Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The ability of epithelial cells in gastrointestinal (GI) track to absorb selected nutrients while secreting electrolytes, enzymes, and digestive factors depends on intracellular trafficking mechanisms that establish and maintain the polarized disruption of different transport proteins and surface receptors on the apical and basolateral membranes. A combination of intracellular sorting operations, vectorial delivery mechanisms, plasmalemma-specific fusion, and retention processes are responsible. Well-defined signals that specify polarized sorting or retention of proteins have been described, and the intracellular machinery that decode and act on these signals are becoming better understood. In this chapter, we provide an up-to-date review of the trafficking routes, molecular machinery, and mechanisms involved in protein sorting and trafficking.
Epithelial cells exhibit tremendous diversity and specialization along the GI tract, yet share many common morphological features. These include a basement membrane, a complex set of cell junctions that demarcate the boundary between the apical and the basolateral membrane, a cytoskeleton with a specific spatial distribution, and a set of membrane compartments that serve as platforms for polarized sorting and delivery of the apical and basolateral membrane proteins. Although a detailed description of these structures exceeds the purpose of this chapter, a brief summary of their distribution and functional significance is highlighted to provide a context for understanding the mechanisms of membrane polarity (see Fig. 45.1 ).
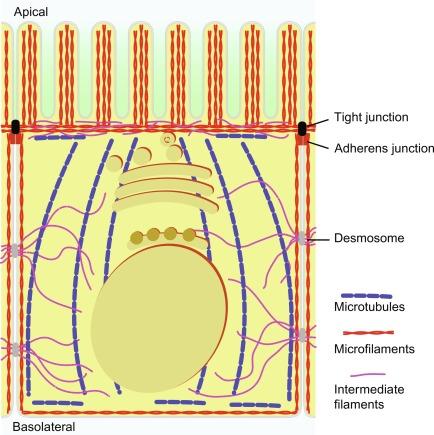
The tight junction complex at the apex of the cell forms a relatively impermeable barrier between the solutions on either side of the epithelium, and acts as a molecular “fence” to partition the contents of the apical and basolateral membranes from each other. . It is formed of integral membrane proteins, such as occludin, the claudins, and the junction adhesion molecule (JAM), which assemble as a network of strands and interact with opposing membranes of adjacent cells through their extracellular domains. A number of regulatory and scaffolding proteins associate with the cytoplasmic surface of the tight junction, including ZO-1, -2, and -3, cingulin, 7H6 and symplekin, providing a means to connect the junction to the actin cytoskeleton. They also allow recruitment of signal transduction proteins and polarity generating complexes, creating de facto signaling platforms. Importantly, components of the exocyst, a multiprotein complex involved in polarized protein trafficking, also associate with or near tight junctions.
The adhesion between epithelial cells is mostly due to the Zonula adherens , a belt-like structure that encircles the cell just below the tight junctions. Cadherins are the main component of the Z. adherens , with their extracellular domains likely mediating a calcium-dependent trans-dimerization process that provides adhesion between neighboring epithelial cells. In all, the apical junctional complexes are dynamic structures that undergo dramatic rearrangement and redistribution during embryonic development, cell migration, and proliferation, closely integrating these processes to an equally dynamic cytoskeleton. The exocyst plays an important role in maintaining adherens junctions. In intestinal epithelia, cholera toxin inhibits exocyst-mediated trafficking of E-cadherin, thus compromising epithelium barrier integrity, which contributes to diarrhea. In addition to the apical junctional complexes, desmosomes, composed of desmosomal cadherins, associate to intermediate filament (IF) attachment proteins and provide additional anchoring points throughout the basolateral membranes, contributing to the maintenance of the epithelium integrity.
