Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The lower female genital tract includes the vulva, the vagina, the cervix, and the uterine cavity. It is in direct communication with the external environment, and prone to various non-infectious and infectious inflammatory reactions. Although most of these infections remain confined locally; they can progress and the organisms may ascend to the fallopian tubes and the ovaries. Occasionally, microbial infections from the lower genital tract can disseminate via the peritoneal space or the hematogenous or lymphatic routes.
It must be appreciated that while a large number of women harboring genital infections may remain asymptomatic, vaginal infection can produce a number of clinical symptoms. Increased vaginal secretions, along with sloughed vaginal epithelial cells, other infective organisms, bacteria, and inflammatory cells, constitute acute vulvovaginitis. Inappropriate use of over-the-counter medications, personal hygiene, tight clothing, impermeable panty hose (panty hose vulvitis), reaction to various laundry detergents, and washed clothes may contribute to the symptoms of vulvovaginitis. Often, personal and social reasons may delay medical intervention.
Increased vaginal secretions
Sloughed vaginal epithelial cells
Bacteria
Infectious organisms
Inflammatory cells.
Vaginitis accounts for nearly 6 million visits to the healthcare providers per year, with an annual cost of over a billion dollars to society. Vulvovaginal irritation, itching, pain, ulceration with bleeding, dyspareunia, and warty growths are some of the common presenting features of vaginal infections. Most often, a woman may remain minimally symptomatic and may not seek medical help for various personal and social reasons.
Among healthy women, the vaginal milieu is polymicrobial and contains a large number and variety of aerobic as well as obligate and facultative anaerobic organisms. The most frequently recovered bacteria include lactobacilli, Streptococcus viridans , and Staphylococcus epidermidis ; none of which cause symptoms. Bacteroides and Gardnerella vaginalis may be culturable from 20–30% and 50% of the asymptomatic women, respectively. Staphylococcus occurs infrequently in the healthy vaginal flora. Table 7-1 lists the microorganisms that can be commonly recovered from vaginal specimens.
| Lactobacilli | Bacteroides species |
| Diphtheroids | Peptococcus species |
| Staphylococcus species | Peptostreptococcus species |
| Streptococcus species | Fusobacterium species |
| Enterobacter (not group A) | Clostridium species |
| Gardnerella vaginalis | Bifidobacterium |
Pregnancy, besides causing a growth of lactobacillary flora, does not appear to affect the microbial composition of the vagina significantly. Estrogenic hormones and similar substances help in the epithelial maturation of the vagina and support the growth of an extraordinary number of microbes. Transplacental hormonal exchanges also influence the vaginal epithelium of the newborn infant. Bacterial composition and an adult type of microenvironment may occur in a newborn female infant.
Menarche and menopausal changes also affect the bacterial makeup of the vagina. Hormone or hormone-like medications, contraceptives, intrauterine contraceptive devices (IUDs), barrier diaphragms, pessaries, and other similar substances, and contraception, may directly or indirectly influence the microbial balance of the lower genital tract. Common factors influencing the vaginal microbial flora are presented in Table 7-2 . It must be appreciated that the vaginal flora is in a dynamic state physiologically and in health. It contains a large number of organisms that, under poorly understood conditions, may become pathogenic and cause disease.
| Physiologic | Diseases and Drugs | Local Factors |
|---|---|---|
|
|
|
A number of general cytologic features represent the various effects of infective processes. These include the changes listed in Table 7-3 . Specific cytologic changes frequently are associated with certain infections and are described in their respective areas. Only some of these responses may occur under specific inflammatory conditions.
| General | Cellular Degenerative Changes | Cellular Reactive |
|---|---|---|
|
|
|
Overgrowth of microbes in the vaginal milieu may result in obscuring of morphologic details in the smear ( Fig. 7-1 ). In such smears, numerous polymorphonuclear leukocytes occur, often interspersed with a large number of histiocytes. Excessive bacterial growth may also contribute to cellular obscuring. It must be realized that no meaningful evaluation of cellular change may be possible on such smears. In all such cases, it is almost mandatory that appropriate therapy be initiated and a repeat smear examined before an opinion is rendered. Atrophic epithelium of the vagina is particularly prone to inflammatory changes ( Fig. 7-2 ) that may mimic atypia. In such cases local hormonal application generally helps in proper interpretation of cells. In specimens with overwhelming inflammatory exudates, it may not be possible to differentiate vaginitis from cervicitis and endocervicitis. Inflammatory exudates and vaginal microbial flora are considerably altered in liquid-based gynecologic slides (LBGSs).
