Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Transfer of solutes across the placenta is essential for fetal growth and development. Placental transfer occurs in both directions, with maternal nutrients transferred to the fetus and fetal wastes to the mother. A wide range of nutrients and wastes must be transported across the placenta requiring multiple different transport processes. Insufficient placental transfer of nutrients or wastes can impair fetal growth and development. When poor placental function affects fetal development, this can impact health in utero around birth and across the life course.
Placental nutrient uptake from the maternal circulation must be sufficient to meet both placental and fetal metabolic requirements. The fetus needs oxygen, nutrients, and maternal immunoglobulin G (IgG) to sustain its development and prepare for postnatal life. At the same time, the placenta has to clear waste products, such as carbon dioxide, from the fetal circulation. The placenta must also mediate uptake of the metabolites required for its own metabolic demands to support placental functions including nutrient transport and hormone secretion. While there is selective placental transfer of nutrients and wastes, the placenta must also protect the fetus from maternal hormones, metabolites, pharmaceutical drugs, and environmental toxins. The requirements for placental transfer will vary in line with changes in fetal demand across gestation.
Transfer of nutrients and wastes across the placenta can occur by passive diffusion and transporter-mediated processes as well as by endocytosis and transcytosis. Other factors that affect nutrient and waste transfer include placental metabolism, structure, and blood flow. The placenta does not operate in isolation and maternal and fetal physiology and metabolism will affect drivers of placental solute transfer. In particular, maternal and fetal blood flow play a key role in determining the concentrations of substances available for transport and the gradients that may drive their transfer across the placenta. In addition, regulatory signals from the mother or fetus will target the placenta and regulate its function. The way in which these processes interact will also be key in determining what crosses the placenta.
This chapter will address the mechanisms that underpin placental solute transfer and how these mechanisms are regulated. Key determinants of placental transfer include whether an ion or molecule can diffuse across the placenta, whether it can be transported, and whether there are any metabolic processes in the placenta that sequester or degrade it.
The structure of the placenta provides the foundations for its function as a barrier and as a mediator of selective transport. It should be noted that placental structure varies across species in terms of both the macro- and micro-architecture.
The term human placenta is typically disc shaped and formed of 15 to 20 functional units called lobules or cotyledons , each with its own maternal and fetal blood supply. The fetal blood vessels are contained within placental villi, which are tree-like branching structures with an outer surface in direct contact with maternal blood. This hemochorial placentation reduces the diffusion distances and the number of layers that solutes must cross between maternal and fetal circulations.
The villous tree originates from the chorionic plate and is anchored to the basal plate by specialized anchoring villi. Stem villi emerge first and branch into intermediate villi and finally into terminal villi, which mediate the majority of placental solute exchange. Within the villi, arteries and veins are present in stem villi. Venules and arterioles are located in intermediate villi, while capillaries are found in intermediate and terminal villi. The arteries and veins in stem villi contain an outer smooth muscle layer, while arterioles, venules, and capillaries are surrounded by pericytes.
In the first trimester of pregnancy, the embryo implants within the maternal endometrium and villous formation occurs followed by villous vascularization. Maternal blood flow to the placenta does not begin until 10 to 12 weeks of gestation, before which histotrophic nutrition is provided by the uterine glands. In the second trimester, villous branching increases the size and surface area of the placenta, to support increasing fetal demand. In the third trimester, increasing placental efficiency is mediated by dilation of capillaries within the terminal villi. This brings the capillary endothelium into closer contact with the trophoblast and reduces diffusion distances between the maternal and fetal circulations. Placental development across gestation is reviewed in detail elsewhere. Poor placental development in the early stages of gestation, such as villous branching or vascularization, can have a significant impact on later placental structure and function.
Maternal blood flow carries nutrients to the placenta and waste products away, whereas the fetal vasculature is important for solute delivery to the fetus and removal of wastes. Blood flow through the maternal and fetal circulations within the placenta maintains the concentration gradients necessary for transfer of many substances.
Maternal blood spills out of maternal spiral arteries into the intervillous space and percolates between the villi until returning to the maternal circulation through venous outflows. The number of spiral arteries per placental lobule and the number and location of decidual veins leaving each lobule have not been clearly established. While the anatomic location of decidual venous outflows is unclear, computer modeling of blood flow suggests that they would be located peripherally for optimal efficiency. Work with Doppler ultrasonography and magnetic resonance imaging (MRI) is leading to a better understanding of maternal blood flow through the intervillous space.