The cytoskeleton in epithelial cells is closely associated with both the apical and basolateral membranes, playing important structural and functional roles in protein sorting and trafficking. The building blocks of the cytoskeleton, namely microfilaments (MF), microtubules (MT), and IF, are organized as depicted in Fig. 45.1 (reviewed in Ref. ). The MF are formed by the polymerization of β- and γ-actin monomers, with a fast-growing plus-end (“barbed”) and slow-growing minus end (“pointed”). Multiple MF associate to form filamentous networks, for which some cross-linking proteins such as α-actinin or filamin are required. In addition, other actin-binding proteins control polymerization dynamics, bundling, nucleation, branching, actin-membrane interaction, cell-extracellular matrix (ECM) interaction, contractility, scaffolding, and signaling. MT are composed of α- and β-tubulin monomers that combine to produce αβ-dimers, prior to being added in a head-to-tail fashion to the fast growing plus-end of an existing MT. This intrinsic polarity is of great importance in defining the directionality of their transport capabilities. In epithelial cells (such as Caco-2, MDCK, or enterocytes) MT emanate from multiple noncentrosomal organizing centers (MTOC) located below the apical membrane (pericanalicular region in hepatocytes), and assemble into polarized bundles running along the apical-to-basolateral axis, with their rapidly changing plus-ends projecting toward the basal membrane. However, a group of MT is oriented with their plus-ends toward the apical membrane and appears to play an important role in polarized vesicle transport in MDCK cells (see Section 45.7.1 ). IF in intestinal epithelial cells are composed mostly of keratin types 8, 18, and 19, and have important structural roles.
At the apex of the lateral membranes, running along the cell-adhesion junction, densely packed antiparallel arrays of actin filaments associate to myosin II in order to generate a contractile ring. This structure is closely associated with the apical junctional complexes via E-cadherin interactions, and creates an extensive transcellular network important, for example, in canalicular contraction during bile secretion. Underling the apical membrane, a meshwork of parallel arrays of actin filaments cross-linked by myosin and fodrin, provides a physical structure to anchor secretory vesicles in proximity to the plasma membrane during epithelial cell secretion and exocytosis. A set of randomly organized MT, with their minus end and multiple MTOC embedded in a meshwork of cytokeratins, runs parallel to the apical (and basolateral) domain. Subapical IF also play an important role in maintaining cell integrity in digestive epithelia. Intestinal microvilli emerging from the apical membrane are supported by the apical actin meshwork and contain well-organized actin MF bundled by actin-binding proteins including villin, fimbrin (or plastin-1), and espin. However, the structural role play by actin-binding proteins has been recently questioned by the discovery that knockout mice lacking villin, fimbrin, and espin still produced microvilli, but have deficient retention of apical proteins into microvilli of enterocytes. The basolateral membrane is also lined by a meshwork composed of cross-linked arrays of actin. Focal adhesions connect this actin meshwork to the extra cellular matrix through integrins and their associated cytosolic actin-binding proteins. In addition, in the small intestine, keratin-associated proteins interact with desmosomes to establish cell-cell interactions. MT and their motors (dyneins and kinesins) are important in vesicle trafficking, in addition to organizing and positioning various organelles, such as Golgi, endosomes, and lysosomes. MF also contribute to scaffolding and motility of cytoplasmic components, providing also tracks for the myosin motor proteins involved in vesicle trafficking.
Polarized sorting operations largely occur within the Golgi and endosomes. These membrane organelles have a characteristic subcellular distribution. The Golgi, the chief biosynthetic sorting station, is located in the apical pole in close proximity to the nucleus, with tubular structures of the trans-Golgi network (TGN) projecting further toward to apex of the cell. Between the TGN and the apical membrane, an array of small membrane compartments or endosomes constitutes other key sorting stations. These compartments are generally named after their primary function. Apical early endosomes or basolateral early endosomes (AEEs or BEEs, respectively) are a heterogeneous population of endosomes that localize immediately under their respective membranes, have moderately acid internal pH, and contain the small GTPase Rab5. Common (recycling) endosomes (CEs or CREs), termed also subapical compartment (SAC) in hepatocytes, are marked by the small GTP-binding protein Rab8. In fully differentiated epithelial cells, a subapical cluster of membrane tubules is identified by the presence of Rab11 and named apical recycling endosome (ARE). Finally, late endosomes (LE) contain Rab7. A detailed description of the specific function of each compartment in protein trafficking is provided in Section 45.3 and is summarized in Fig. 45.2 .