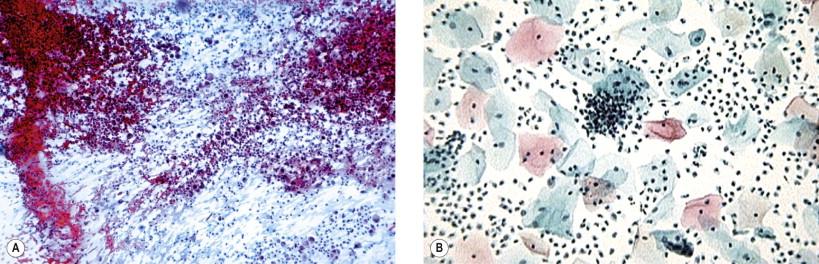
Background, inflammatory exudate and microbiologic flora may not be obvious in LBGSs.
Important to treat with local estrogens
Repeat cytologic examination after 6 weeks
Immediate colposcopy not recommended.
Cellular specimens from specific areas, e.g., the vaginal, cervical, and endocervical smear, or vulval or lateral vaginal wall scrapings, may reveal inflammatory response in one or more preparations that can help localize the infective process within the lower genital tract. A specific cervical inflammation with predominant lymphohistiocytic reaction may occur in follicular cervicitis (discussed later). Granulomatous reaction may occur in the presence of foreign bodies (e.g., suture material, surgical clips, IUD) or specific infections such as tuberculosis.
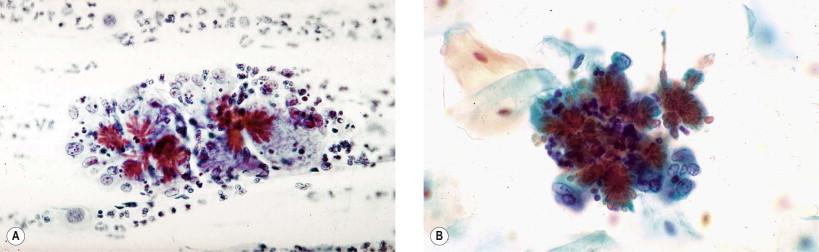
In infectious processes, both fresh and old blood may be observed. Postmenopausal women with atrophic, thin vaginal mucosa may bleed more easily. Similar changes may occur in Trichomonas vaginalis infection, which produces the typical “strawberry” cervical lesion. Whereas the fresh bleeding is recognizable without much difficulty, old bleeding, observed as fibrin, should be differentiated from mucus. In direct smear preparations, fibrin threads are uniformly thick and reveal nodal formations at the points of intersections of interlacing threads. Sometimes, hemosiderin pigment may be observed within the macrophages and extracellularly. Sometimes, old hemorrhage may contain hematoidin crystals that appear as “cockleburs,” as described by Hollander and Gupta ( Fig. 7-3 ).
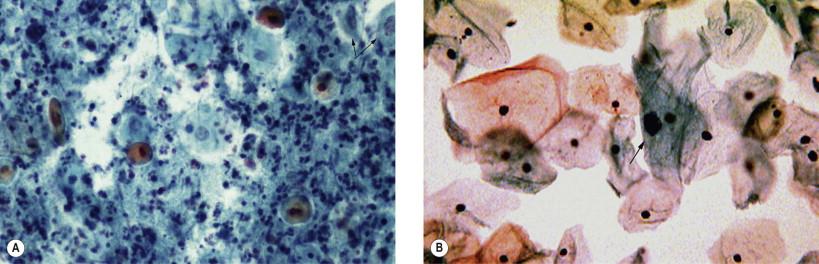
Cytolysis, the process of cellular degeneration due to bacterial overgrowth, commonly affects the intermediate squamous epithelial cells. The process is believed to be glycogen dependent. Late menstrual cycle and pregnancy as well as hormonal contraceptives often cause lactobacilli overgrowth. Pale staining, vesicular nuclei with little or no cytoplasm of the intermediate cells predominate in such smears. Numerous lactobacilli may occur interspersed with the remaining cellular remnants ( Fig. 7-4 ).
