Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
By the end of this chapter the reader should:
Understand the biochemistry of metabolism, including urea cycle, Krebs cycle and fatty acid cycle
Understand the pathophysiology of metabolic disorders, e.g. electrolyte and acid–base disturbance, hyperammonaemia and hypoglycaemia
Know the genetic and environmental factors in the aetiology of metabolic disorders
Be aware of the metabolic disorders identified on neonatal screening
Understand the investigations that are used to diagnose metabolic disorders
Understand the principles of dietary and pharmacological treatment of metabolic diseases
Intermediary metabolism is the term given to the biochemical reactions that degrade, synthesize or interconvert molecules within the cells. There are numerous metabolic pathways, which serve the following aims:
Generation of energy
Catabolism of organic molecules
Synthesis of cellular building blocks
Excretion of harmful substances.
These pathways require enzymes, which, if absent or deficient, can give rise to an inborn error of metabolism (IEM). This chapter describes the key metabolic pathways and links them with their associated diseases.
The key terms are outlined in Table 29.1 .
| Terminology | Definition |
|---|---|
| Acid | A proton or hydrogen ion donor. It can dissociate to yield H + and the corresponding base. |
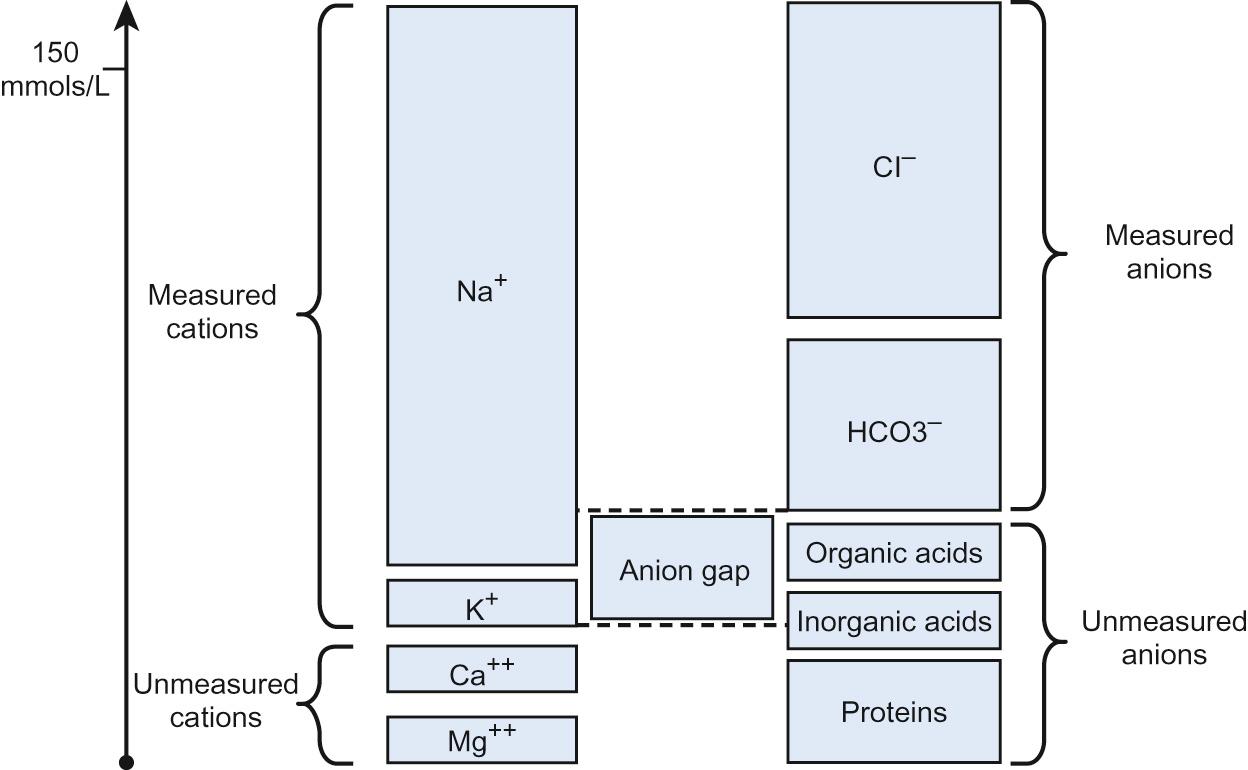
| Anion gap (see Fig. 29.1 ) | [Na + + K + ] − [Cl − + HCO 3 − ]. Normal = 10 − 16 mmol/L. Reflects concentration of those anions not routinely measured, e.g. organic acids (see Question 29.1 ). |
| Base | A proton or hydrogen ion acceptor. Can accept H + to form corresponding undissociated acid. |
| Base excess | Measures the change in the concentration of a buffer base from the normal value. Normal range = +/− 2 mmol/L. |
| Buffer | Consists of a weak acid in the presence of its base. A buffer serves to minimize changes in H + concentration in response to the addition of an acid or base. Examples of buffers in: Plasma – bicarbonate, proteins, inorganic phosphate (Pi) Erythrocytes – haemoglobin, bicarbonate, Pi Kidneys – bicarbonate, Pi, ammonium |
| pH | The logarithm to the base 10 of the reciprocal of the hydrogen ion concentration. pH = −log [H + ] |
| pKa | The pH of a buffer at which half the acid molecules are undissociated and half are associated. |
Acid–base balance is essential for correct cellular functioning. Blood gas measurement can identify the primary disturbance ( Table 29.2 ). In general:
Metabolic disturbances are compensated acutely by changes in ventilation and chronically by renal responses
Respiratory disturbances are compensated by renal responses.
| Abnormality | Primary disturbance | Effect on | Base excess | Compensatory response | |