Within the intervillous space there is no fixed direction of maternal blood flow, either between the placental villi or in relation to fetal blood flow within the villous tree. This mixed or multivillous flow pattern differs significantly from the counter current flow system that might exist if the placenta had been designed by an engineer for optimal efficiency. Computer modeling of blood flow in the human placenta based on a multivillous system of flow produces data similar to in vivo experimental data, providing support for this hypothesis.
Maternal blood flow to the placenta may be altered in maternal disease states. In preeclampsia, blood flow to the placenta is believed to be reduced or modified due to impaired spiral artery remodeling. Maternal heart disease is also associated with altered uteroplacental blood flow and a higher incidence of poor fetal outcomes. Impaired maternal flow may reduce the efficiency of nutrient delivery to the fetus, removal of fetal wastes, and the delivery of placental signals (hormones, microparticles, nutrients) back to the mother.
Fetal blood flow is dependent on the capacity of the fetal heart and the resistance of the placental vascular network. Thus the development and maturation of placental villi could affect vascular resistance and therefore blood flow. Impaired placental blood flow will primarily affect the transfer of oxygen and carbon dioxide, whose transport is highly dependent on flow to maintain transplacental partial pressure gradients.
Within the villous tree, fetal arterial blood, which is deoxygenated and depleted in nutrients, will travel up the villi to the capillaries in terminal villi where solute exchange is most efficient. The oxygen- and nutrient-enriched blood will then travel back down the venous system to the fetus. While limited exchange may occur in larger vessels within stem and intermediate villi, the majority is believed to occur in terminal villi. The regulation of feto-placental blood flow and its effects on nutrient transfer is an area that needs further investigation.
The vascular resistance of the feto-placental circulation is determined by the structure of its vascular network and its vascular tone. Placental vascular tone can be regulated by vasoactive drugs and metabolites. , Interestingly, vascular tone may be regulated in both the arterial and venous vessels, and venous constriction may influence bulk fluid flow between the fetal and maternal circulations. As such, changes in vascular resistance within the fetal circulation may affect both the perfusion of the placenta and bulk flow of fluids between the fetal and maternal circulations. Higher vascular resistance would make it harder for the fetal heart to effectively perfuse the placenta. However, more work is needed to fully understand these dynamics.
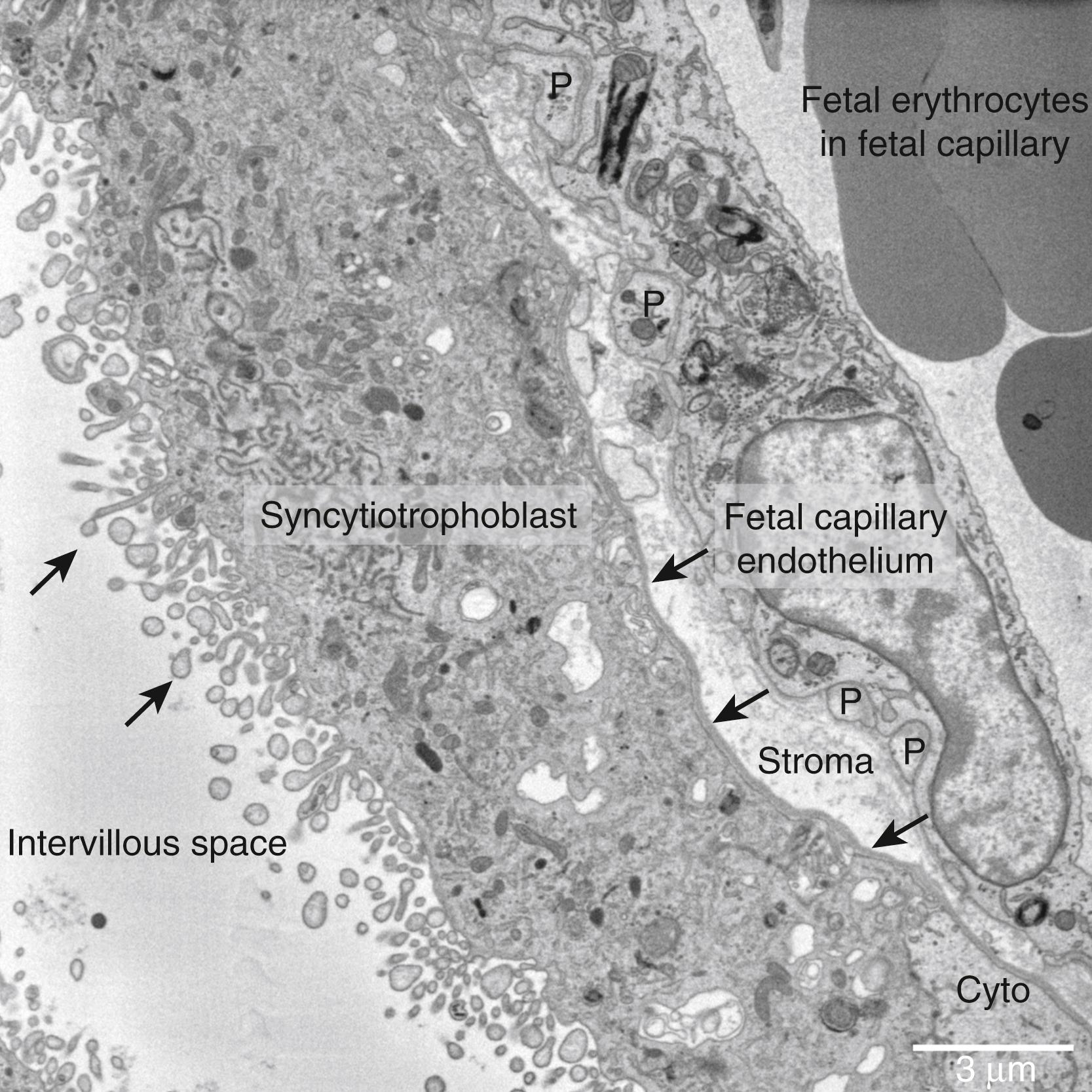
