Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The field of hepatobiliary surgery has evolved dramatically in the past few decades, with improved understanding of the anatomic segments of the liver, advancements in modern imaging techniques, better operative instrumentation, and improved anesthesia care, as well as postoperative management. At the same time, minimally invasive surgery has become an integral part of each surgical subspecialty. However, the application of minimally invasive techniques to liver surgery has been slower to develop, and it is still far from being a standard option for most practicing hepatobiliary surgeons. This reluctance stems in part from the complexity of liver surgery, concerns for significant bleeding or gas embolism, and lack of formal training in minimally invasive surgery for the more “senior” hepatobiliary surgeons.
However, a dramatic progress in minimally invasive hepatic surgery has been made in recent years. Since the First International Consensus Conference in Louisville, Kentucky in 2008 the number of laparoscopic liver resections (LLRs) performed worldwide has increased exponentially. The recently published International Survey on Technical Aspects of Laparoscopic Liver Resection (INSTALL) study demonstrated an expanding indication for LLR that includes larger tumor size, increased number of tumors, and lesions in difficult locations. LLR is now considered a safe option when performed by experienced surgeons in a major hepatobiliary center. In addition to the laparoscopic approach for liver resection, the current minimally invasive techniques also include robotic liver resections (RLRs). Large reviews and series have reported the safety and feasibility of LLR for both benign lesions and malignant tumors, including anatomic right hepatectomies, left hepatectomies, and even extended hepatectomies. In a world review of 2804 cases of LLR, 50% of the resections were done for malignancy, with the majority being performed for hepatocellular carcinoma (HCC) or colorectal cancer (CRC) metastases. A plan for conducting a randomized clinical trial comparing laparoscopic with open hepatic resection for cancer had initially been discussed at the First International Conference in Louisville. There are currently two randomized clinical trials of laparoscopic versus open liver resection (OLR) being conducted in Europe. They are the ORANGE II PLUS trial in the Netherlands and the OSLO CoMet study in Norway. In the latter trial the primary outcome is 30-day perioperative morbidity. The secondary outcomes include 5-year survival (overall, disease-free, and recurrence-free), resection margins, recurrence pattern, postoperative pain, health-related quality of life, and evaluation of the inflammatory response.
An important principle of minimally invasive surgery is that the availability of this technique does not alter the indication. Therefore an LLR should be considered only for lesions that would otherwise be treated with open hepatic surgery. Indications and contraindications for laparoscopic liver surgery are shown in Table 125.1 . Malignant liver lesions, symptomatic hemangioma or focal nodular hyperplasia (FNH), and hepatic adenomas larger than 4 cm should be resected. Ideally suited lesions are masses that are located in the right anterior segments (V and VI) or left lateral segments (II and III). However, experienced groups have shown that even laparoscopic major hepatectomies can be safely accomplished. Although approximately 36% of LLRs are performed in cirrhotic patients, postoperative liver insufficiency has always been an important factor in deciding extent of hepatic resection, whether in open or laparoscopic hepatectomy. In a multiinstitutional Japanese propensity matched study, Takahara et al. reported that the frequency of postoperative liver failure after LLR for HCC is lower than after OLR (0.5% vs. 1.8%). To avoid postoperative liver failure, for minor LLR (≤2 segments), the majority of surgeons set the upper limit of a total bilirubin at 2.0 mg/dL, whereas for major resection (≥3 segments) the threshold is lowered to 1.5 mg/dL or less. The INSTALL study reported that more than 80% of surgeons set the cutoff at 40% volume for future liver remnant when the liver is found to be cirrhotic.
| Indications | Contraindications |
|---|---|
|
|
| Live donor hepatectomy for liver transplant | — |
| Indeterminate lesions—cannot rule out cancer | — |
In liver surgery the main differences between benign and malignant lesions are related to achieving adequate margins and avoidance of tumor rupture. Malignant liver tumors, lesions abutting major vasculature, or tumors that are too large to be manipulated laparoscopically should be resected by an open approach. Perihilar cholangiocarcinomas are often challenging even by an open approach, and in general should not be done with a minimally invasive technique. The presence of dense adhesions that prevent safe dissection, unexpected difficulty in manipulating the liver, or failure to make progress are indications for conversion to an open technique. Such a decision is never considered a failure but rather a good judgment call, used to prevent avoidable complications. Another less common indication for LLR is live donor hepatectomy for liver transplantation. Only 5% of LLRs are performed for this indication around the world. Laparoscopic live donor hepatectomy has been described for left lateral sectionectomy (LLS) and adult-to-adult right hepatectomy. Caution is noted in that these operations should only be done by transplant teams with extensive open live donor liver transplant expertise, as well as minimally invasive liver resection experience.