In cervicovaginal smears, intermediate and superficial cells occur predominantly among healthy and normally menstruating women. Heavy inflammation frequently causes an exfoliation of parabasal cells in the vaginal smear. Although often present in postmenopausal women and in the immediate postpartum period, occurrence of these three types of cells in the premenopausal age group should be carefully evaluated. Caution need to be exercised in rendering hormonal evaluation in smears with excessive inflammation. The parabasal cells may exfoliate from ulceration of the squamous epithelium of the vagina and the ectocervix.
Under the persistent effect of various microbial infections and inflammatory reactions, both squamous and columnar epithelial cells may undergo degenerative changes. Almost all these changes are nonspecific, but their identification helps in the proper interpretation of more serious cellular alterations. This is critical because most degenerative changes may be accompanied by concurrent regenerative, reactive, and metaplastic changes. An extremely heterogeneous group of cellular changes that includes reactive, degenerative, metaplastic, and neoplastic features is termed atypical squamous cells (ASC). Morphologic features of these cells overlap and generally are nonspecific, meaning that they cannot be precisely separated between neoplastic and non-neoplastic changes. Additional studies are often necessary for further characterization of these changes.
In inflammatory states cytoplasm of the squamous and columnar cells may be completely or partially disintegrated. However, the major changes are observed within the nuclei. These have been detailed by Frost. Briefly: the nuclei may become compact, dense, and pyknotic with loss of all chromatin details ( Fig. 7-5 ). Such nuclei may have a distinct circumferential cytoplasmic clearing or hollow, causing a perinuclear “halo” ( Fig. 7-6 ), often seen in association with Trichomonas and other infections. Nuclei undergoing degenerative changes frequently lose the sharp details of their nuclear envelope, the chromatin, and the interphase. The nuclear chromatin may clump irregularly or appear beaded along the nuclear margins. They may become blurry and opaque. Other changes include nuclear swelling, with partial or total disintegration of the nuclear envelope, karyorrhexis, and karyopyknosis ( Fig. 7-7 ).
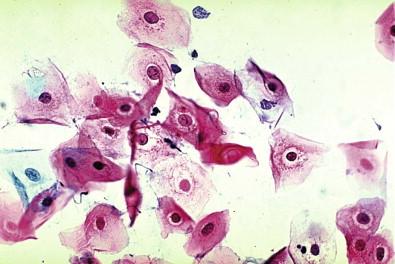
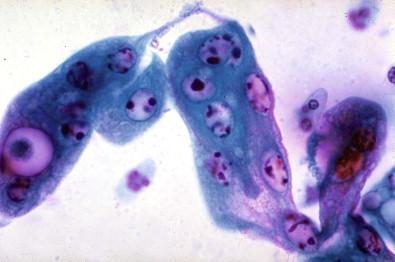
The epithelial cells of the lower genital tract (ectocervix, endocervix, and transformation zone), under the influence of persistent irritation (infectious and non-infectious) and repair, undergo morphologic changes commonly referred to as hyperplasia and metaplasia. These essentially reflect a benign process of tissue repair. An overestimation of reparative changes is a most common error in interpretive cytology. Geirsson and coworkers referred to these as atypical reparative changes (ARCs). The majority of these changes are believed to be derived from the columnar and squamous epithelia, but reserve or pluripotential cells may be involved in the genesis of the metaplasia. It must be appreciated that there is a continuum of changes observed in the healing phase. Cytomorphologic changes, for convenience, are grouped under the term metaplasia. Some of these morphologic changes can be indistinguishable from “early” intraepithelial dysplastic alterations, observed within both the squamous and the glandular epithelia. These cellular features are grouped as “atypical squamous or glandular cells of undetermined significance” (ASC, AGC).