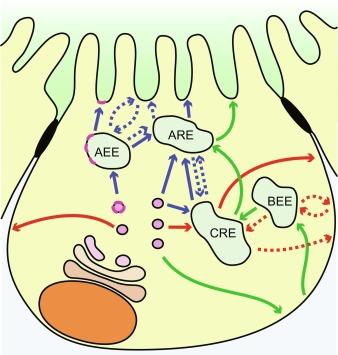
Newly synthesized proteins can travel via many different routes to reach their final apical or basolateral membrane destination. By maintaining multiple polarized trafficking routes, which can overlap with endocytotic sorting and recycling pathways, epithelial cells attain a fast and precise mechanism to regulate physiologically the location and density of cell surface proteins. For sake of simplicity, we first describe the three main biosynthetic pathways used for trafficking from Golgi to the apical and basolateral membranes, followed by a description of the postendocytic pathways. Both types share multiple compartments, as shown in Fig. 45.2 .
Newly synthesized proteins can follow a direct route from the TGN to their final apical or basolateral membrane destination ( Fig. 45.2 ). In this scenario, the apical and basolateral membrane proteins are sorted soon after their synthesis. The TGN has been classically considered to be the main sorting station of the biosynthetic pathway in epithelial cells, but it is becoming evident that sorting events in this route can also take place at various endosomal sorting compartments. In addition to sorting mechanisms, which segregate apical proteins from the basolateral membrane proteins before they reach their final destinations, other mechanisms are involved in the direct delivery pathway, including directional transport and specific docking of vesicle carriers with the appropriate membrane domains. Once delivered to the proper membrane, domain-specific retention may also contribute to maintaining appropriate polarity and cell surface density of specific membrane proteins.
Steady-state polarity may also arise by a circuitous routing process, often called the indirect trafficking pathway. In this case, newly synthesized proteins are initially targeted to one plasma membrane domain and then either selectively retained or selectively retrieved and resorted to the opposite membrane domain. Such a pathway is favored for many apical membrane proteins in hepatocytes. In these cells, the majority of membrane proteins are first targeted from the TGN to the basolateral membrane, and then apical membrane-destined proteins are rerouted to the apical membrane by a process called transcytosis. Transcytosis is not limited to hepatocytes; in MDCK cells, subsets of newly synthesized proteins, including some glycosylphosphatidylinositol-anchored (GPI-anchored) proteins, are delivered first to the basolateral membrane and then, following a transcytotic route, are redirected to the apical membrane. Similarly, the polymeric IgA receptor (pIgR) is endocytosed from the basolateral membrane of MDCK cells and routed to the apical membrane via recognition of a specific transcytosis signal. As shown in Fig. 45.2 , the pIgR transcytotic route appears to involve sorting steps at BEE, CRE, and ARE, before being delivered to the apical membrane.
Finally, newly synthesized membrane proteins can be randomly targeted to both plasma membrane domains and then be selectively retained or degraded, so that polarized sorting is achieved at the plasmalemma itself. For example, the alpha2B-adrenergic receptor has been reported to be delivered to both the apical and basolateral membranes of MDCK cells, but then are selectively retained exclusively at the basolateral membrane. Postendocytic pathway may also play an important role in the random pathway by selectively recycling specific proteins to the proper polarized membrane.
It is important to consider that protein-sorting pathways can be both cell- and protein-specific. For example, the intestinal brush border protein lactase-phlorizin hydrolase (LPH) is directly delivered to the apical surface of Caco-2 cells, while the human neurotrophin receptor p75 reaches its final apical destination via a basolateral transcytotic pathway. Certain pathways may predominate in a certain cell type, but less-traveled routes may be taken, depending on the specific protein. For example, while hepatocytes seem to favor the indirect pathway, canalicular (apical) ABC (ATP-binding cassette) transporters MDR1, MDR2 (multidrug resistance proteins 1 and 2), and SPGS (sister of p-glycoprotein) have been shown to follow a direct biosynthetic delivery pathway. In some cases, the type of epithelial cell defines the route taken by a specific protein. For example, gp80 is sorted to the basolateral membrane of T84 human colonic adenocarcinoma cells, but is expressed on the apical plasma membrane of MDCK cells. In other instances, the same protein may be targeted to identical destinations regardless of the epithelial cell type, albeit through completely different trafficking pathways. Such is the case with the human neurotrophin receptor p75, which follows an indirect route to the apical membrane of intestinal Caco-2 cells, but is directly routed to the apical membrane in MDCK cells.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here