There are two main approaches for performing minimally invasive liver resection—pure laparoscopic and hand assisted. A third option is using the laparoscopic technique for mobilization of the liver before opening the abdomen and completing the resection through a relatively small laparotomy incision (so-called hybrid technique). Some authors use a hand port for all cases, whereas others use it selectively and some never use it at all. The benefits of the hand-assisted technique are the relative ease in manipulation of the liver, direct palpation for improved tactile sensation, and the ability for faster control of bleeding in the case of a major vascular injury. Because most specimens mandate a utility incision for intact specimen extraction, the main difference between hand-assisted and pure laparoscopy is the position of the incision. There have been no comparative studies to support benefit or inferiority of the three techniques, and the choice is strictly surgeon's preference. The hand-assisted and hybrid methods are claimed by their proponents to be beneficial for larger lesions, posteriorly located lesions, donor hepatectomy, and for training of surgeons in major LLR techniques. In a large review of more than 2800 LLRs, the majority of minimally invasive liver resections were pure laparoscopic (75%), the hand-assisted approach was the next most common (17%), and the “hybrid” technique was used rarely (2%) ( Table 125.2 ).
| Total No. of Reported Cases | 2804 |
| INDICATIONS FOR LAPAROSCOPIC LIVER RESECTION | |
| Malignant lesions | 1395 (49.8%) |
| Benign lesions | 1253 (44.7%) |
| Live donor hepatectomies for liver transplant | 49 (1.7%) |
| Indeterminate | 107 (3.8%) |
| MINIMALLY INVASIVE APPROACHES TO LIVER RESECTION | |
| Totally laparoscopic | 2105 (75.1%) |
| Hand-assisted laparoscopic | 463 (16.5%) |
| Laparoscopy-assisted open (hybrid) | 60 (2.1%) |
| Gasless laparoscopic | 52 (1.8%) |
| Thoracoscopic | 5 (0.2%) |
| Robotics-assisted | 3 (0.1%) |
| Converted to open | 116 (4.1%) |
| TYPES OF RESECTIONS PERFORMED LAPAROSCOPICALLY | |
| Wedge resection/segmentectomy | 1258 (44.9%) |
| Left lateral sectionectomy | 570 (20.3%) |
| Right hepatectomy | 253 (9.0%) |
| Bisegmentectomy | 209 (7.4%) |
| Left hepatectomy | 191 (6.8%) |
| Deroofing/enucleation | 142 (5.1%) |
| Extended right hepatectomy | 19 (0.7%) |
| Caudate lobectomy | 18 (0.6%) |
| Central hepatectomy | 8 (0.3%) |
| Extended left hepatectomy | 3 (0.1%) |
| Other | 16 (0.6%) |
| Not documented | 117 (4.2%) |
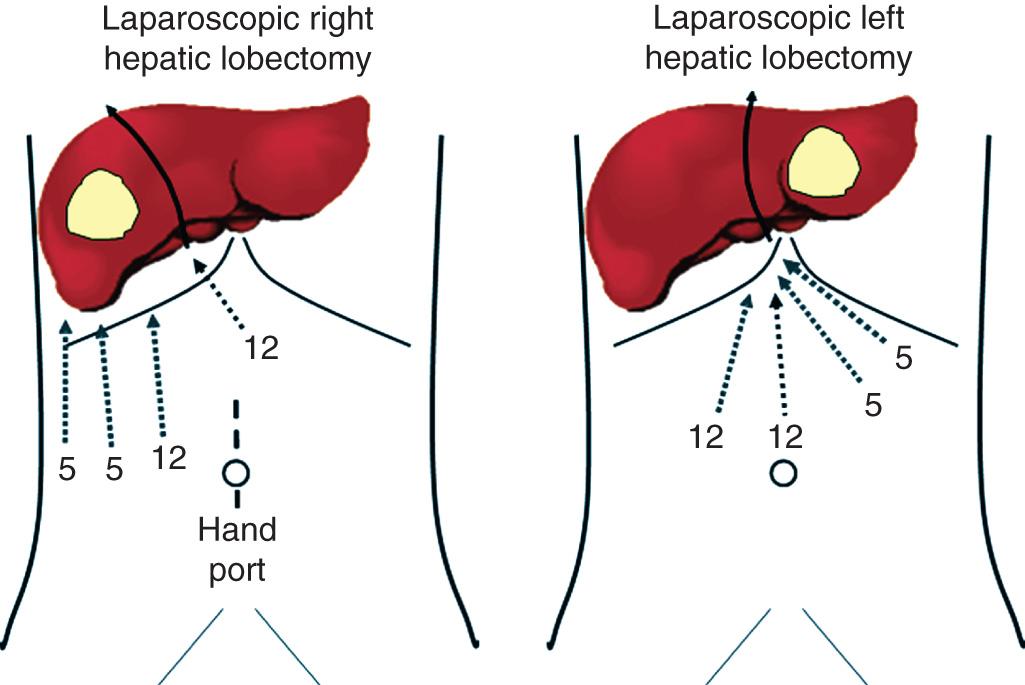
In the operating room the patient is placed in the supine position with both arms extended. Some authors favor the French lithotomy position. The preparation is similar to that of major liver resection, including line placement, bladder catheterization, and orogastric tube insertion. We use a foot board and strapping that allow for steep rotational manipulations of the table during the operation. In the case of a planned major hepatectomy, we use a hand port and place it at the beginning of the procedure, as a supraumbilical midline incision ( Fig. 125.1 ). In the case of a small patient (e.g., <68 inches in height) the incision can be infraumbilical. The pneumoperitoneum is established via a trocar inserted through the hand port. The hand port incision may be used for a rapid conversion, by extending it to a longer midline.