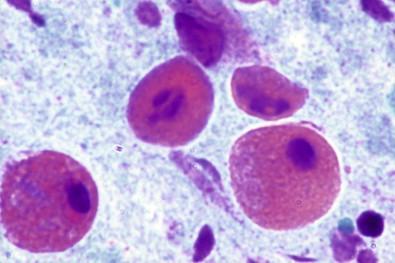
The earliest discernible changes are referred to as presquamous metaplasia: columnar differentiation phase, or type I ARCs of the cervical columnar epithelium. Under the effect of chronic irritation and repair processes, the surface cells of the columnar epithelium continue to mature, and there is a proliferation of the basal or reserve cells. These small, undifferentiated cells commonly occur in small tissue fragments. They have high nucleus-to-cytoplasmic ratios and prominent nucleoli ( Fig. 7-8 ). The nuclear chromatin is fine and uniformly distributed, and the nuclear membrane is well delineated and thin. These may have an inflammatory background. The cells of subluminal origin (progenitor cells) can often be mistaken for undifferentiated neoplasia.
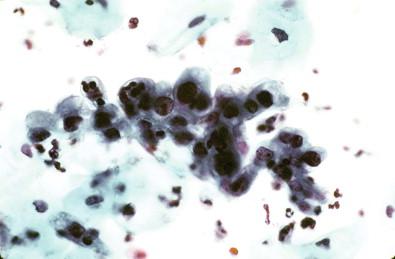
As the changes evolve, the subluminal cells differentiate from the germinal layers upward. These changes reflect the immature squamous metaplastic epithelium and have been referred to as ARC II. The cells may appear to be columnar and have excessive goblet cell proliferation and mucus production. Signet-ring forms may be recognized. These cells can have numerous macronucleoli, coarse chromatin, and modest but pale cytoplasm. If not carefully examined, the changes can be mistaken for a neoplastic lesion of the endocervix ( Fig. 7-9 ). Presence of heavy acute inflammation generally helps in the correct interpretation of these changes. In the LBGS, inflammation is less obvious and these cells often occur as small tissue fragments and may be reported as AGC.
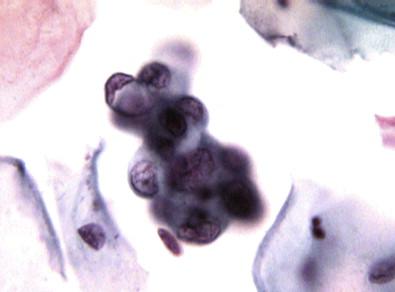
As the changes progress from the subluminal, via the presquamous, to the keratinizing stratified squamous phase , the cells become oval or polygonal with sharp borders and dense cytoplasm. They lie in sheets and reveal no obvious cilia and mucus. Intercellular bridges may be seen at times. These cells with their metaplastic changes have been called ARC type III. The nuclear changes may be reactive or degenerative with pyknosis. These cells may be mistaken for squamous cell carcinoma ( Fig. 7-10 ).
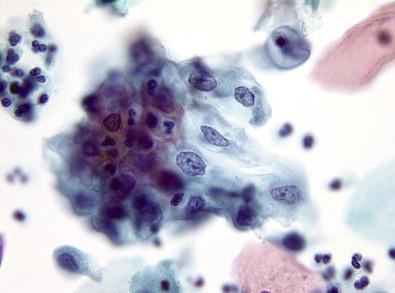
When stressed, as by a chronic irritation or an infective injury, squamous epithelium responds in a number of ways. These changes essentially represent alteration of functional differentiation of the affected cells and are mostly cytoplasmic in nature. Proper identification of these cytoplasmic features is necessary, as they may mask a more serious underlying disease process. These changes–hyperkeratosis, parakeratosis, basal cell hyperplasia, pseudoparakeratosis, and dyskeratosis–have been discussed elsewhere in this book. They reflect abnormalities of maturation with normal keratin formation in cells that normally do not reveal these changes.
Hyperkeratosis
Parakeratosis
Basal cell hyperplasia
Pseudoparakeratosis
Dyskeratosis.
Dyskeratosis is mentioned here because of its relationship with viral infections and developing cancer. This represents an abnormality of the squamous cells in which the cytoplasmic maturation is altered. The affected cells reveal premature, hypermature, or atypical keratinization. It is a common occurrence in the presence of chronic infections, such as those caused by human papillomavirus (HPV). The cytomorphologic features are further detailed in the appropriate sections.
In addition to the squamous metaplasia discussed earlier, endocervical cells may undergo other morphologic changes including columnar cell hyperplasia and hyperplastic polyp formation.
