Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the past 15–20 years, the practice of contrast-enhanced ultrasound (CEUS) has moved from a technique used in a few centres to a widely employed imaging modality that is used worldwide. This change in practice has been facilitated by an appreciation of the importance of the technique in day-to-day ultrasound practice, together with the development and increased availability of both CEUS-capable equipment and the contrast agents themselves.
The principle of ultrasound microbubble contrast agents is based on the peculiar augmentation of echo strength by small bubbles of gas. Unlike other imaging modalities, the majority of which have benefited from contrast-enhancing agents for many years, contrast enhancement in ultrasound was developed relatively recently. The phenomenon of gas-induced ultrasound contrast was first observed when during echocardiography an injection of indocyanine green through a catheter resulted in transient echo enhancement in the region of the catheter tip. This observed enhancement was the result of small air bubbles forming in the catheter tip and strongly scattering ultrasound energy back towards the transducer. Following this there has been progress in understanding the physics of ultrasound contrast enhancement and applying it to the development of encapsulated microbubble agents and imaging technologies for clinical use.
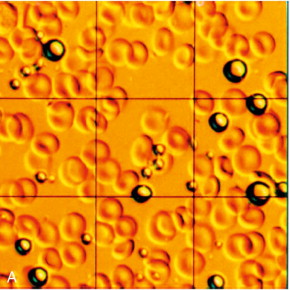
Ultrasound contrast agents in current use are typically small (3–5 μm diameter) gas-filled bubbles, which are slightly smaller than an erythrocyte ( Fig. 17-1 ). Microbubble contrast agents are physiologically inert, non-toxic and pass through the pulmonary circulation following intravenous injection. The microbubbles contain either air or an inert gas, encapsulated either by a thin shell composed of a biocompatible material such as a lipid, protein or more recently, synthetic polymer.
The intense echo enhancement observed with microbubbles is a result of the high compressibility of the gas they contain. A microbubble undergoes volumetric oscillation while being insonated, to a greater degree than a rigid sphere of similar size, and consequently scatters more energy. In addition there is a fortuitous relationship between the size of microbubble that is able to pass through a capillary and that which will resonate at the frequencies typically used in general ultrasound imaging (3–5 MHz). As such, at the resonant frequency, the returning echoes from a microbubble are maximised in such an effective manner that these microbubbles behave as though they are many orders of magnitude larger than their actual physical size.
When microbubbles are forced by the ultrasound imaging beam into resonant oscillation, they exhibit nonlinear motion, much as does the string of a musical instrument when plucked or struck. This results in the generation of harmonics in the ultrasound echoes, analogous to the overtones produced by a musical instrument. Just as these give the instrument its particular recognizable timbre when playing the same note, so the harmonic echoes from bubbles contain a characteristic pattern of frequencies that allows them to be distinguished from those of tissue, even though they arise from the same ultrasound beam. Ultimately, as acoustic power is increased physical disruption of the microbubbles begins to occur, producing echoes at fractions of harmonic intervals which can also be identified by the receiving instrument. The disruption of bubbles may be used to therapeutic advantage, such as drug delivery and cell membrane poration.
Concern over potential bio-effects associated with the use of ultrasound contrast agents has led to many experimental studies assessing whether the presence of microbubbles can act as cavitation seeds, thereby potentiating bio-effects. This has been reviewed by ter Haar and by the World Federation for Ultrasound in Medicine and Biology. , Although it has been shown that adding contrast agents to blood decreases the threshold for cavitation and related bio-effects (e.g., haemolysis, platelet destruction), no significant effects have been reported in circumstances that are comparable to the bubble concentrations and ultrasound exposure used in a low Mechanical Index (MI) diagnostic clinical examination. Nonetheless, it remains prudent to apply the ALARA ( As Low As Reasonably Achievable ) exposure principle to contrast ultrasound examinations by using the lowest MI, the shortest acoustic exposure time, the lowest contrast agent dose and the highest ultrasound frequency that is consistent with obtaining adequate diagnostic information.
At least 5 million injections of microbubble contrast for clinical diagnosis have been performed worldwide. These injections are very well tolerated and have an excellent safety record, with post-market surveillance suggesting that the predominant cause of severe adverse events is an anaphylactoid reaction, with an estimated rate of 1 in 7000 for both the perflutren microspheres approved for cardiac indications in the United States and the sulphur hexafluoride microspheres approved in Europe. This level of adverse events is comparable to that of most analgesics and antibiotics and lower than that for other imaging contrast agents, such as those used in CT imaging. A study by the Italian Society for Ultrasound in Medicine and Biology of more than 23 000 injections of a sulphur hexafluoride microsphere showed no deaths and two serious adverse events, giving a serious adverse event rate of less than 1:10 000.