In humans, the placental barrier at term consists of three main layers: the trophoblast, connective tissue, and capillary endothelium.
The trophoblast layer consists of a continuous syncytiotrophoblast in contact with maternal blood, overlying a discontinuous layer of cytotrophoblast. The syncytiotrophoblast is a transporting epithelium with a maternal facing microvillous membrane (MVM) and a fetal facing basal membrane (BM). The maternal facing villous surface is covered in microvilli, which are evenly distributed across different regions of villi and increase surface area by fivefold. While the BM is often represented as flat, it does contain highly complex folded regions that indicate that its surface area is larger than generally believed. The syncytiotrophoblast forms the maternal-fetal interface and while it covers the surface of the villi, there are regions of damage where it is replaced by fibrin deposits. At term gestation, the cytotrophoblast lie underneath approximately 30% of the syncytiotrophoblast, as visualized by electron microscopy. The cytotrophoblast are involved in syncytiotrophoblast renewal and continue to fuse with the syncytiotrophoblast until term. Whether the cytotrophoblast play an active role in nutrient transfer is uncertain, although some studies implicate them in lipid transfer.
The trophoblast basal lamina is a layer of dense connective tissue that separates the trophoblast from the villous stroma. The barrier properties of the trophoblast basal lamina are not well characterized, unlike the renal glomerular basement membrane that allows free diffusion of low-molecular-weight molecules and restricts the transfer of larger molecules such as proteins. While the renal glomerular basement membrane effectively stops diffusion of albumin (molecular weight 66.5 kDa), IgG (molecular weight 150 kDa) can cross the trophoblast basal lamina. This suggests that the trophoblast basal lamina may be more permeable than the renal glomerular basement membrane.
The villous stroma consists of a loose connective tissue that is unlikely to provide a significant barrier to the diffusion of hydrophilic molecules. The villous stroma could however provide a more significant barrier to diffusion of hydrophobic molecules (e.g., fatty acids, cortisol), which are normally bound to carrier proteins in plasma. Experimental evidence suggests that placental to fetal transfer of fatty acids and cortisol is much lower than placental to maternal transfer. This is consistent with slower diffusion across the villous stroma; however, further evidence is needed to prove this hypothesis. The fibroblasts and macrophages within the villous stroma may also provide a diffusive barrier and will consume nutrients destined for the fetus. However, the overall effect of stromal cells on solute transfer has not been determined.