Endocervical cells frequently enlarge and produce excessive mucus. Such changes occur with chronic irritation of the endocervical canal, such as among women using IUDs or hormonal contraceptives, and who are exposed to certain infections; these are discussed separately.
Hyperplastic endocervical columnar cells may proliferate to produce finger-like epithelial processes–polyps. As described by Ramzy and Frost, these polyps are 3-dimensional structures with three distinct planes: a floor or base composed of a sheet of polygonal cells; a middle plane that makes up the sides of the polyp; and a top or surface layer that, like the base, is also a sheet of polygonal cells. In the center of the polyp, a connective tissue core that contains fibroblasts, collagen, and capillary vessels may be recognizable.
Generally, endocervical epithelium may contain ciliated columnar cells only in small numbers (5–10%). Under conditions of chronic irritation, sheets of ciliated cells representing tubal metaplasia may occur in the specimens collected from the transformation zone and the adjacent endocervix. These cells may show pseudostratification and atypia and can be a common source of misinterpretation as neoplastic cells. Tubal metaplastic cells may stain positively with p16INK4A antibodies, which may lead to confusion with neoplastic processes.
Hyperplasia
Polyp formation
Squamous metaplasia
Tubal metaplasia
Heavy acute inflammation, with pronounced reactive, degenerative, and metaplastic changes may be observed in these cells during the latter half of menstrual bleeding, following instrumentation, in the postpartum period, and in association with the usage of an IUD. Retained gestational products and foreign bodies may result in extensive squamous metaplasia, multinucleated giant cell reaction, and calcification. Some of these changes are discussed later in this chapter.
Bacteria most commonly infect the female genital tract. Bibbo and Wied reported nonspecific organisms including mixed bacteria and coccobacilli in nearly 20% of patients. Among children, these infections occur commonly and may be hormonally dependent. Vaginal or cervicovaginal smears often reveal a number of bacilli and coccoid organisms (mixed infections), as detailed by Wied and Bibbo. These organisms, although diffusely scattered, may occur in clumps and as microcolonies. Appropriate microbiologic isolation techniques are necessary for specific species identification but are generally not considered necessary for clinical management of the disease.
Bacterial vaginitis (nonspecific vaginitis) was first described by Gardner and Dukes. They stated, “Any woman whose ovarian activity is normal and who has a gray, homogenous, malodorous vaginal discharge with a pH of 5.0 to 5.5 that yields no Trichomonads is likely to have Haemophilus vaginalis vaginitis.” It is also known as nonspecific vaginosis/vaginitis, so named by Blackwell and Barlow, or bacterial vaginosis (BV), a term used by the International Agency for Research on Cancer. This is the most common cause for the clinical entity of bacterial vaginosis. Instead of making a specific diagnosis of G. vaginalis infection, reporting of a “shift in the bacterial flora” is the current term used to describe the organism variously named as Haemophilus vaginalis and Corynebacterium vaginalis . Gardner and Dukes first described these organisms. Regarding the etiology of BV, the statement by Fredricks and Marrazzo that “BV probably results from infection with complex communities of bacteria that consist of metabolically interdependent (syntrophic) species” appears true.
Morphologically, the organisms are Gram-negative or Gram-variable, are 0.1–0.8 nm in diameter, and appear bacillary or coccobacillary. The microbe, although it shares many characteristics with Corynebacterium , is catalase-negative and is now classified separately. Petersdorf and colleagues and Ledger and associates found that as many as 40–50% of women may have vaginal infection with G. vaginalis and be asymptomatic. Among symptomatic women, leukorrhea and pruritus with inflamed vaginal mucosa and occasional punctate hemorrhages are commonly observed. Increased growth and concentration of these organisms may not denote pathogenicity. It is believed that patients with pure G. vaginalis infection are asymptomatic when the vaginal pH is <4.5. Secondary organisms interplay with G. vaginalis and alter this synergistic relationship. A raised pH >4.5 (5.0–6.5) and an interaction with various bacteroides and peptococci may produce clinical disease. Recently, considerable interest has been exhibited in the study of BV. Molecular identification of the associated bacteria has revealed the presence of three bacteria in the Clostridiales order. These are named as bacterial vaginosis-associated bacteria (BVAB1, BVAB2, and BVAB3). BVAB are considered highly specific for BV infection.
