see the needle tip entering the vessel lumen ( 2 ). Fig. 10.2, Demonstration of the “triangulation” technique typically applied with ultrasound-guided (UG) venous and/or arterial cannulation in the transverse imaging approach. The echo imaging plane and the needle plane can be viewed as the two sides of a triangle that should meet and intersect at the depth of the structure (eg, radial artery [red line] ) for which cannulation is attempted. The experienced operator will change the angle (α) between the two planes (ultrasound and needle) and the distance (needle insertion site vs imaging plane), depending on the depth of the structure. To follow the needle tip in the transverse approach (vessel viewed in short axis), the echo plane or needle insertion angle has to be further adjusted from needle entry through the skin to the perforation of the vessel. A greater angle is used (echo plane angled toward the skin [1] ) to visualize the needle tip after it penetrates the skin, and then a more perpendicular angle relative to the skin is applied to see the needle tip entering the vessel lumen ( 2 ).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MonitoringoftheHeartandVascularSystem/1_3s20B9780323497985000103.jpg)
If a longitudinal (“in-plane”) approach is chosen (ie, the vessel is viewed in its long axis), the needle tip can be followed more easily as it is advanced; however, structures adjacent to the ultrasound plane (lateral to the vessel) cannot be viewed simultaneously. Exactly aligning the needle and vessel axis together in a 2D echo plane, particularly with a tortuous atherosclerotic artery, is technically more difficult. Fig. 10.4 shows the arterial catheter entering the radial artery using the longitudinal (in-plane) approach. A high-frequency linear array ultrasonic transducer (8 to 12 MHz) is optimal for UG arterial catheter placement, since higher frequencies are needed for high-resolution imaging of the near field. Box 10.4 summarizes the potential benefits and concerns related to UG arterial catheter placement.
Higher success rate on first attempt
Fewer overall attempts
Increased patient comfort (fewer attempts)
Fewer complications (eg, anticoagulated patients)
Demonstration of vessel patency, anatomic variants
Low pulsatile or nonpulsatile flow (eg, nonpulsatile assist devices, extracorporeal membrane oxygenation, shock)
Nonpalpable or weakly palpable pulses (eg, peripheral edema, hematoma)
Emergency access (eg, catheter placement during resuscitation)
Risk of catheter-related infections if poor aseptic technique is applied
Additional training required
Costs involved with equipment required
Central venous pressure (CVP) catheters are used to measure the filling pressure of the RV, give an estimate of the intravascular volume status, assess right ventricular function, and serve as a site for volume or drug infusions. For accurate pressure measurement, the distal end of the catheter must lie within one of the large intrathoracic veins or the right atrium (RA). As in any pressure monitoring system, having a reproducible landmark, such as the midaxillary line with a closed chest or the left atrium (LA) during surgery, as a zero reference is necessary. Frequent changes in patient positioning without proper leveling of the transducers relative to the heart produce proportionately larger errors compared with arterial pressure monitoring.
The normal CVP waveform consists of three upward deflections (A, C, and V waves) and two downward deflections (X and Y descents). The A wave is produced by right atrial contraction and occurs just after the P wave on the electrocardiogram (ECG). The C wave occurs because of the isovolumic ventricular contraction, forcing the tricuspid valve (TV) to bulge upward into the RA. The pressure within the RA then decreases as the TV is pulled away from the atrium during right ventricular ejection, forming the X descent. Right atrial filling continues during late ventricular systole, forming the V wave. The Y descent occurs when the TV opens and blood from the RA empties rapidly into the RV during early diastole. The CVP waveform may be useful in the diagnosis of pathologic cardiac conditions. For example, the onset of an irregular rhythm and loss of the A wave suggest atrial flutter or fibrillation. Cannon A waves occur as the RA contracts against a closed TV, as occurs in junctional (atrioventricular [AV] nodal) rhythm, complete heart block, and ventricular arrhythmias. This occurrence is clinically relevant because nodal rhythms are frequently seen during anesthesia and may produce hypotension attributable to a decrease in stroke volume (SV).
The CVP is a useful monitor if the factors affecting it are recognized and its limitations are understood. Thromboses of the vena cavae and alterations of intrathoracic pressure, such as those induced by positive end-expiratory pressure (PEEP), also affect measurement of the CVP. The correlation with left-sided heart filling pressures and assessment of left ventricular preload is poor. Clinically, following serial measurements (trends) rather than individual numbers is often more relevant. The response of the CVP to a volume infusion, however, is a useful test.
