Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Minimally Invasive Parathyroidectomy.
Subsequent to the first parathyroidectomy documented in the literature (in 1925 by Felix Mandl of Vienna), four-gland exploration via a generous incision was the standard technique until the 1970s and 1980s. The advent of various localization modalities since that time, coupled with the fact that approximately 85% of patients with primary hyperparathyroidism (PHPT) have a solitary adenoma, has led many surgeons to prefer minimally invasive parathyroidectomy (MIP) as their operative approach of choice. The general goals of MIP are to limit operative dissection, reduce incision length, and improve postoperative recovery. Perhaps one of the greatest benefits of MIP is the potential for reduced scarring of deeper tissues, allowing for future operations, if needed, to be safer and less difficult.
There is considerable variability in the literature as to what exactly is defined as MIP; in general, however, it refers to any surgical approach that preoperatively aims to identify and remove a single enlarged gland (i.e., focused parathyroid exploration ) and may, in select circumstances, allow examination of the ipsilateral gland as well (i.e., unilateral exploration ). Choices for incision in MIP include a standard collar incision, smaller midline incision, ectopic or lateral incision, or video-assisted approach (see Chapter 55 , Principles in Surgical Management of Primary Hyperparathyroidism, Chapter 56 , Standard Bilateral Parathyroid Exploration, and Chapter 58 , Minimally Invasive Video-Assisted Parathyroidectomy). Intraoperative parathyroid hormone (PTH) level monitoring can be employed to confirm that excision of the gland identified preoperatively results in biochemical cure (see Chapter 59 , Intraoperative PTH Monitoring During Parathyroid Surgery). Many groups recommend that intraoperative PTH monitoring be employed routinely in all cases of MIP.
The most imperative prerequisite to MIP is adequate preoperative localization by imaging to guide the operation. This can be accomplished with a variety of modalities including ultrasound, computed tomography (CT), Tc-99m sestamibi, and positron emission tomography (PET). Imaging accuracy can be variable based on region and institution; thus these factors should be considered in the development of preoperative localization protocols.
There are several advantages to MIP compared with traditional four-gland exploration in appropriate patients. These include the option to perform surgery under local anesthesia, reduced operative time, decreased postoperative pain, improved cosmesis, shorter time to hospital discharge, reduced complication rate, reduced deep tissue scarring, and decreased overall costs. In addition, the majority of data would suggest long-term outcomes are comparable to the standard multigland exploration.
This chapter addresses considerations for performing MIP, including anatomic factors, the importance of a parathyroid localization nomenclature system, the role of preoperative imaging studies, and operative preparation and techniques.
Many patients with PHPT may be candidates for MIP. The ideal patient should have evidence to suggest the presence of a single parathyroid adenoma. Key elements include biochemical proof of PHPT, preoperative imaging that is highly suggestive of single-gland disease with identification of parathyroid adenoma location, and access to an experienced parathyroid surgeon. Patients suspected to have multi-gland disease, such as those with multiple endocrine neoplasia type 1 (MEN 1), other familial syndromes, lithium use, chronic renal failure, or a possible diagnosis of parathyroid cancer may not be good candidates for MIP (see Chapter 60 , Surgical Management of Multiglandular Parathyroid Disease; Chapter 61 , Surgical Management of Secondary and Tertiary Hyperparathyroidism; Chapter 62 , Parathyroid Management in the MEN Syndromes; and Chapter 64 , Parathyroid Carcinoma). Pediatric patients and those younger than age Chapter 45 were originally thought to be at higher risk for multigland disease and, thus, ineligible for MIP; however, recent data suggest favorable outcomes with MIP in these populations. Concurrent thyroid pathology that could require concomitant thyroid gland resection should be absent. A more comprehensive list of contraindications to MIP is presented in Table 57.1 .