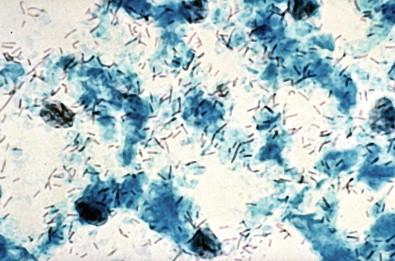
Patients with a high pH of the vagina have a vaginal discharge with a distinct fishy odor. When the pH is further raised by potassium hydroxide (KOH), this odor is manifest in the “whiff test.” Such preparations of vaginal reactions and KOH, when examined microscopically, have the diagnostic “clue cells” ( Fig. 7-11 ). These refer to normal polygonal squamous cells having thin, transparent cytoplasm covered by tiny coccobacillary forms of G. vaginalis . Edges of the “infected” cells reveal the BV changes. The cell borders may be indistinct and on a different plane of focus. Similar clue cells are observed in the fixed and stained Papanicolaou preparations ( Fig. 7-12 ). A variable amount of acute inflammation may be present in the background. More complete or partial covering of the squamous epithelial cells by the organisms ( Fig. 7-13 ) or their sticking to the cellular margins ( Fig. 7-14 ) per se should not be considered diagnostic for G. vaginalis . To be diagnostic, clue cells should have bacterial organisms not only covering the surfaces of the affected cells but also spreading beyond the margins of the squamous cells. In LBGS preparations, organisms appear in a clean background ( Fig. 7-15 ). Detection of BV is reported to be considerably less in LBGS preparations than in conventional slides. This may not be an entirely true observation. A high degree of diagnostic accuracy exists in cytologic detection of clue cells and culture confirmation for G. vaginalis . Schnadig and coworkers cultured G. vaginalis in nearly 90% of the cases that contained clue cells. This infection is believed to be sexually transmissible, and an accurate diagnosis is necessary.
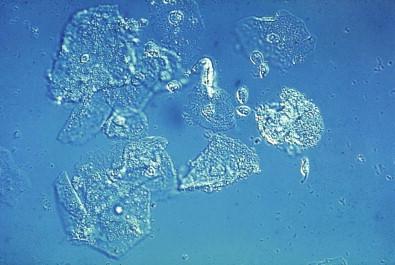
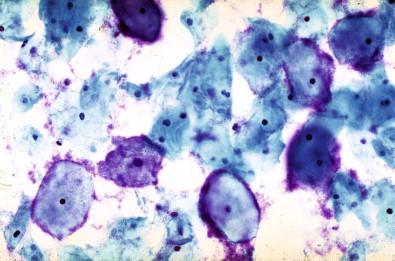
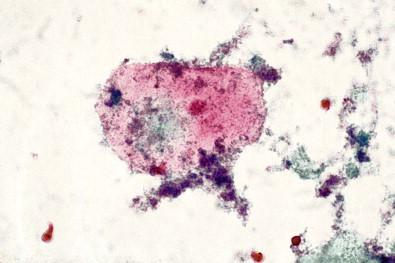
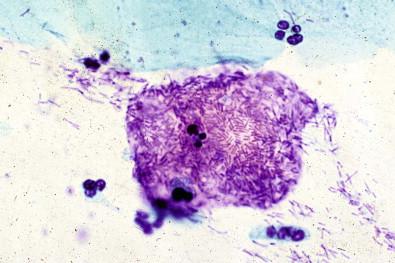
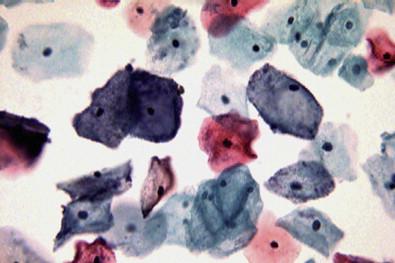
This entity is now rarely observed in current practice. This group of microbes includes a large number of Gram-positive coccoid organisms commonly observed in female genital tract smears, and Gram-negative diplococci. Staphylococcus aureus may be recovered from the vagina in about 5% of normal women. These organisms frequently cause vaginitis and vaginal discharge and may produce toxic shock syndrome. This association was documented by Shands and coworkers in 1980. These organisms characteristically occur singly and can be seen within the polymorphonuclear leukocytes or other infected epithelial cells. In vaginal smears, occasionally fragments of tampon fibers may be observed ( Fig. 7-16 ). However, the finding of coccoid organisms or tampon fibers in the vaginal smear does not have any correlation with the clinical occurrence of toxic shock syndrome.