The most recent development in minimally invasive liver surgery is robotic hepatectomy. The first report of robotic-assisted liver resection was published in 2006 by Ryska et al. The known advantages of robotic technology include improved precision, dexterity, degree of movement, and visual magnification, as well as decreased tremor and fatigue. Robotic liver surgery has gained significant popularity because of its potential to overcome limitations of conventional laparoscopy. Over the past 5 years, many case reports and single-institutional case series of RLRs have emerged. In 2014 Tsung et al. reported their experience of robotic versus laparoscopic hepatectomy with a 1 : 2 matched analysis. The patients were matched for presence of background liver disease, extent of hepatic resection, diagnosis, body mass index, age, gender, and American Society of Anesthesiologists (ASA) class. With the exception of higher operative time and overall room time in the robotic group, there were no significant differences between perioperative outcomes of robotic and laparoscopic groups. R0 negative margin status was also comparable, which indicates that the oncologic outcome is not compromised by either approach. However the technical advantages associated with robotic approach allows completion of a greater percentage of minor and major hepatectomies using the purely minimally invasive technique. Ninety-three percent of the RLRs were accomplished without the need for hand-assist ports or the hybrid technique, compared with only 49.1% of those performed with the laparoscopic approach. This finding suggests that robotic system may offer technical ease for surgeons in accomplishing purely minimally invasive liver resections. Robotic approach may also facilitate better vascular control during major liver resections compared with its laparoscopic counterpart. Pretransection extrahepatic inflow control is more easily achieved with the robot. Laparoscopic stapling of the portal vein extrahepatically is sometimes difficult to accomplish with the laparoscopic approach due to a poor stapler angle. Increased degree of freedom with the robotic instrumentation mitigates this problem by allowing control of the portal vein extrahepatically using suture ligation. Similar issues apply to the extrahepatic outflow control. During hepatic parenchymal transection, improved three-dimensional magnification provided by the robotic camera may allows surgeon to identify individual vessels more clearly for precise control and ligation. The downside of robotic approach is added costs for the robotic system, in addition to the longer operative time. However the overall perioperative outcomes are comparable between the laparoscopic and RLRs. A future, larger scale, prospective multicenter study is needed to objectively determine the ultimate superiority of robotic over laparoscopic technique. Based on the most recent international consensus in Morioka, Japan, major robotic liver surgery is still recommended to be done within institutional review board–approved registry.
Despite established advantages of laparoscopy, hemorrhage during LLR remains a major concern. The initial slow development of laparoscopic liver surgery is partly explained by the fear of inability to control bleeding. This important question was discussed among a panel of 34 experts covering five continents during the Second International Conference on Laparoscopic Liver Resection Surgery in Morioka, Iwate, Japan, in October 2014. Several studies have been designed to elucidate potential factors responsible for reduced blood loss during LLR. It is widely accepted that the main reasons for reduced blood loss are the positive pressure of the CO 2 pneumoperitoneum (10 to 14 mm Hg), low central venous pressure (≤5 mm Hg), the emergence of new transection devices, and the facilitation of inflow and outflow controls. Experts present in Morioka agreed that low central venous pressure and pneumoperitoneum act in a synergistic manner to reduce bleeding. Image magnification provided by a laparoscope may also allow more precise dissections and facilitates good control of segmental or subsegmental portal pedicles. In cases of severe bleeding, increasing the pneumoperitoneum pressure and decreasing the airway pressure by a brief pause in the artificial ventilation are maneuvers that can be used to decrease back bleeding. Decailliot et al. demonstrated in a nonrandomized study that Pringle maneuver during LLR is as efficient as Pringle maneuver during open liver surgery in decreasing blood loss. Types of instruments by energy sources and technique used in parenchymal transection vary among each surgeon and institution. We recommend that hepatobiliary surgeons should select techniques and instruments based on their familiarity and complete understanding of the system for each specific LLR case. There have been no randomized controlled trials that answer the question of the best technique or device for laparoscopic hepatic parenchymal transection. All studies on this subject have been case-controls, case series, case reports, experimental studies, and reviews. In addition to the use of multiple hemostatic methods, such as bipolar cautery (for vessels ≤2 mm), vessel sealing device or clips (for vessels 3 to 7 mm), locked clips or staplers (for vessels ≥7 mm), thermofusion, and precoagulation, experts advocate that liver surgeons should master intracorporeal suturing techniques when performing laparoscopic liver surgery. Careful inspection of the transection surface for bleeding and bile leak after decreasing the pneumoperitoneal pressure should be performed routinely prior to ending the operation.