| Absolute Contraindications | Relative Contraindications |
|---|---|
|
|
|
|
|
|
The focused approach of MIP requires a thorough knowledge of cervical anatomy and embryology (see Chapter 2 , Applied Embryology of the Thyroid and Parathyroid Glands). Parathyroid gland location follows definite embryologically influenced patterns. Because the superior parathyroid glands share an embryologic origin with the lateral thyroid tissue in the primordium in the fourth branchial pouch, nondiseased superior parathyroid glands are invariably found close to the dorsum of the upper thyroid lobe. When a superior parathyroid gland becomes heavy, enlarged, and adenomatous, it tends to be found more posteriorly and caudally. If closely associated with the thyroid capsule, the gland will remain in contact with the posterior surface of the thyroid, in continuity with thyroid tissue. The pedicle of the superior parathyroid gland is located lateral and posterior/dorsal to the oblique course of the recurrent laryngeal nerve (RLN).
The inferior parathyroid glands share their embryologic origin with the thymus, and both arise from the third branchial complex. An enlarged inferior parathyroid gland usually remains in close proximity to the lower pole of the thyroid, often just lateral or posterior to the lower pole. If the enlarged inferior parathyroid gland becomes heavy or caudal embryologic migration is excessive, it may descend into the thyrothymic ligament. The enlarged gland may also be present deeper within the thymus and may even reside in the mediastinum.
Experienced surgeons use the RLN as an important landmark for identifying parathyroid glands. The inferior parathyroid gland is consistently anterior or ventral to the RLN, whereas the pedicle to the superior parathyroid gland is always posterior or dorsal to the RLN.
Enlarged superior parathyroid glands are almost always found in specific sites; these are, in order of frequency of occurrence, (1) the dorsum of the upper pole of the thyroid, (2) the paraesophageal space posterior to the thyroid parenchyma (a less common location is the retropharyngeal space), and (3) descent into in the paraesophageal space caudal to the inferior pole of the thyroid.
An enlarged inferior parathyroid gland most commonly rests at the lower pole of the thyroid, within the thyrothymic ligament or in the superior-most portion of the cervical thymic tongue. Occasionally, the inferior parathyroid gland descends within the thymus into the upper mediastinum.
A common misconception is that a parathyroid gland located high in the neck is always a superior gland and that one located low in the neck is an inferior gland. In fact, a superior enlarged parathyroid gland may descend caudally and be located near the lower pole of the thyroid or even below the inferior pole of the thyroid. In this situation, the gland frequently is suspended by a long, vascular pedicle from the inferior thyroid artery. The superior gland in this case may actually be “inferior” to the inferior gland base. Surgeons must always pay attention to the location of the gland relative to the course of the RLN. Enlarged superior glands that have descended caudally still retain their normal position posterior or dorsal to the RLN, whereas enlarged inferior glands always remain anterior or ventral to the RLN. The importance of this positioning is that the superior gland will be dorsal in the neck in proximity to the deeper tracheoesophageal region.
The relationship of an enlarged parathyroid gland to the thyroid capsule is also critical to the potential position of a diseased gland. When located within the fibrous thyroid capsule, the diseased parathyroid gland expands but remains within the confines of the surgical capsule of the thyroid. When located external to the thyroid capsule, enlarged parathyroid glands may become subject to gravity and the repetitive forces of deglutition and become displaced posteriorly behind the gland in the tracheoesophageal region. A summary of anatomic “pearls” for MIP is presented in Box 57.1 .
Superior glands can be inferior to the inferior gland.
Ninety percent of parathyroid glands are within 1 cm of the junction of the inferior thyroidal artery and recurrent laryngeal nerve.
Within the inferior triangle (viewed from the front, laterally defined by the RLN, with base inferior) lie most inferior glands (see Figure 57.1 , A ).
Within the superior triangle (viewed from the front, medially defined by the RLN with base superior) lie most superior parathyroid glands (see Figure 57.1 , A ).
The recurrent laryngeal nerve is more oblique on the right side.
