Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The application of new techniques in molecular biology and genetics continues to revolutionize the understanding of the mechanisms underlying both normal and abnormal embryonic development. It is impossible to have a contemporary understanding of embryonic development without integrating fundamental molecular and morphological aspects of embryology. This chapter introduces the most important families of molecules known to direct embryonic development and some important genetic techniques that help dissect developmental processes.
One of the most important realizations has been the conservatism of the genes that guide development. Sequencing studies have shown remarkably few changes in the nucleotide bases of many developmentally regulated genes that are represented in species ranging from worms to Drosophila to humans. Because of this phylogenetic conservatism, it has been possible to identify mammalian counterparts of genes that are known from genetic studies to have important developmental functions in other species ( Box 3.1 ). ∗
∗ By convention, names of genes are italicized, whereas the products of genes are printed in roman type. Abbreviations of human genes are all capitalized (e.g., HOX ); those for other species are written with only the first letter capitalized (e.g., Hox ).
It is also clear that the same gene may function at different periods of development and in different organs. Such reuse greatly reduces the total number of molecules that are needed to control development. Before and after birth, specific genes may be expressed in normal and abnormal processes. One of the principal themes in contemporary cancer research is the role of mutant forms of developmentally important genes (e.g., protooncogenes) in converting normal cells to tumor cells.
Despite the discovery and characterization of many developmentally important genes in mammals, the basic framework for understanding the molecular basis of embryonic development still rests largely on studies of developmental genetics in Drosophila . Although the earliest stages of human development occur under less rigid genetic control than those of Drosophila, an exposure to the fundamental aspects of early Drosophila development nevertheless sets the stage for a deeper understanding of molecular embryogenesis in mammals.
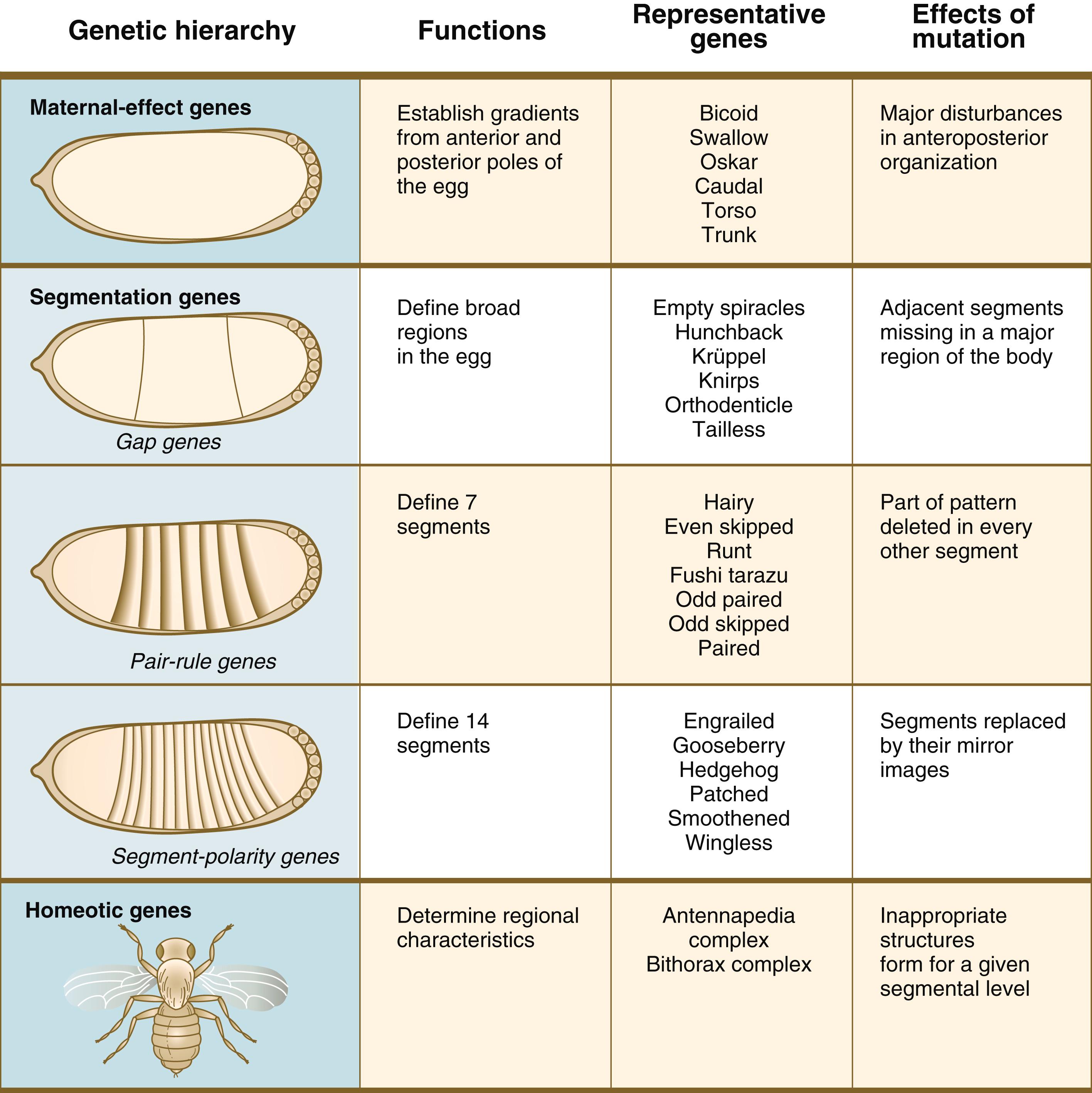
Embryonic development of Drosophila is under tight genetic control. In the earliest stages, the dorsoventral and anteroposterior axes of the embryo are established by the actions of batteries of maternal-effect genes ( Figure 3.1 ). When these broad parameters have been established, the oval embryo undergoes a series of three sequential steps that result in the segmentation of the entire embryo along its anteroposterior axis. The first step in segmentation, under the control of what are called gap genes, subdivides the embryo into broad regional domains. Loss-of-function gap mutants result in loss of structure, or gaps, in the body pattern several segments in width. In the second step, a group of pair-rule genes is involved in the formation of seven pairs of stripes along the craniocaudal axis of the embryo. The third level in the segmentation process is controlled by the segment-polarity genes , which work at the level of individual segments and are involved in their anteroposterior organization. ∗
∗ In Drosophila , each stripe (segment) is subdivided into anterior and posterior halves. The posterior half of one segment and the anterior half of the next are collectively known as a parasegment. The genetics and developmental aspects of insect parasegments are beyond the scope of this text, but in Chapter 6 , when formation of the vertebral column is discussed, a similar set of divisions of the basic body segments in vertebrate embryos is introduced.
