Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A severe burn elicits a stress response that initially assists the body in compensating for and adapting to a traumatic injury. There is an elevation in circulating concentrations of catecholamines such as epinephrine and norepinephrine alongside a concurrent increase in inflammatory cytokine production. This stress response is also associated with a significant increase in metabolic rate. Altered protein and glucose metabolism are key factors contributing to burn-induced hypermetabolism. This hypermetabolic, hyperinflammatory state promotes muscle protein catabolism and organ failure, among other detrimental effects. If this response continues unchecked, as occurs in severely burned patients, the resultant hypermetabolic state hinders patient recovery and reintegration into society. Thus the mechanisms underlying the development and persistence of the post-burn stress response, as well as interventions to ameliorate these responses, remain areas of intense research.
One of the most profound and well-documented components of the burn-induced hypermetabolic response is cardiac dysfunction. Immediately following injury, patients experience shock accompanied by reduced heart rate, cardiac output, and contractility. By 2–3 days after injury, the cardiovascular system rebounds, and heart rate and cardiac work increase significantly above normal levels. Post-burn heart rates approach 160% of those in nonburned, healthy patients. Systolic dysfunction and increased myocardial energy demand also occur. These alterations in cardiac function are associated with longer stays in the ICU in addition to increased morbidity, as quantified by number of surgical interventions. Furthermore tachycardia and energy expenditure remained elevated for up to 3 years after the external wounds were healed, underscoring the longevity of the cardiovascular response to severe burns.
Post-burn pathophysiological skeletal muscle catabolism and loss of lean body mass (LBM) significantly prolong rehabilitation. Muscle wasting occurs due to an imbalance in the ratio of protein synthesis to protein breakdown. Catabolism of LBM correlates with increased morbidity and mortality in burn victims. After an LBM loss of 10%, marked delays in wound healing and higher infection rates proportionally increase as the percentage of LBM loss increases. Acutely the net LBM losses from muscle wasting lead to prolonged mechanical ventilation, inhibition of cough reflexes, and a delay in mobilization, contributing to increased mortality in these patients. Chronically these losses reduce strength and the possibility for full rehabilitation. Similar to other characteristics of hypermetabolism, burn-related cachexia can continue for several years following injury. Furthermore, persistent protein catabolism has been hypothesized to account for the growth delay that frequently occurs in our pediatric burn patients.
LBM wasting is hypothesized to be due to a redistribution of protein as well as use of the skeletal muscle as a fuel source. Nitrogen balance studies (whole-body and cross-leg) show persistent muscle breakdown for nearly a year post-burn. Our patients experience an average nitrogen loss of 20–25 grams per square meter of total body surface area (TBSA) per day, a rate at which lethal muscle cachexia becomes imminent in less than 1 month if left untreated. Since a significant portion of insulin-stimulated glucose uptake occurs in the skeletal muscle, significant LBM loss may contribute to post-burn insulin resistance. Flakoll and coworkers showed that increased plasma glucose levels stimulated whole-body proteolysis in the absence of changes in leucine oxidation or nonoxidative disposal. While LBM catabolism is elevated, a decrease in regenerative capacity occurs, further reducing LBM. Satellite cells, the muscle stem cells that regenerate skeletal muscle, are impacted by burn injury. Within the skeletal muscle tissue, although there is an increase in satellite cell proliferation, there is a concurrent increase in apoptosis, leading to a net reduction in satellite cells. The end result of the increase in net protein breakdown alongside a decrease in satellite cells appears to be a reduction in total LBM.
In addition to the alterations in skeletal muscle protein synthesis, breakdown, and regeneration, oxygen consumption is also greatly increased after burn injury. However the molecular mechanism underlying these alterations is not clearly defined. Recently Porter and colleagues reported burn-induced derangements of skeletal muscle mitochondrial function and posited that these changes are a key contributor to the mechanism of burn-induced hypermetabolism. Mitochondrial respiration in severely burned patients is uncoupled for more than 1 year post-burn, resulting in increased heat production. This heat production accounts for nearly a third of the total energy expenditure of the patient and provides a novel therapeutic target to alleviate hypermetabolism.