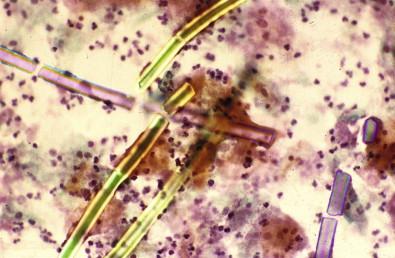
Lactobacilli are a heterogeneous group of organisms normally present in the vaginal flora. They occur in abundance in the late luteal phase and in pregnancy, prefer an acid environment, and are common among women using hormonal preparations (contraceptives and replacements) and in the premenarchal and menopausal age groups. They are Gram-positive, immobile, non-spore-forming anaerobes or facultative anaerobes. Certain species may be aerobic in their growth characteristics. In the presence of lactobacilli, glycogen-rich intermediate cells are often lysed. Smears in such cases show cellular crowding, cytolysis with cytoplasmic debris, and numerous bare nuclei occurring in a predominantly bacillary background. False clue cells can be reported in these cases as the lactobacilli adhere to the edges of squamous cells. Lactobacilli may be observed in up to 50% of healthy women depending on the day of the menstrual cycle. In the symptomatic population, the observed figure may be lower, about 20%. It is debatable whether pure lactobacilli (an unlikely occurrence) produce vaginitis, although vaginal discharge and leukorrhea may occur as a result of excessive cytolysis.
These Gram-negative diplococci cause abundant, purulent vaginal exudates. The infection affects the urethra and the perivaginal glands. On the surface of squamous cells, these organisms occur as bean-shaped diplococci. The gonococci are better observed in the air-dried areas of the smears, such as the edges of the smear. This is an uncommon occurrence in properly prepared LBGSs. Within the air-dried distended polymorphonuclear leukocytes, diplococci may be present in large numbers ( Fig. 7-17 ). Gonococcus vaginitis is a venereal infection with important social and medical implications. Although it is detectable cytologically, we do not advise rendition of such a diagnosis on cytologic examination of Papanicolaou-stained smears alone; they may be indistinguishable from other cocci organisms, phagocytosed debris, or Chlamydia organisms.
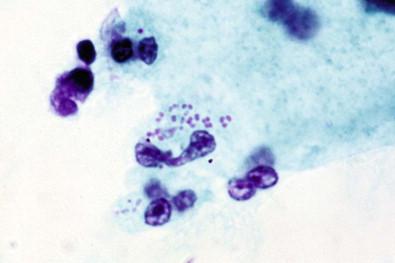
These motile, anaerobic, rod-shaped organisms resemble Wolinella and have been recognized as a cause of nonspecific vaginitis by Hjelm and colleagues. In the Papanicolaou-stained smears, these bacteria cannot be easily diagnosed but are better detected in wet-mount preparations. Clinically, the presentation is of nonspecific vaginitis.
A recently recognized clinical picture has been reported among women who have used antifungal local medications for genital Candida infection for a prolonged period of time, generally more than 20 months. These result in the proliferation of giant lactobacilli often accompanying the yeast forms of Candida organisms. A correct morphologic recognition of this condition is important for specific treatment with appropriate antibiotics ( Fig. 7-18 ).
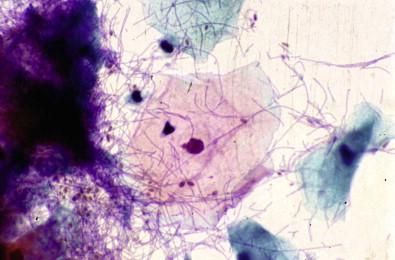
A forgotten tampon is the most common cause of this type of vaginitis, in which there is a secondary overgrowth of anaerobic organisms. The tampons may irritate and ulcerate the vaginal wall and ectocervix. Occasionally, fragments of tampons can be observed in vaginal smears. Their presence is not diagnostic of vaginitis. Heavy acute inflammation, mucus, and foreign-body giant cells may be observed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here