The segmentation process results in a regular set of subdivisions along the anteroposterior axis of the early Drosophila embryo, but none of the previously mentioned developmental controls imparts specific or regional characteristics to the newly formed segments. This function is relegated to two large clusters of homeotic genes found in the antennapedia complex and the bithorax complex. The specific genes in these two complexes determine the morphogenetic character of the body segments, such as segments bearing antennae, wings, or legs. Mutations of homeotic genes have long been known to produce bizarre malformations in insects, such as extra sets of wings or legs instead of antennae (hence the term antennapedia ).
From a functional standpoint, many of the important molecules that guide embryonic development can be grouped into relatively few categories. Some of them remain in the cells that produced them and act as transcription factors ( Figure 3.2 ). Transcription factors are proteins possessing domains that bind to the DNA of promoter or enhancer regions of specific genes. They also possess a domain that interacts with RNA polymerase II or other transcription factors and consequently regulates the amount of messenger RNA (mRNA) produced by the gene.
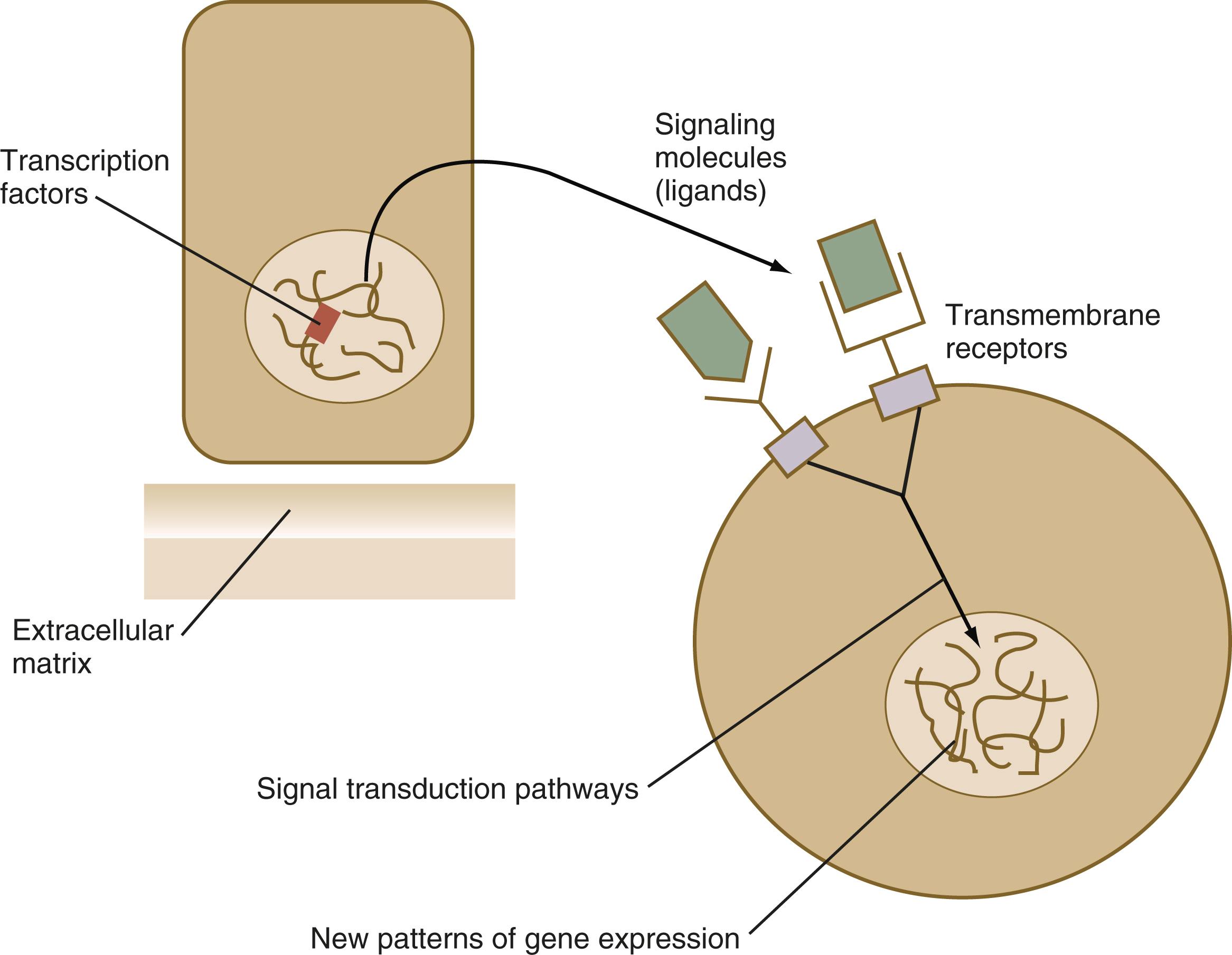
Other molecules act as intercellular signaling molecules . Such molecules leave the cells that produce them and exert their effects on others, which may be neighboring cells or cells located at greater distances from the cells that produce the signaling molecules. Many signaling molecules are members of large families of related proteins called growth factors . To exert their effect, signaling molecules typically bind as ligands to receptor molecules , which are often transmembrane proteins. When these receptor molecules form complexes with signaling molecules, they set off a cascade of events in a signal transduction pathway that transmits the molecular signal to the nucleus of the responding cell. This signal influences the nature of the gene products produced by that cell and often the cell’s future course of development.