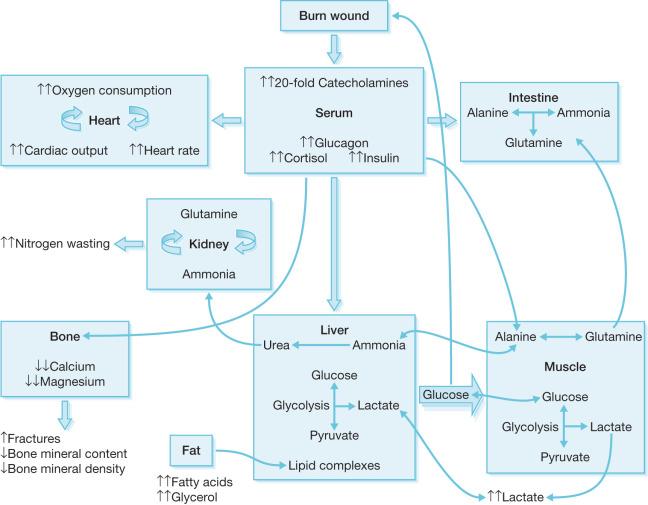
Hyperglycemia is another common metabolic derangement in response to burn injury that occurs in both pediatric and adult burn patients and persists well after the initial discharge from the ICU. Insulin resistance and hyperglycemia contribute to poor wound healing as well as muscle catabolism. Elevated cortisol and catecholamine levels increase the delivery of glucose to vital organs, thus inhibiting insulin's anabolic functions. Catecholamines impair glucose disposal and contribute to peripheral insulin resistance by inhibiting both insulin release and glucose uptake ( Fig. 29.1 ). The availability of gluconeogenic substrates such as glycerol lactate and alanine is increased by lipolysis of adipose tissue, glycogenolysis, and proteolysis of skeletal muscle post-burn, which augments hepatic glucose production ( Fig. 29.1 ). Moreover, elevated blood glucose levels fail to suppress hepatic glucose release, exacerbating hyperglycemia in burn patients. This is further complicated by catecholamine-mediated glycogen breakdown. Impaired mitochondrial function in the liver and the skeletal muscle has been associated with altered lipolysis and insulin signaling post-burn by dampening insulin's inhibition of glucose production in the liver and altering glucose uptake into skeletal muscle. Glucagon and pro-inflammatory cytokines such as interleukin-6 (IL-6) also play a role in modulating glycogenolysis, gluconeogenesis, and insulin signal transduction, resulting in further augmentation of hyperglycemia and insulin resistance.
Catecholamine-induced lipolysis increases plasma free fatty acid concentrations, which contributes to organ steatosis and insulin resistance in patients with severe burns. Kraft et al. showed that increased plasma triglyceride levels in severely burned children correlated with poorer outcomes, including impaired organ function, thus confirming an earlier report linking elevated triglycerides with morbidity. Loss of peripheral subcutaneous fat may also play a role in the development and persistence of insulin resistance after a severe burn. Recently browning of white adipose tissue (the adoption of a thermogenic brown phenotype by white adipocytes) and the implications this potentially has for patients with diabetes and/or metabolic syndrome has become the topic of intense research. White adipose tissue isolated from severely burned patients underwent browning, where the development of smaller adipocytes with more mitochondria in addition to elevated expression of uncoupling protein 1 were observed. This response has since been confirmed by others, affirming that increased adrenergic and inflammatory stress in white adipose tissue post-burn is associated with browning. Browning of white adipose tissue has been proposed to play a role in burn-induced hypermetabolism, where adipose tissue becomes a more thermogenic tissue in response to severe burn trauma. Emphasis of future research should be placed on developing both nonpharmacologic and pharmacologic therapeutic approaches to attenuate or reverse this hypermetabolic response in adipose tissue.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here