The fetal capillary endothelium is the final barrier to nutrient transfer or the initial barrier to waste transfer, depending on the direction of transfer. The fetal capillaries are partially covered by a layer of pericytes in close association with endothelial cells ( Fig. 10.1 ). Transfer across the vascular endothelium can occur by a transcellular route (e.g., oxygen) or by a paracellular route via endothelial cell-cell junctions. The fetal capillary endothelium has a similar diffusive permeability to skeletal muscle. It is assumed that many water-soluble solutes cross the endothelium via the paracellular route; however, there is little direct evidence of this. The endothelial junctions will be less permeable to hydrophobic solutes or larger solutes such as IgG. However, proteins associated with IgG and cholesterol transfer are expressed in the fetal capillary endothelium. ,
Placental metabolism can act as a barrier to placental transfer, for example, the inactivation of maternal cortisol by placental 11β-hydroxysteroid dehydrogenase 2 prevents it from reaching the fetus. Placental metabolism can impact transfer levels across the placenta by consuming nutrients so that they are not available to the fetus. Placental metabolism also determines the intracellular concentration of many substrates and therefore their availability to membrane transporters. Nutrients such as amino acids and fatty acids can be sequestered in placental intracellular pools (e.g., protein or phospholipid) with both the uptake and release of these nutrients from the pools affecting their availability for transport out of the placenta.
There is diffusion of lipophilic substances across the placenta by a transcellular route and transfer of hydrophilic substances by a poorly defined paracellular route. Oxygen, carbon dioxide, and small lipophilic drugs (such as anesthetics) can readily diffuse across the different layers of the placenta. Because diffusion of these substances across the placenta is rapid, transfer of these substances will be flow limited.
Clear functional evidence for size selective diffusion across the human placenta suggests that there must be a paracellular route; however, the anatomic basis for this remains unclear. , Transtrophoblastic channels have been proposed to explain the observed diffusion, and while potential channels are seen in human placenta, full width channels have not been directly observed. Alternatively, there is evidence that regions of syncytial damage may provide a route for paracellular diffusion.
Most solutes require transport across the plasma membranes of placental cells including the syncytiotrophoblast and potentially the fetal capillary endothelium. Proteins that facilitate transfer of molecules and ions across plasma membranes include membrane transporters and channels. While the role of these membrane proteins is essential, it is important to recognize that other processes such as metabolism can influence transfer and may also be rate limiting.
Membrane transport proteins operate by binding one or more substrates on one side of the membrane, followed by a conformational change that translocates those substrates to the other side of the membrane. Membrane transport proteins can be grouped into transporter families. The P-type ATPase (e.g., sodium potassium ATPase) and ATP-binding cassette protein (ABC, e.g., multi-drug resistance protein) families mediate ATP-dependent active transport, whereas members of the solute carrier family (SLC, e.g., GLUT1/ SLC2 A 1 ) mediate secondary active and facilitate transport. , The activity of the ATP-dependent transporters, particularly the sodium potassium ATPase, is ultimately responsible for creating the gradients that drive the activity of SLC family members. The ABC transport proteins play a role in transporting solutes such as cholesterol, inorganic ions, as well the efflux of drugs to potentially protect the fetus. The SLC superfamily of transport proteins contain 55 families, although not all are expressed or functional in placenta. The majority of nutrient transporters in the placenta membranes belong to the SLC superfamily.
Facilitated transport proteins mediate transport of solutes in both directions across the membrane, with net transport down the concentration gradient. Examples of facilitated transporters in the human placenta include the glucose transporter GLUT1/ SLC2A1 , amino acid transporters (TAT1/ SLC16A10 , LAT3/ SLC43A1 , and LAT4/ SLC43A2 ), and monocarboxylate transporters (e.g., MCT1/ SLC16A1 and MCT4/ SLC16A3 ).