Of the more than 21,000 genes in the human genome, more than 3000 encode transcription factors. Some transcription factors are general ones that are found in virtually all cells of an organism. Other transcription factors are specific for certain types of cells and stages of development. Specific transcription factors are often very important in initiating patterns of gene expression that result in major developmental changes. They typically do so by acting on promoters or enhancers to activate or repress the transcription of specific genes. Based on their structure and how they interact with DNA, transcription factors can be subdivided into several main groups, the most important of which are introduced here.
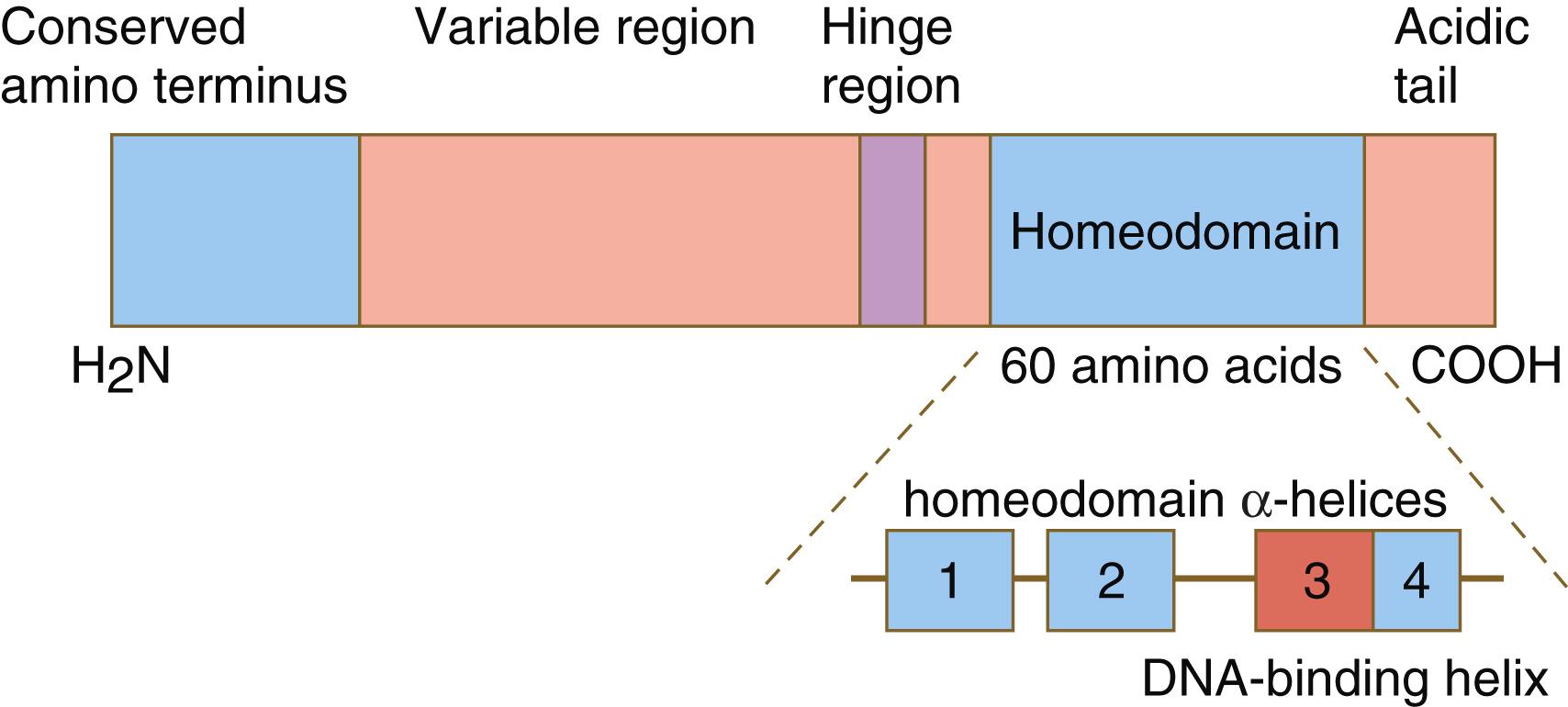
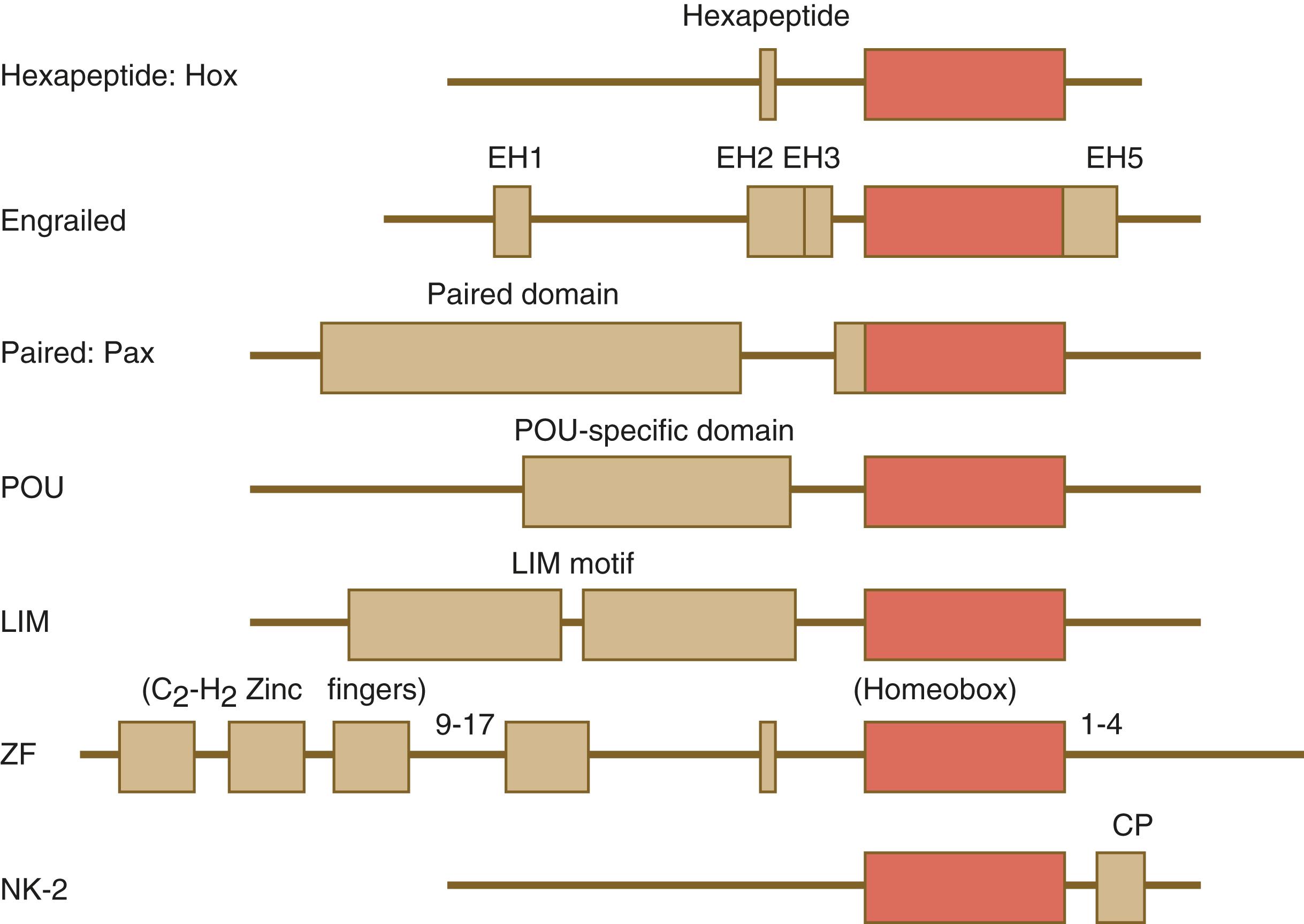
One of the most important types of transcription factors is represented by the homeodomain proteins . These proteins contain a highly conserved homeodomain of 60 amino acids; a homeodomain is a type of helix-loop-helix region ( Figure 3.3 ). The 180 nucleotides in the gene that encode the homeodomain are collectively called a homeobox . Homeobox regions were first discovered in the homeotic genes of the antennapedia and bithorax complex in Drosophila (see Figure 3.1 ), hence their name. This designation sometimes confuses students because after their initial description, homeoboxes have been found in several more distantly related genes outside the homeotic gene cluster. Many other gene families contain not only a homeobox but also other conserved sequences ( Figure 3.4 ).
The Drosophila antennapedia–bithorax complex consists of eight homeobox-containing genes located in two clusters on one chromosome. Mice and humans possess at least 39 homologous homeobox genes (called Hox genes in vertebrates [ HOX in humans]), which are found in four clusters on four different chromosomes ( Figure 3.5 ). The Hox genes on the four mammalian chromosomes are arranged in 13 paralogous groups.