Standardizing parathyroid nomenclature offers a universal, consistent language to communicate the precise locations of enlarged parathyroid glands among members of the multidisciplinary team, which includes radiologists, surgeons, anesthesiologists, endocrinologists, pathologists, ultrasonographers, and nuclear medicine physicians. One such classification system was developed and used at the MD Anderson Cancer Center to allow a systematic approach to the most frequently encountered positions of enlarged parathyroid glands. This clinically useful classification scheme encompasses information about the parathyroid gland’s pedicle and the surrounding structures, and it incorporates the relation of the RLN to the glands as discussed previously. Based on the premise that superior glands have a pedicle that originates lateral to the RLN and that inferior glands have a pedicle that originates medial to this nerve, the system considers natural descent patterns (both embryologic and acquired): superior glands descend posteriorly in the tracheoesophageal groove, and inferior glands descend anteriorly in the plane of the trachea.
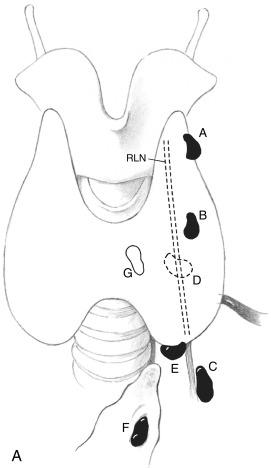
The classification system is alphabetical and uses the letters A through G to describe exact parathyroid gland locations.
This system not only offers a concise, reliable means of communicating the exact location of enlarged parathyroid glands but also can facilitate surgical planning and selection of approach. The gland’s letter designation has, therefore, implications for placement of the incision, patient positioning, the scope of the operation, and the selection of anesthesia. For example, precise information about the expected location of the adenoma allows for better planning by the anesthesiologist, who can determine the type of anesthesia needed based on operative complexity, depth of dissection, and projected duration of the procedure. Knowledge of the adenoma’s location also helps operating room personnel anticipate technical requirements, such as the need for nerve monitoring devices, instrumentation for sternotomy or thymectomy, or intraoperative ultrasound. Common descriptors of adenoma location also facilitate communications between physicians (e.g., reviewing the report of an operation performed by another surgeon, particularly if reoperation is being planned) and in documenting an excised gland’s exact location for the pathologist. In addition to describing common locations of glands, the alphabetic letters A through G are used as reminders of locations to explore when a diseased gland cannot be identified intraoperatively.
A common nomenclature also facilitates communications among institutions in situations such as registering patients for clinical trials and reporting results in the medical literature. At the MD Anderson Cancer Center, this nomenclature provides primary terminology for use in imaging reports and has been expanded and incorporated into anesthesia, nursing care, operative, and pathology reports. The authors of the system have found that it greatly improves communication, removes ambiguity, and obviates lengthy descriptions of parathyroid gland locations.
The alphabetical classification system for identifying and locating adenomatous parathyroid glands is presented here and in Box 57.2 (also see Figure 57.1 ):
Type A. A dherent to the posterior thyroid parenchyma, a type A parathyroid gland is in the expected location of a normal parathyroid gland and is in apposition to the posterior surface of the thyroid parenchyma. The gland may be compressed within the capsule of the thyroid.
Type B. B ehind the thyroid parenchyma, a type B gland is a superior gland that is exophytic to the thyroid parenchyma and has fallen posteriorly into the tracheoesophageal groove. There is minimal or no contact between the gland and the posterior surface of the thyroid tissue. On coronal views, the gland is posteriorly located near the esophagus. An undescended gland high in the neck near the carotid bifurcation or mandible also may be classified as a type B gland.
Type C. C audal to the thyroid parenchyma, a type C gland is a superior gland that has descended posteriorly and caudally into the tracheoesophageal groove. On anterior images, a type C gland is inferior to the inferior pole of the thyroid parenchyma. The type C gland is posterior to and in many cases inferior to the oblique angle of the RLN. Glands in the carotid sheath are either type B or C glands, depending on their craniocaudal relationship to the inferior pole of the thyroid gland (cranial, type B, caudal, type C).