After inserting the hand port and establishing the pneumoperitoneum to a pressure limit of 14 mm Hg, four additional trocars are placed, as depicted (see Fig. 125.1 ). The falciform ligament is divided with endoshears and the round ligament divided using a stapler or with LigaSure or harmonic scalpel. The falciform ligament is left long on the liver side to facilitate retraction. Intraoperative ultrasound is performed to identify the lesion and mark the parenchymal transection line. After taking down the right coronary and triangular ligaments, the right lobe is gradually rotated off the retroperitoneum and lifted from the inferior vena cava (IVC). Short hepatic veins are clipped with 5-mm hemolocks. Small veins can be divided with the LigaSure. At that stage the right hepatic vein is exposed and can be divided with a vascular stapler. If the exposure of the right hepatic vein is not optimal, it can be divided inside the liver at the end of the procedure after the parenchymal transection. The next step is the hilar dissection. It is started with the cholecystectomy and exposure of the right hepatic artery, right portal vein, and bile duct. The right hepatic artery is doubly secured with locking clips. The right portal vein is dissected and encircled. It can be transected with the vascular stapler; however, if the angle precludes safe stapling, it can be left to the end of the procedure and controlled with a small bulldog clamp inserted through the hand port to allow for an ipsilateral Pringle maneuver. Next, the parenchymal transection is started with an ultrasonic dissector or LigaSure. The deeper parenchyma with crossing middle hepatic vein branches is divided with a vascular stapler. Some surgeons use a bipolar pinching forceps and/or Cavitron ultrasonic surgical aspirator (CUSA) or hydrojet to help divide the parenchyma. During the parenchymal transection, as in an open hepatic resection, the central venous pressure is kept low to minimize blood loss. If not already done, the right portal vein and right hepatic veins are divided as the parenchymal transection is deepened, along with the right hepatic duct inside the liver. If a hand port is used, the hand can provide a laparoscopic hanging maneuver to facilitate exposure and transection. Wakabayashi et al. and Soubrane et al. advocated limited liver mobilization before transection, as a potential technique to decrease bleeding during LLR. The anterior approach provides the advantage of performing LLR without having to mobilize the liver before transection, which is not always easy and safe to accomplish laparoscopically, particularly in case of heavy liver weight or a large-sized tumor. The caudal approach is the main conceptual change in LLR. The caudal approach, which relies on visual magnification, offers improved exposure around the right adrenal gland and the vena cava. This approach also greatly facilitates identification of the Laennec capsule and Glissonian pedicle at the hilar plate. Using this technique the IVC from caudal to cranial can be efficiently exposed, which is then followed by division of the short hepatic veins before parenchymal transection. A meticulous caudal to cranial parenchymal transection with laparoscopic magnification results in better identification of intraparenchymal structures for optimal liver parenchymal division. Placement of patients in reverse Trendelenburg position helps to lower the venous pressure and improve gravitational shifting of visceral structures away from the liver hilum. A recently introduced concept of superior and lateral approaches with or without the use of intercostal and transthoracic trocars requires patients to be placed in the left lateral decubitus position or even prone position. This new technical advancement in LLR offers better exposure of the right posterosuperior segments and lifts the right hepatic vein higher than the vena cava to reduce hepatic venous back bleeding. Any oozing from the cut edge is controlled with cautery or TissueLink sealing device. Any visible bile leaks are oversewn with a 4-0 absorbable suture. The specimen is extracted through the hand port, and the abdomen is reinspected for bleeding. A closed-suction drain is left next to the cut surface of the liver and brought out through one of the 5-mm trocar sites.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here