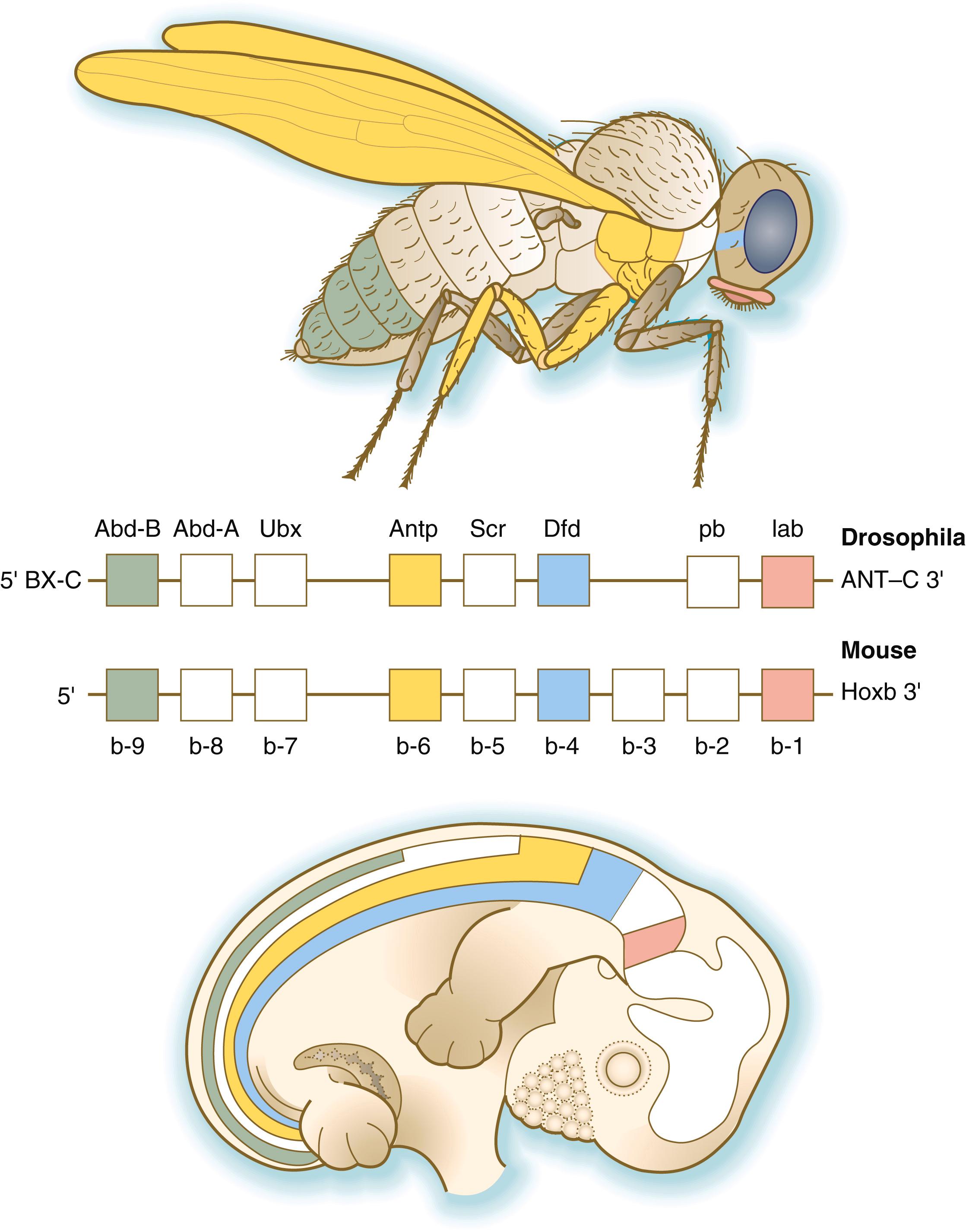
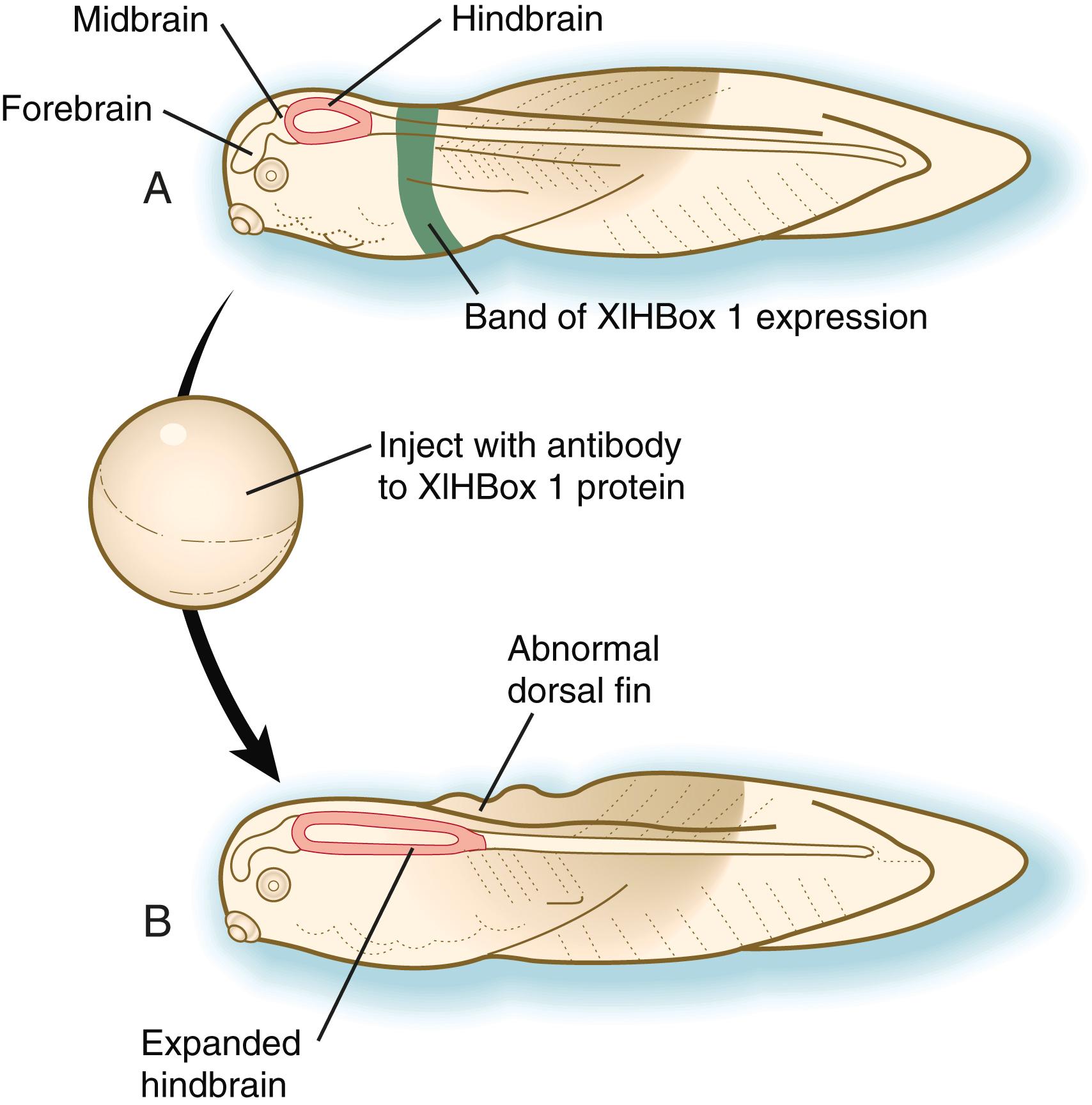
Vertebrate Hox genes play a prominent role in the craniocaudal segmentation of the body, and their spatiotemporal expression proceeds according to some remarkably regular rules. The genes are activated and expressed according to a strict sequence in the 3′ to 5′ direction, corresponding to their positions on the chromosomes. Consequently, in Drosophila and mammals, 3′ genes are expressed earlier and more anteriorly than 5′ genes ( Figure 3.6 ). Mutations of Hox genes result in morphological transformations of the segmental structures in which a specific gene is normally expressed. Generally, loss-of-function mutations result in posterior-to-anterior transformations (e.g., cells of a given segment form the structural equivalent of the next most anterior segment), and gain-of-function mutations result in anterior-to-posterior structural transformations. Figure 3.7 illustrates an experiment in which injection of an antibody to a homeodomain protein into an early frog embryo resulted in the transformation of the anterior spinal cord into an expanded hindbrain.
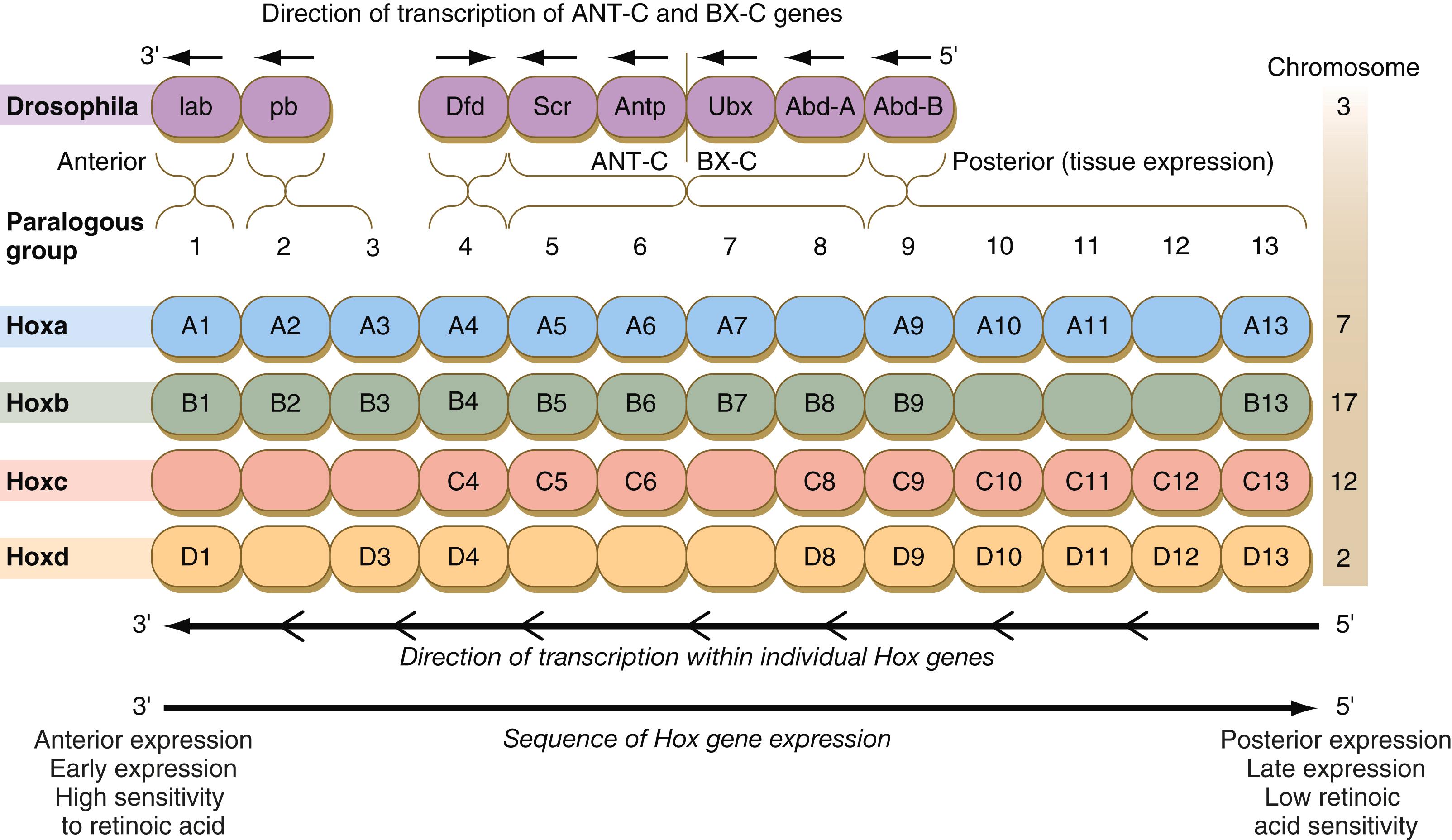
Although Hox genes were originally described to operate along the main body axis, sequential arrays of expression are found in developing organs or regions as diverse as the gut, the limbs, and the internal and external genitalia. The expression of isolated Hox genes also occurs in locations such as hair follicles, blood cells, and developing sperm cells. The principal function of the Hox genes is to help establish structures along the main body axis, but ordered groups of Hox genes are later reused in guiding the formation of several specific nonaxial structures. In mammals, individual members of a paralogous group often have similar functions, so that if one Hox gene is inactivated, the others of that paralogous group may compensate for it. If all members of a paralogous group are inactivated, profound morphological disturbances often result (see p. 170).