|---|---|---|---|---|---|
| pH | pCO 2 | ||||
| Respiratory acidosis | ↑ pCO 2 | ↓ | ↑ | Negative | ↑ [HCO 3 − ] |
| Metabolic acidosis | ↓ [HCO 3 − ] | ↓ | N or ↓ | Negative | ↓ pCO 2 |
| Respiratory alkalosis | ↓ pCO 2 | ↑ | N or ↓ | Positive | ↓ [HCO 3 − ] |
| Metabolic alkalosis | ↑ [HCO 3 − ] | ↑ | N or ↑ | Positive | ↑ pCO 2 |
In the case of metabolic acidosis, calculation of the anion gap will determine if there is the presence of an unmeasured anion such as an organic acid, e.g. methylmalonic or propionic acid ( Table 29.3 and Fig. 29.1 ). Acidosis with a normal anion gap is often associated with hyperchloraemia because the loss of base is buffered by an increase and/or retention of chloride.
| With normal anion gap | With raised anion gap |
|---|---|
|
|
Metabolic acidosis is a common finding. In the majority of cases, it reflects severe illness rather than an inborn error of metabolism (IEM). The latter should be considered if the acidosis is out of keeping with the clinical picture, is persistent despite standard management and there is no identifiable acid present, e.g. lactate or ketones.
Metabolic acidosis is typically non-specific in presentation. Signs may include a reduced conscious level, vomiting or those associated with the underlying aetiology, e.g. non-blanching rash in the case of meningococcal sepsis. Many patients will display an increased respiratory rate, Kussmaul respiration, reflecting the compensatory hyperventilation that occurs to promote removal of carbon dioxide.
The blood gas is key to identifying the primary disturbance in acid–base balance. In addition to calculating the anion gap, ketones and lactate should be measured as potential causes of acidosis. When investigating for an IEM, urine organic acids and plasma amino acids and acylcarnitines are required. It is important to measure an ammonia level as this can be elevated in an organic acidaemia due to the metabolites inhibiting the urea cycle.
The underlying aetiology, when known, should be treated. If acidosis is severe, normalization of acid–base balance can be achieved with administration of sodium bicarbonate.
Normal plasma lactate is <2 mmol/L. A raised level has a wide differential ( Table 29.4 ). In terms of IEM, mitochondrial disorders are classically associated with a raised lactate, with levels often fluctuating. When considering the possibility of mitochondrial disease, measuring cerebral spinal fluid for a raised level can be helpful. However, a normal lactate does not exclude a mitochondrial disorder.
A 6-day-old 3 kg term baby boy, born after a normal pregnancy and delivery, presents with reduced feeding and tachypnoea (respiratory rate 80/minute) over the last 24 hours. On examination, he is encephalopathic.