Although there is currently no Food and Drug Administration (FDA)-approved radiologic indication in the United States, there is extensive experience with ultrasound contrast in echo-cardiology. A review of more than 18 671 hospitalised patients undergoing echocardiography in an acute setting in a single centre reported no effect on mortality from using contrast in this group. Furthermore, an analysis of registry data from 4 300 966 consecutive patients who underwent transthoracic echocardiography at rest during hospitalisation showed that 58 254 of these patients were given the contrast agent Definity™. Acute, crude mortality was no different between groups, but closer analysis revealed that in patients undergoing echocardiography, those receiving the contrast agent were 24% less likely to die within 1 day compared with patients not receiving contrast. Nonetheless, after four deaths of acutely ill cardiac patients, North American labelling currently advises caution when using microbubble agents in patients with severe cardiopulmonary compromise. At least one contrast agent is currently undergoing clinical development in the United States and seeking the first FDA approval for a radiologic indication in the USA.
(See Table 17-1 .)
| Brand name | Producer | Gas | Stabilisation | Approved Applications |
|---|---|---|---|---|
| Sonazoid™ | GE Healthcare | Perfluorobutane | Hydrogenated egg phosphatidyl serine | Abdominal |
| Optison™ | GE Healthcare | Perfluoropropane | Albumin | Cardiac |
| SonoVue™ | Bracco | Sulphur hexafluoride | Phospholipid | Cardiac Vascular Hepatic Breast |
| Definity™ | Lantheus Medical Imaging | Octafluoropropane | Phospholipid | Cardiac Abdomen Pelvis |
The need to form biologically and physically stable microbubbles in commercial quantities in a simple, cost-effective fashion has led to the development of a variety of microbubble technologies. At the time of writing, commercially available contrast agents approved for intravenous administration include the agents listed in Table 17-1 , the majority of experience and data are derived from studies employing Sonovue™ in Europe and Asia, Definity™ in North America and Sonazoid™ in Japan.
Optison ™ uses perfluoropropane gas which has slow diffusion into and low solubility in blood, extending the life of the agent. Excretion of the gas is ultimately through the lungs. Albumin provides the soft shell of the microbubble for which the only licensed clinical indication is cardiac imaging.
SonoVue ™ consists of sulphur hexafluoride encased by a phospholipid. The microbubbles are prepared by mixing the provided carrier fluid and freeze dried powder. Once prepared, the bubbles remain stable in the vial for several hours. The stability of this formulation in vivo allows real time imaging to occur for several minutes following administration. Licensed clinical indications for use of this agent include cardiac, macrovascular, liver and breast applications.
Definity ™ is licensed in the United States for cardiac use and comprises lipid-stabilised octafluoropropane gas. The indications include opacification of the cardiac chambers and improved delineation of the endocardial borders; in Canada, Definity™ is additionally approved for radiological applications in the abdomen and pelvis.
Sonazoid ™ is a lipid-stabilised perfluorobutane microbubble that allows both continuous vascular and late-phase Kupffer cell imaging. First licensed for use in Japan, it is envisaged that Sonazoid™ will be increasingly employed in the field of focal hepatic lesion detection and follow up post hepatic radio-frequency ablation.
The physical characteristics of microbubbles have allowed the development of a variety of contrast-specific imaging techniques. Equipment manufacturers attach a variety of proprietary names to the techniques employed; many of these share the same principles of operation and the majority of high-specification ultrasound machines have contrast-specific modes available.
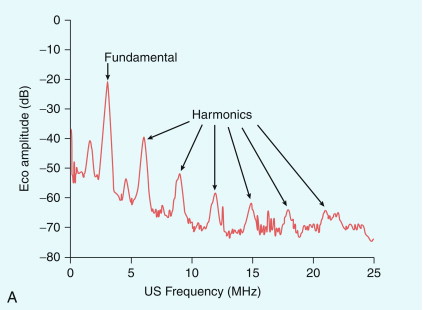
One simple method to distinguish bubbles from tissue is to excite the bubbles so as to produce harmonics and then detect these in preference to the fundamental frequency echo from tissue ( Fig. 17-2 A). Key factors in the harmonic response of an agent are the incident pressure of the ultrasound field, the frequency, the size distribution of the bubbles, and the mechanical properties of the bubble capsule – a stiff capsule will dampen oscillations and attenuate the nonlinear response. Although simple and effective, this method halves the bandwidth of the image, reducing its resolution and can be confounded by the propagation of a second ‘tissue’ harmonic echo which results from nonlinear propagation of ultrasound by non-bubble bearing structures, so reducing the image contrast. It is rarely used in modern systems.