The regulation of Hox gene expression is complex. A major regulator along parts of the anteroposterior axis of the developing central nervous system is retinoic acid, but this effect is mediated by other genes. At a different level, Hox expression is influenced by modifications of chromatin and the three-dimensional organization of the chromosomes. Aggregations of regulatory materials into topologically associated domains (TADs) appear to be important in the expression of Hox gene functions, with one TAD regulating the expression of 3′ Hox genes (paralogous groups 1–9) and another regulating 5′ Hox genes (paralogous groups 10–13). A major regulator at the chromatin level is the group of Polycomb proteins, which silence Hox genes through chromatin remodeling. Defects in Polycomb regulation can result in homeotic transformations of the axial skeleton (see p. 171). Polycomb regulation of chromatin also plays a major role in X-chromosome inactivation (see p. 61). Even after the transcription has occurred, microRNAs (miRNAs) may cleave Hox mRNAs and inactivate them.
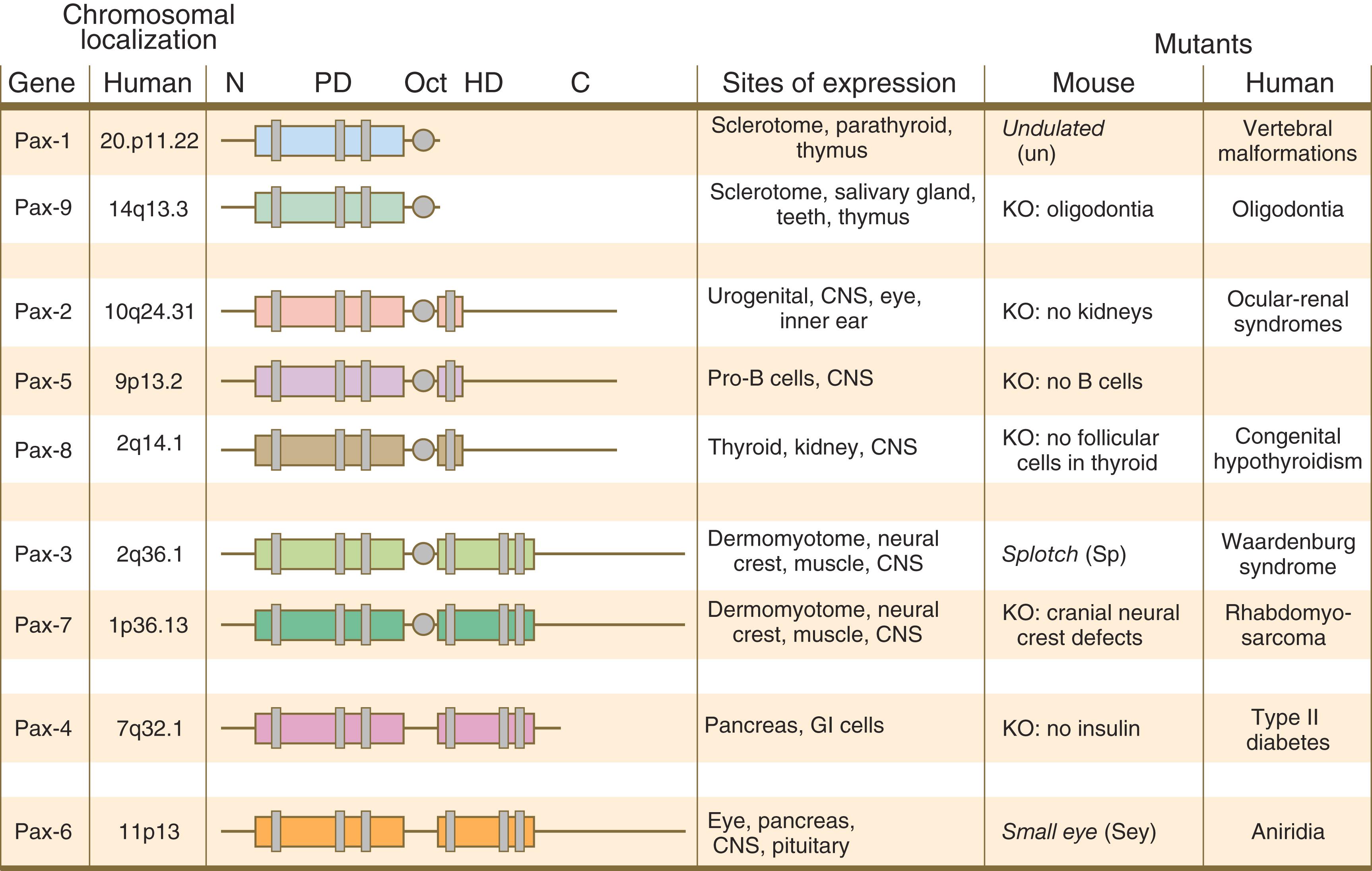
The Pax gene family , consisting of nine known members, is an important group of genes that are involved in many aspects of mammalian development ( Figure 3.8 ). The Pax genes are homologous to the Drosophila pair-rule segmentation genes (see Figure 3.1 ). All Pax proteins contain a paired domain of 128 amino acids that binds to DNA. Various members of this group also contain entire or partial homeobox domains and a conserved octapeptide sequence. Pax genes play a variety of important roles in the sense organs and developing nervous system, and outside the nervous system they are involved in cellular differentiative processes when epithelial–mesenchymal transitions occur.