Investigations:
| Full blood count | mild pancytopenia |
| Sodium | 136 mmol/L |
| Potassium | 3.6 mmol/L |
| Chloride | 110 mmol/L |
| Lactate | 8 mmol/L (1–2.8) |
| Ammonia | 60 µmol/L (normal <100) |
| C-reactive protein | 6 mg/L |
| pH | 7.29 |
| pCO 2 | 2.0 kPa (15 mmHg) |
| pO 2 | 13 kPa (98 mmHg) |
| Bicarbonate | 10 mmol/L |
| Base excess | −18 mmol/L |
Which of the following is the most likely diagnosis? Select ONE answer only.
Group B streptococcal septicaemia
Hypoxic–ischaemic encephalopathy
Organic acid disorder
Surfactant protein B deficiency
Urea cycle defect
C. Organic acid disorder.
There is a marked anion gap. The anion gap = (136 + 3.6) − (110 + 10) = 31.6 mmol/L.
In this patient, the gas normalizes with intravenous 10% dextrose and two half corrections of sodium bicarbonate. Further investigations: urine organic acid analysis reveals methylmalonic acidaemia (MMA).
Group B streptococcal septicaemia is possible, but is more likely to present with shock and a much more abnormal blood count, including low or high white blood cell count and thrombocytopenia. The low CRP in spite of being ill for 24 hours is also against this diagnosis. Hypoxic–ischaemic encephalopathy would present before 6 days. Surfactant protein B deficiency would present with increasing respiratory distress from birth. A urea cycle defect is possible, but the ammonia level is normal for a neonate.
Methylmalonic and propionic acidaemia are the most common organic acidaemias
Can cause pancytopenia because of effects on the bone marrow at times of decompensation
pH can be maintained with hyperventilation
A lactate of 8 mmol/L would not by itself generate such a large anion gap
To calculate a half correction (using this case as an example):
| Metabolic | Non-metabolic |
|---|---|
|
|
Ammonia is a highly neurotoxic chemical detoxified by the urea cycle ( Fig. 29.2 ), which principally occurs in the liver. Ammonia is formed from:
Nitrogen produced from amino acid metabolism
Glutamate by the action of glutamate dehydrogenase
Glutamine by the action of glutaminase.
Alanine and glutamine produced by muscle turnover
Urease-positive gut bacteria
Ingested protein not utilized in biochemical processes
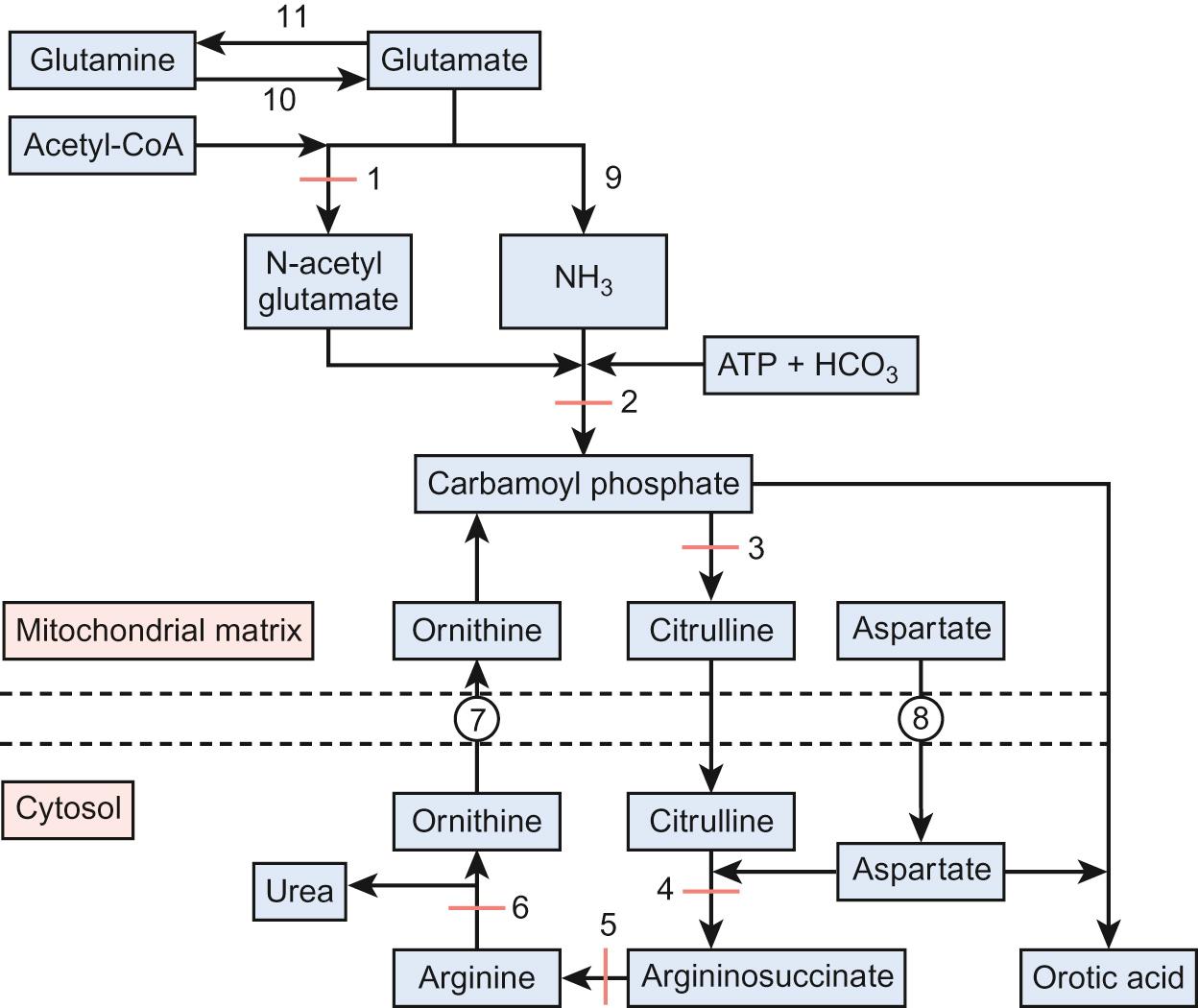