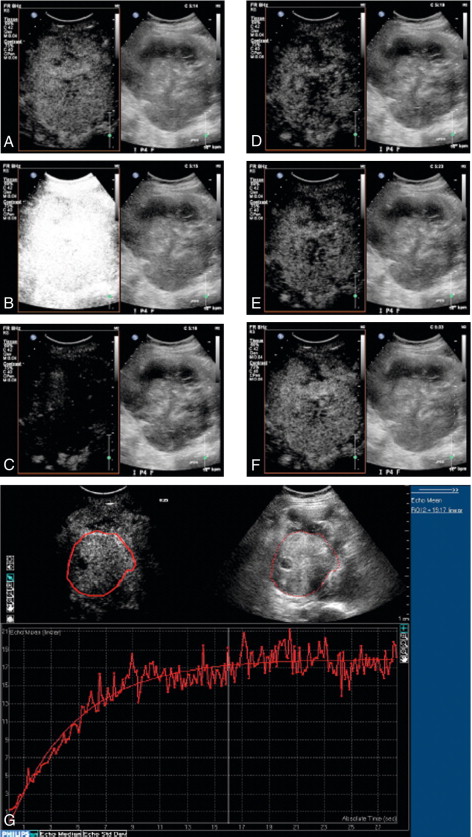
Phase or pulse inversion imaging (PII) achieves a better differentiation between bubbles and tissue without loss of resolution. PII involves transmission of an initial imaging pulse followed by a second pulse which is an inverted version of the first. The two resulting echoes are then summed: if they are scattered from a linear target, this sum is zero. However, because microbubbles produce non-linear scatter, the sum of the two signals from a microbubble will not be zero ( Fig. 17-2 B). In addition to allowing a wider bandwidth to be used than in harmonic imaging, PII also allows relatively low insonation pressures (low MI) which reduces the risk of microbubble disruption. This technique allows for continuous real time imaging during vascular phases, allowing detection of vascular patterns peculiar to different liver tumours. Inclusion of a third pulse of lower amplitude than the other two pulses allows detection of nonlinear echoes at the fundamental frequency, which improves penetration of the contrast image. This method is sometimes called ‘power modulation pulse inversion’ (PMPI) or ‘contrast pulse sequence’ (CPS).
Quantification of microbubbles is complicated by the fact that there is no simple unit of quantification of the contrast echo which can be measured reliably and reproducibly. Furthermore, contrast imaging using the multi-pulse sequences such as PMPI and CPS described above are subject to multiple artifacts including motion artifact from patient or operator movement which can be difficult to avoid in long examinations. Initial work in the field of quantification of microbubbles focused on assessment of a number of variables including time to arrival, wash-in/wash-out characteristics, time to peak and the area under the curve ( Fig. 17-3 ). With advances in technology, quantification techniques have progressed to allow microbubbles to be used for true, functional imaging in the form of dynamic contrast-enhanced ultrasound (DCE-US). Unlike other functional imaging techniques, DCE-US avoids the need for ionising radiation and so may be used repeatedly to monitor, for example, tumour response to treatment.
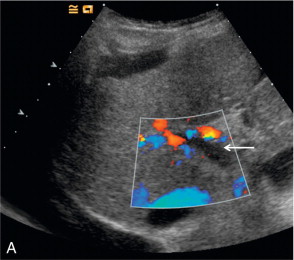
Artifacts occur predominantly with the older high MI imaging techniques. One of the most commonly seen artifacts when using microbubbles to enhance conventional Doppler studies is that of ‘blooming’ in which colour pixels appear to extend beyond the bounds of the vessel ( Fig. 17-4 ). This phenomenon may prove problematic when objective computer analysis of contrast enhancement is required. While the problem arises partly from multiple re-reflections between adjacent microbubbles, it is also in part due to limitations of the hardware analysis of the Doppler signal.
A further artifact resulting from limitations in the ultrasound hardware is the apparent reversal of Doppler flow following administration of contrast. This effect represents overloading of the Doppler circuitry and once appreciated is easily recognised.
Intravascular contrast may make high-velocity shifts detectable, which pre-contrast were not detectable. Whilst this may initially appear to be a desirable effect, it can be problematic when absolute angle-corrected values are required for clinical decision making, for example in assessment of internal carotid artery stenosis.
Sharp spikes of Doppler ‘noise’ may occasionally be seen, which may arise from collapse of the microbubbles.
‘Pseudo-enhancement’ may arise from echogenic structures which are non-perfused but lie distal to a body of bubbles. This artefact is commonly seen after radio-frequency ablation of liver lesions where it can be misinterpreted as an incomplete ablation.
Cardiology represents the single greatest area of use of microbubble contrast agents with several established and evolving applications.
Patient factors including obesity and lung disease render a significant number of echocardiograms non-diagnostic, particularly in the case of stress echocardiography. Microbubble contrast agents allow improved delineation of the endocardial border and detection of wall motion abnormalities, as a result, up to 74% of otherwise non-diagnostic studies may be ‘rescued’. Use of microbubbles allows additional information regarding regional and global left ventricular function to be acquired during a stress echocardiography examination. Furthermore, valvular disease may be more accurately assessed by contrast-enhanced Doppler.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here