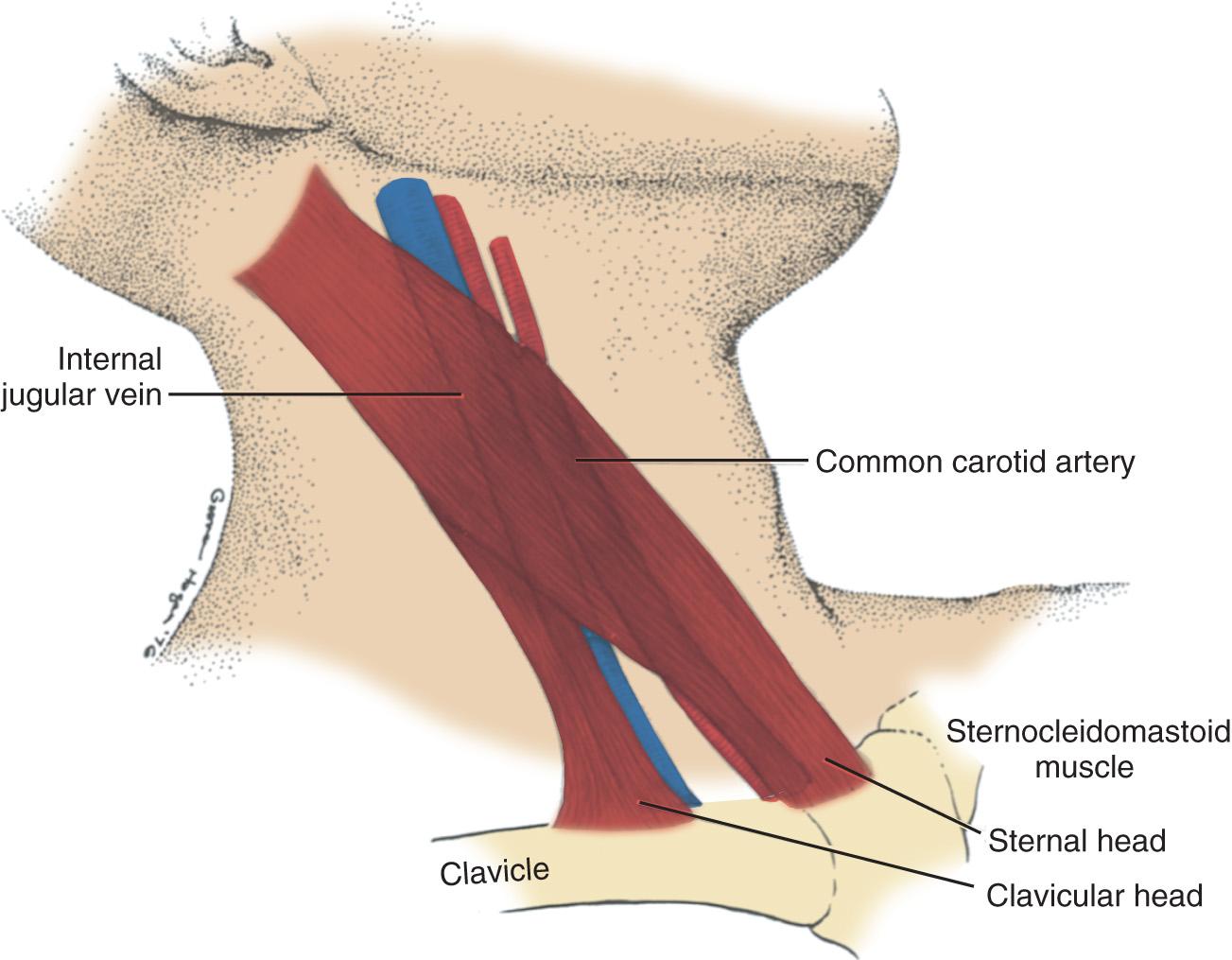
Cannulation of the internal jugular vein (IJV) has multiple advantages, including the high success rate as a result of the relatively predictable relationship of the anatomic structures: a short, straight course to the RA that almost always ensures RA or superior vena cava (SVC) localization of the catheter tip; and easy access from the head of the surgical table. The IJV is located under the medial border of the lateral head of the sternocleidomastoid (SCM) muscle ( Fig. 10.5 ). The carotid artery is usually deep and medial to the IJV; however, this spatial relationship can vary, and puncture of the carotid artery is best avoided by using an UG technique. The right IJV is preferred, because this vein takes the straightest course into the SVC, the right cupola of the lung may be lower than the left, and the thoracic duct is on the left side.