The POU gene family is named for the acronym of the first genes identified: Pit1 , a gene uniquely expressed in the pituitary; Oct1 and Oct2 ; and Unc86 , a gene expressed in a nematode. Genes of the POU family contain, in addition to a homeobox, a region encoding 75 amino acids, which also bind to DNA through a helix-loop-helix structure. As described in Chapter 4 (see p. 65), Oct-4 plays an important role during early cleavage.
The Lim proteins , products of the LHX family of genes, constitute a large family of homeodomain proteins, some of which bind to the DNA in the nucleus and others of which are localized in the cytoplasm. Lim proteins are involved at some stage in the formation of virtually all parts of the body. The absence of certain Lim proteins results in the development of headless mammalian embryos (see p. 84).
The Dlx gene family, similar to the Hox gene family, is a group of genes that have been phylogenetically conserved. The six members of this group in mammals are related to the single distalless gene in Drosophila, and they play important roles in patterning, especially of outgrowing structures, in early embryos. The mammalian Dlx genes operate in pairs, which are closely associated with Hox genes. In humans, the DLX 1/2 cluster is linked to the HOXD cluster on chromosome 2; the DLX 3/4 cluster is linked to the HOXB cluster on chromosome 17; the DLX 5/6 cluster is linked to the HOXA cluster on chromosome 7. In mammals, the HOXC genes are not associated with a DLX cluster, which was presumably lost at some point during evolution. DLX products are heavily involved in the formation of the jaws and ear, as well as the early development of the placenta.