The urea cycle (see Fig. 29.2 ) consists of six enzymes, with each full cycle disposing of two nitrogen atoms: one from ammonia and one from aspartate. The cycle progresses as:
N -acetylglutamate forms from the condensation of glutamate with acetyl-CoA catalysed by N -acetylglutamate synthetase
Condensation of ammonia with bicarbonate forms carbamoyl phosphate catalysed by carbamoyl phosphate synthetase. The latter is only active in the presence of N -acetylglutamate.
Carbamoyl phosphate condenses with ornithine to form citrulline
Citrulline is transferred into the cytoplasm and combines with aspartate to form argininosuccinate, catalysed by argininosuccinate synthase.
Argininosuccinate lyase cleaves argininosuccinate to arginine
Arginine is hydrolysed to urea, which is excreted in urine. Each urea molecule contains two nitrogen atoms and one carbon atom. Ornithine is transported back into the mitochondrion by the ornithine transporter.
Ammonia is also buffered by the conversion of glutamate to glutamine via the action of glutamine synthetase. At times of hyperammonaemia, the glutamine concentration increases and thus can be used as an indicator of insufficient urea synthesis and is indicative of longer term metabolic control.
Hyperammonaemia (normal plasma ammonia levels are <100 µmol/L in neonates and <50 µmol/L thereafter) has a wide differential ( Table 29.5 ). Urgent measurement of ammonia should therefore take place in any baby, child or adult presenting with unexplained encephalopathy or illness. The urea cycle disorders (UCD) arise due to deficiency of one of the six main urea cycle enzymes.
| Inborn errors of metabolism | Acquired |
|---|---|
|
|
The classic presentation is the term baby who becomes increasingly sleepy and encephalopathic on day 3–5 of life with poor feeding and vomiting (see Question 29.2 ). Ammonia levels can rise rapidly. Urgent investigation is required to clarify the diagnosis and guide management.
The urea cycle disorders are inherited in an autosomal recessive manner, except for ornithine transcarbamylase (OTC) deficiency, which is X-linked (see Genetics of metabolic disorders , below). Male infants are severely affected and many do not survive the neonatal period. Female carriers have a varied phenotype; the majority remain asymptomatic but approximately 15% will require treatment.