Exchange transporters, or antiporters, transport one solute into the cell in exchange for another leaving the cell. Examples in the placenta include amino acid transporters (e.g., LAT1/ SLC7A5 and LAT2/ SLC7 A 8 ) and the organic anion transporter (OAT4/ SLC22A 1 1 ). Exchange transporter activity will lead to equilibration of the proportions of their different substrates across the membrane with no change to the overall number of molecules. Increasing the number of exchange transporters in the membrane will mean that the equilibrium is reached faster. Therefore increasing exchanger activity will have minimal effect on a system that is already at or near equilibrium.
Active transporters actively pump solutes into or out of cells and can transport against the concentration gradient. These may be primary active transporters that are powered by ATP or secondary active transporters that are driven by previously established membrane gradients. While these transporters can accumulate substrate, biophysical constraints will limit the extent to which they can do this. There is a point at which increasing the number of transporters in the membrane will no longer increase transport across the membrane because the gradient against which they need to pump is too great.
Plasma membrane primary active transporters in the placenta include members of the P-class (sodium potassium ATPase and plasma membrane calcium ATPase [PMCA]) and the ABC transporter superfamily. , The sodium potassium ATPase is located on both the MVM and BM of the syncytiotrophoblast, with the greatest activity on the MVM. Secondary active transporters in the placenta include co-transporters such as members of the SLC38 gene family that mediate sodium-dependent amino acid uptake.
Endocytosis is the process whereby solutes or particles are transported into the cell within fluid-filled membrane-bound vesicles that form via invagination of the cell membrane. This is an important active transport mechanism in the placenta that is used to transport essential molecules, such as IgG, to the fetus. Endocytotic processes include phagocytosis, whereby larger particles are engulfed, pinocytosis that transports small solutes and water or receptor-mediated endocytosis. Endocytosis can therefore be nonselective or selective via the receptor-mediated internalization of specific extracellular ligands. Substrates can interact with specific cell surface receptors and form clathrin-coated pits that are released from the membrane into the cytoplasm via dynamin action. IgG, iron, low-density lipoprotein, and vitamin B 12 are known to be taken up by receptor-mediated endocytosis and other molecules such as vitamin D may also be transported this way. ,
Membrane channels provide a selective pore that mediates transfer of substrates, usually ions, across the membrane. Net transport occurs in the direction of the concentration gradient. There are many channels in the placental cell plasma membranes; however, these typically mediate cellular homeostasis rather than transplacental transfer with the exception of aquaporins.
The composition of maternal and fetal plasma are important determinants of placental function. Both solute concentration and concentration gradient can be important determinants of placental transport. In addition, the presence of binding proteins may influence availability of solutes for placental uptake.
The composition of maternal plasma is determined by maternal dietary intake, nutritional reserves, and metabolic state, as well as maternal lung, liver, and kidney function, for example, maternal plasma oxygen and carbon dioxide levels will be determined by lung function. Maternal metabolism is altered by placental hormones during pregnancy to increase nutrient availability to the fetus. However, maternal metabolic conditions during pregnancy could affect placental transfer, including diabetes mellitus, phenylketonuria, and intrahepatic cholestasis of pregnancy. These conditions may increase the gradient driving placental transfer and may affect the transfer of other substrates by competitive inhibition of their transporters. For instance, high phenylalanine levels with phenylketonuria may impair placental amino acid transport.
Compounds in the maternal blood may also be harmful to the fetus, including pharmaceutical drugs (e.g., metformin, thalidomide), environmental toxins (e.g., phthalates), metabolites or wastes (e.g., bile acids), and maternal hormones. The levels of environmental toxins and pharmaceutical drugs in the maternal blood will be determined by maternal exposure or production versus the clearance of these compounds via the lungs, liver, and kidneys.
The composition of the fetal plasma will be determined by both placental transfer and fetal metabolism. Fetal metabolism will affect placental transfer by controlling the solute gradients driving or opposing transfer. Higher metabolism will increase fetal carbon dioxide levels and reduce fetal glucose levels increasing the gradient driving the transfer of these substances across the placenta. Conversely, reduced fetal metabolism will reduce the gradients driving placental transfer of these solutes. This may provide an autoregulatory mechanism with a decrease in fetal plasma nutrients acting as a “signal” to the placenta to increase transfer to the fetus. Fetal metabolism may also be required to convert wastes into less toxic or more soluble forms to aid their placental transfer away from the fetus. For instance, sulfating many compounds will aid their solubility, and fetal production of urea and glutamate may aid transport of excess nitrogen to the mother.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here