The Msx genes (homologous to the muscle-segment homeobox [ msh ] gene in Drosophila ) constitute a small, highly conserved family of homeobox-containing genes, with only two representatives in humans. Nevertheless, the Msx proteins play important roles in embryonic development, especially in epitheliomesenchymal interactions in the limbs and face. Msx proteins are general inhibitors of cell differentiation in prenatal development, and in postnatal life they maintain the proliferative capacity of tissues.
The T-box (Tbx) genes take their names from the brachyury (T) locus, which was recognized as early as 1927 to cause short tails in heterozygotic mice. In 1990, the gene was cloned and found to contain a conserved region (the T-box), coding 180 to 200 amino acids, which binds to a specific nucleotide sequence in the DNA. Initially thought to be a single gene, an entire family of T-box genes with more than 100 members (18 genes in the human genome) has been described. T-box genes are evolutionarily ancient, and some are even found in protozoa and fungi. Genes of this family play important roles in development. In addition to brachyury, the T-box gene family contains eomesodermin , a gene that plays a critical role in specifying cell lineages—especially endoderm and types of anterior mesoderm during gastrulation. Another prominent group consists of the Tbx genes, which among other functions coordinates outgrowth of both the arm and leg.
The transcription factors of the basic helix-loop-helix type are proteins that contain a short stretch of amino acids in which two α-helices are separated by an amino acid loop. This region, with an adjacent basic region, allows the regulatory protein to bind to specific DNA sequences. The basic regions of these proteins bind to DNA, and the helix-loop-helix domain is involved in homodimerization or heterodimerization. This configuration is common in numerous transcription factors that regulate myogenesis (see Figure 9.41 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here