Diagnosis of urea cycle disorders ( Table 29.6 ) is based upon plasma amino acid analysis and the presence or absence of urine orotic acid, which is produced when carbamoyl phosphate passes into the pyrimidine pathway. The absence of orotic acid in a urea cycle disorder implies N -acetylglutamate synthetase (NAGS) or carbamoyl phosphate synthetase (CPS) deficiency. Orotic acid is classically very elevated in OTC because of the accumulation of intracellular carbamoyl phosphate. The remaining defects are associated with a much smaller or negligible amount of orotic aciduria.
| Enzyme | Disorder | Plasma amino acid concentrations relative to reference range | Urine orotic acid | ||||
|---|---|---|---|---|---|---|---|
| Alanine | Glutamine | Citrulline | ASA | Arginine | |||
| NAGS | NAGS def | ↑ | ↑ | Normal | |||
| CPS | CPS def | ↑ | ↑ | ↓ | ↓ | Normal | |
| OTC | OTC def | ↑ | ↑ | ↓ | ↓ | ↑↑↑ | |
| ASS | Citrullinaemia | ↑ | ↑ | ↑↑ | ↓ | ↑ | |
| ASL | ASA def | ↑ | ↑ | ↑ | ↓ | ↑ | |
| Arginase | Arginase def | ↑ | ↑ | ||||
This can be thought of in terms of acute and long term.
Acute:
Stop feeds and commence 10% dextrose to reduce nitrogen load on the cycle
Commence intravenous ammonia scavenging medications (see Principles of pharmacological treatment , below)
Commence intravenous arginine to replenish the urea cycle
Transfer to specialist centre in preparation for haemofiltration
Chronic:
Low protein diet to reduce nitrogen load on the cycle
Ammonia scavenging medications to aid excretion of excess nitrogen
Arginine (except in arginase deficiency) to replace arginine not produced by the urea cycle
A 5-day-old baby girl is born at term after a normal pregnancy and delivery. She presents with a 24-hour history of increasing sleepiness and poor feeding. On examination, she is encephalopathic with an irritable cry.
Investigations:
| Haemoglobin | 136 g/L |
| White cell count | 10.0 × 10 9 /L |
| Platelets | 360 × 10 9 /L |
| CRP | 10 mg/L |
| Glucose | 4.0 mmol/L |
| Ammonia | 875 µmol/L (<100) |
| Lactate | 5 mmol/L (1–2.8) |
| Urea and electrolytes | normal |
| Liver function tests | normal |
| Calcium, phosphate, ALP | normal |
| pH | 7.5 |
| pCO 2 | 2.5 kPa |
| pO 2 | 11.3 kPa |
| Base excess | −5 mmol/L |
| Bicarbonate | 22 mmol/L |
Which of the following is the most likely diagnosis? Select ONE answer only.
Hyperinsulinaemia of the newborn
Intrapartum hypoxia
Pyridoxine dependency
Septicaemia
Urea cycle defect
E. Urea cycle defect.
A urea cycle defect is the most likely because of the elevated lactate level and the extremely high ammonia. Hyperinsulinaemia is possible but ruled out by the normal blood glucose. Intrapartum hypoxia would have presented earlier with seizures and possibly renal failure. Pyridoxine dependency would result in intractable seizures. Septicaemia is unlikely with the virtually normal blood count.
Intravenous sodium benzoate and sodium phenylbutyrate are commenced. She is transferred to the paediatric intensive care unit (PICU) for haemofiltration. Ammonia normalizes over 6 hours. While on PICU she suffers a seizure. Investigations: High plasma citrulline and absent urine orotic acid suggest citrullinaemia, subsequently confirmed with mutation analysis. She subsequently recovers and is discharged on a low protein diet, sodium benzoate and arginine.
Ammonia is a respiratory stimulant and can cause respiratory alkalosis
Seizures can be seen in the acute phase due to cerebral oedema secondary to the effects of hyperammonaemia
Early referral to PICU for haemofiltration is essential
High ammonia levels (>1000 µmol/L) are associated with poor prognosis in terms of survival and long-term neurological outcome
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here