The middle approach to the right IJV is shown in Fig. 10.6 . The Trendelenburg position is chosen to distend the IJV. The head is then turned toward the contralateral side, and the fingers of the left hand are used to palpate the two heads of the SCM muscle and the carotid pulse. The needle is inserted slightly lateral to the carotid pulse at a 45-degree angle to the skin and directed toward the ipsilateral nipple until venous blood return is obtained. Alternatively, the use of a small-gauge finder needle can be used to avoid carotid puncture with a large-bore needle. When venous return is present, the whole assembly is lowered to prevent the needle from going through the posterior wall of the central vein and advanced an additional 1 to 2 mm until the tip of the catheter is within the lumen of the vein. Aspiration of blood must be confirmed before the catheter is then threaded into the vein. It is recommended by the ASA practice guidelines, and often mandated by institutional protocols, that the correct intravenous catheter position be confirmed before placing a large-bore introducer sheath. Various techniques have been suggested. The small-bore catheter can be attached to a transducer by sterile tubing to observe the pressure waveform. Another option is to attach the cannula to sterile tubing and allow blood to flow retrograde into the tubing. The tubing is then held upright as a venous manometer, and the height of the blood column is observed. If the catheter is in a vein, then it will stop rising at a level consistent with the CVP and demonstrate respiratory variation. Despite its reported use in the past, color comparison and observation of nonpulsatile flow are notoriously inaccurate methods of determining that the catheter is not in the carotid artery. A guidewire is then passed through the 18-gauge catheter, and the catheter is exchanged for the wire. With the more widespread use of echocardiography, the correct intravenous position can also be confirmed by following the Seldinger wire along its course in the IJV more distally by handheld transcutaneous probes or demonstrated within the RA if the TEE probe was inserted before IJV cannulation. The use of more than one technique to confirm the venous location of the guidewire may provide additional reassurance of correct placement before cannulation of the vein with a larger catheter or introducer. Once it is certain that the guidewire is in the venous circulation, the CVP catheter is passed over it and the wire is removed.
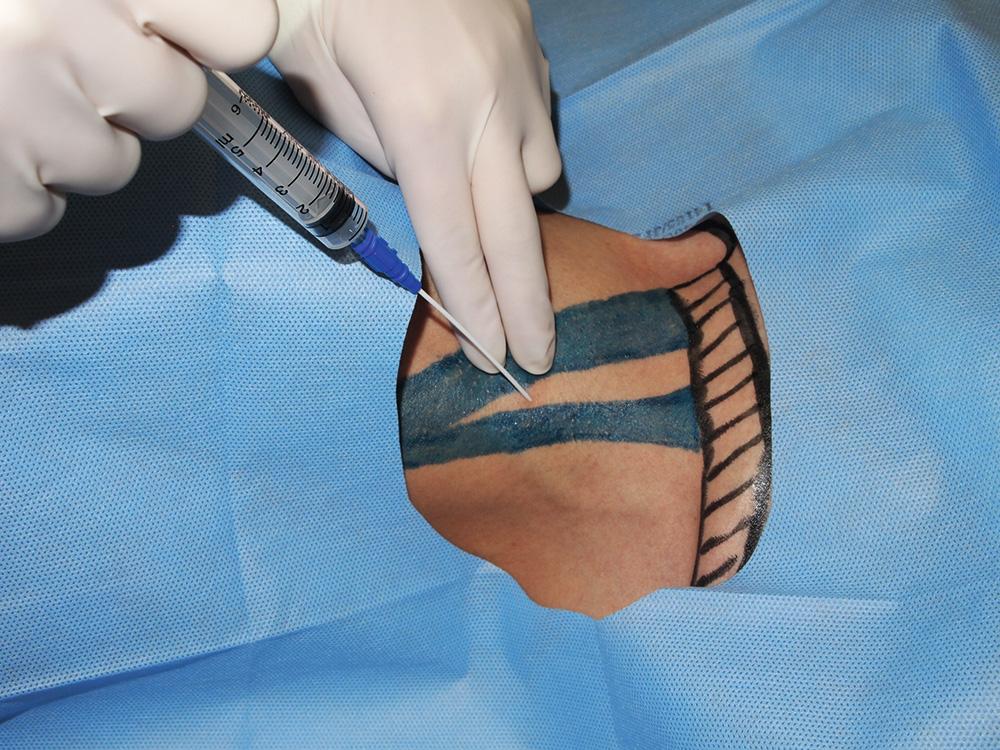
Ultrasound has been increasingly used for central venous access, in particular to guide IJV cannulation and to define the anatomic variations of the IJV. Using ultrasound to guide central venous cannulation increases the success rate and helps prevent complications and thus may ultimately help improve patient outcomes. Most studies have demonstrated that 2D UG IJV cannulation has a higher success rate on the first attempt and fewer complications. Those findings also were confirmed in pediatric patients.
Box 10.5 lists some of the recognized benefits and concerns of UG central venous cannulation. Circumstances in which ultrasound guidance of IJV cannulation can be particularly advantageous include patients with difficult neck anatomy (eg, short neck, obesity), prior neck surgery, anticoagulated patients, and infants.
Higher success rate on first attempt
Fewer overall attempts
Facilitates access with difficult neck anatomy (obesity, prior surgery)
Fewer complications (eg, carotid artery puncture, anticoagulated patients)
Demonstration of vessel patency, anatomic variants
Relatively inexpensive technology
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here