Type D. D irectly over the RLN in the middle region of the posterior surface of the thyroid tissue. The dissection of the type D gland can be “difficult” or “dangerous” because of this gland’s proximity to the RLN. The gland lies in the middle region of the posterior surface of the thyroid parenchyma, near the junction of the RLN and the inferior thyroid artery. The embryologic origin of a type D parathyroid adenoma may be that of either a superior or inferior gland. The distinction is made intraoperatively based on the gland’s relationship to the RLN and the pedicle of the gland. Usually, the position of the pedicle cannot be determined on preoperative imaging studies.
Type E. The type E gland is an inferior gland that is proximal to the inferior pole of the thyroid parenchyma. This gland is more closely aligned with the same anterior-posterior plane of the thyroid tissue and the trachea (as opposed to more posterior glands that relate to the esophagus). The type E adenoma has a pedicle that is medial and anterior to the RLN and is the easiest to resect because of its superficial location relative to the depth of the cervical region.
Type F. The type F gland is an inferior gland that has descended into the thyrothymic ligament or superior thymus. This gland may be in the anterior mediastinum. On anterior-posterior imaging views, the type F gland is in the coronal plane of the thymus. It has “fallen” into the thyrothymic ligament, is below the inferior pole of the thyroid, and is usually superficial or immediately lateral to the trachea. A type F gland is frequently referred to as an ectopic gland, and its resection usually involves transcervical delivery of the thyrothymic ligament or superior portion of the thymus.
Type G. The type G gland is a rare, gauche gland. This gland is an intrathyroidal parathyroid gland that has thyroid tissue circumferentially around it. Resection of a type G gland may require thyroid lobectomy because the gland “got caught” within the thyroid tissue.
Type A. A type A gland is a dherent to the posterior thyroid parenchyma. This gland is in the a ccepted, expected location of a normal gland. It is a lmost a ttached, a lmost intracapsular.
Type B. A type B gland is b ack b ehind the thyroid parenchyma. The surgeon should b e careful not to miss it. It is exophytic to the thyroid parenchyma and lies in the tracheoesophageal groove. An undescended gland high in the neck near the carotid bifurcation or mandible is classified as a type B + gland.
Type C. A type C gland is c ommonly missed; it c ould be mistaken for the esophagus when palpated. It is c audal to the thyroid parenchyma, c loser to the c lavicle, in the tracheoesophageal groove.
Type D. Dissection of a type D gland may be d ifficult because the gland is d angerously close to the recurrent laryngeal nerve. Preoperative images d on’t enable d etermination of origin.
Type E. A type E gland is e asy to resect. It is the most e xternally located gland because it is not deep in the neck. E arly on, its e ase of resection gives the e ndocrine surgeon e xtra confidence.
Type F. A type F gland has f allen into the thyrothymic ligament. F requently it is referred to as ectopic. Resection can be f un because a type F gland can usually be retrieved with delivery of the superior portion of the thymus. For the inexperienced surgeon, however, resection can be f rightening because of the delivery of the gland from the mediastinum via the neck.
Type G. A type G gland g ot caught in thyroid tissue during descent—stuck in the starting g ate. A true intrathyroidal gland is rare— g auche g land!
Preoperative imaging is critical to successful MIP (see Chapter 54 , Guide to Preoperative Parathyroid Localization Testing: Ultrasound, Sestimibi and 4D CT). It provides the surgeon with a roadmap for resection of an adenoma by providing a precise location of the gland and adjacent structures. It should be emphasized that preoperative imaging does not assist with diagnosis or in the decision to proceed with surgery. Modalities may include ultrasound, nuclear medicine studies, and CT. Many surgeons feel that two different modalities with concordant results are necessary to proceed with MIP. Some groups suggest that four-dimensional CT is the most accurate imaging modality.
As mentioned previously, the sensitivity and specificity of a particular modality can vary depending on region and institution. This variability should be considered when deciding which modalities to pursue. We recommend that these imaging techniques be performed by dedicated imaging professionals with experience and an interest in parathyroid disease. For these purposes, securing the services of a dedicated team with a track record of performing repeated studies is desirable. The surgeon should direct the selection and sequence of the preoperative images to be obtained to tailor the most efficient and effective surgical intervention. In addition, surgeons should routinely review the images themselves before an operation; they should not rely on reading the reports only. Surgeon-performed ultrasound has a proven track record of assisting the surgeon in localizing the enlarged gland and should be emphasized in the workup where possible. Accurate anatomic and functional data can dictate incision placement, patient positioning, and can help determine whether intraoperative nerve monitoring (IONM) may be necessary.
Preoperatively determining the accurate location of a suspected parathyroid adenoma can direct the surgical approach, minimizing dissection and maximizing efficiency during parathyroidectomy. Knowledge of the precise location of an enlarged parathyroid gland and its relationship to surrounding structures is essential for MIP and reoperation for persistent or recurrent disease. Simply put, if the imaging studies cannot localize the gland, an MIP should not be performed.
Our general approach is to use a nuclear medicine imaging study (to provide information on gland function) combined with an anatomic imaging study (to provide useful information about surrounding structures), such as ultrasound or CT. It is preferable to be able to “localize” a gland to a specific quadrant rather than just “lateralize” it.
The type of anesthesia to be used in MIP should be determined by the surgeon and anesthesiologist based on the patient’s surgical fitness, comorbidities, body habitus, expected procedure duration, location of the diseased parathyroid gland, and experience of the anesthesia team. Monitored anesthetic care using a combination of local anesthesia and sedation may be appropriate for thin patients who have a type E inferior gland that would be expected to require a minimal surgical procedure with a short operative time. General anesthesia may be best for a deep gland in the tracheoesophageal groove (type B or C gland) in an obese patient with minimal hyperextension and significant comorbidities. Access to a peripheral blood draw site must be identified before induction to allow easy access to peripheral blood for PTH assessment.
Before making an incision, the surgeon performing MIP must ensure the necessary components of a safety checklist are addressed. This includes standard verification of correct patient name, medical record number, procedure, operative laterality, presence of surgical consent, review of patient-specific studies (e.g., electrocardiogram or coagulation profile), and verbalization of anesthesia concerns. In addition to these items, review of documentation confirming biochemical proof of disease and examination of all relevant preoperative imaging should be performed in the operating room.
Operating room team members should be briefed on all necessary equipment before patient arrival. This may include an appropriate nerve monitoring equipped endotracheal tube (ET), associated supplies, and avoidance of paralysis if neural monitoring is planned. Ethylenediaminetetraacetic acid (EDTA), tubes, syringes, and the importance of rapid transport of the intraoperative PTH specimens should be discussed. At the appropriate time, operating room personnel should be clearly told which specific gland is excised to initiate appropriate specimen labels.
The anesthesiologist should be notified about the need for and anticipated timing of peripheral venous blood sampling for PTH. Some groups believe central venous sampling from the internal jugular vein is also feasible and accurate; however, measurement of extended intraoperative PTH values may be required. A central draw may be considered if peripheral access is difficult. If a central draw from the internal jugular vein is performed, all samples should be obtained from the same location along the vein. Our preference is to obtain peripheral venous access that can be used for blood draws as needed. There is no need for intraarterial access; a simple venipuncture at a predetermined location vetted by the anesthesia team before draping is usually adequate. Anesthesia or operating room personnel should be asked to begin a timer once the suspect gland has been excised. This may help notify the surgeon of postexcision time intervals to guide additional PTH draws.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here