Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We sincerely thank Fedorov Lab members Drs. Benjamin Buck and Aleksei Mikhailov, Matthew Fazio, Shane Scott, Megan Subr, and Allison Wilson for valuable input during the preparation of this chapter.
The sinoatrial node (SAN) is the primary pacemaker of the heart and is responsible for regular cardiac rhythm. Since its discovery by Keith and Flack over a century ago, many important advances have been made in understanding the complex structure and function of the SAN and the role these factors have in cardiac physiology and pathophysiology. Decades of research have provided greater understanding of the anatomy, molecular biology, , and electrophysiology , of the human SAN. Three-dimensional (3D) histologic and immunofluorescence reconstructions of the SAN and molecular studies looking into gene and protein expression, have helped to demonstrate the unique structural and molecular heterogeneity of the SAN pacemaker conduction complex. , , Furthermore, recent ex vivo near-infrared transmural optical imaging of the SAN in human and animal hearts has provided important insights into the functional and molecular features of this profoundly complex 3D structure in normal states and SAN dysfunction (SND). Novel data , from ex vivo human SAN studies reveal the existence of highly sophisticated and hitherto unknown backup mechanisms of intranodal pacemakers and conductions pathways that ensure robust, uninterrupted SAN pacemaking and electrical conduction in norm and disease. These mechanisms involve both specific structural and novel molecular features that provide redundant alternative options to preserve SAN function and keep the heart beating. This chapter reviews the current knowledge of SAN structure, excitability, and conduction with specific reference to these recent discoveries in the human heart.
Anatomically located at the junction of the superior vena cava and right atrium in the mammalian heart ( Fig. 28.1A ), the SAN is a complex, multicompartment structure characterized by small clusters of pacemaker myocytes that are arranged in parallel rows that frequently anastomose. , , , , In the normal adult human heart, the SAN is an 11 to 29 mm long, 2 to 6 mm wide, banana-shaped structure that traverses the myocardium. It is normally tilted such that the SAN head (superior third) lays relatively subepicardially and the SAN tail (inferior third) subendocardially. However, its structure is highly variable, making its exact location difficult to define in patients. , The SAN head is typically separated from the epicardium by less than 1 mm of fibrous tissue and fat. , , , The SAN is centered on the SAN artery; in 55% of humans, the SAN arises from the right coronary artery, and it arises from the left circumflex artery in the other 45%. Notably, the SAN artery marks an important anatomical landmark of the SAN complex and is used to help avoid accidental injury to the SAN during surgical procedures , , (see Fig. 28.1A and C ). The SAN pacemaker cells are striated similarly to ordinary myocardial cells, but they are smaller in both length and diameter. The clusters of specialized pacemaker cardiomyocytes are enmeshed within strands of dense connective tissue, nerve fibers, and capillaries, creating the SAN pacemaker complex. These specialized SAN pacemaker cell clusters serve as leading pacemakers that initiate electrical activation, which exits the SAN via the sinoatrial conduction pathways (SACPs) leading to atrial activation (see Fig. 28.1B ). The human SAN pacemaker complex has several SACPs formed by 200- to 400-μm-thick branching myofiber tracts, which provide discrete and continuous electrical connections between the SAN pacemakers and atria (see Fig. 28.1A–C ).
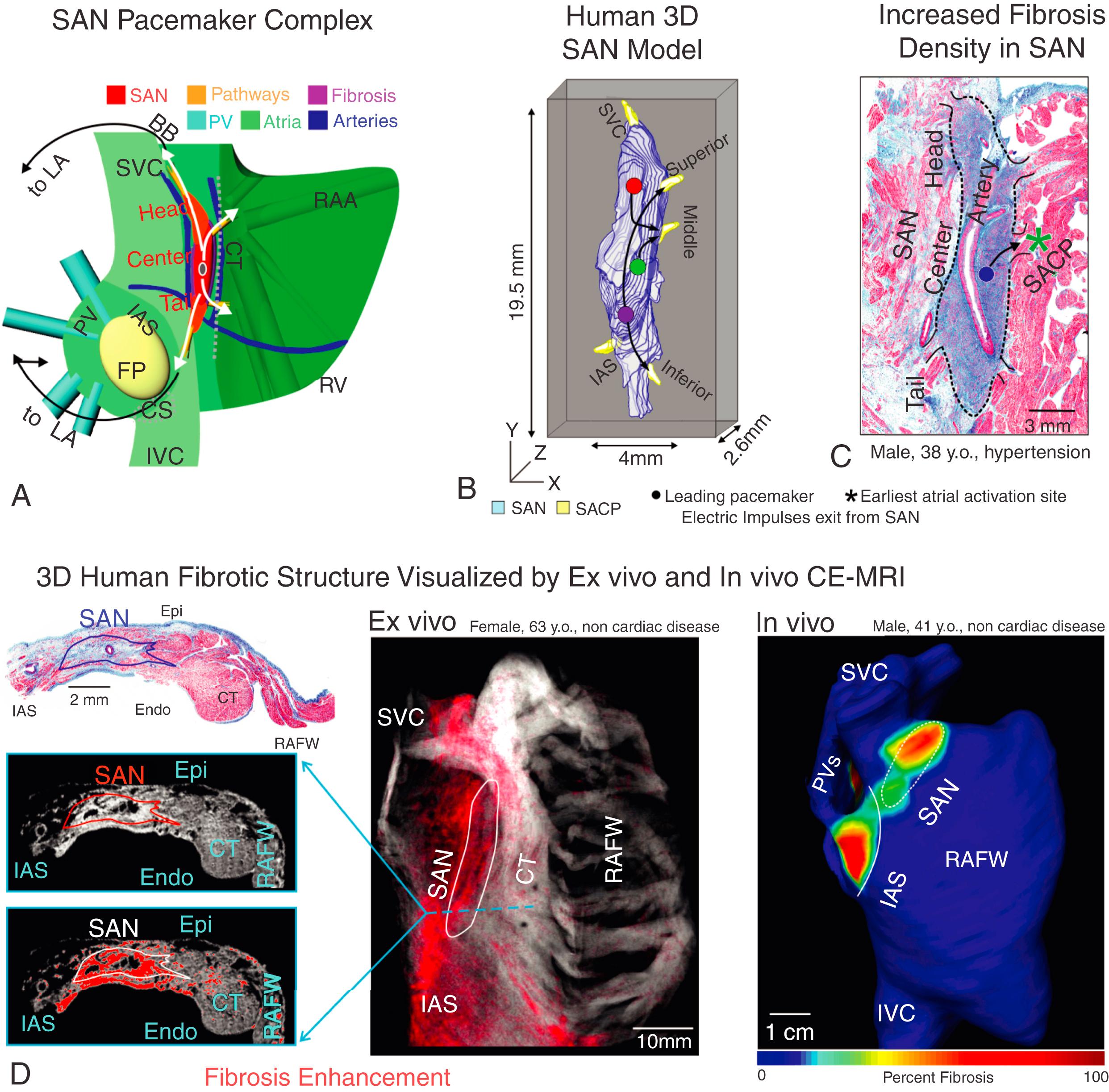
The normal adult SAN structure consists of dense 40% to 55% fibrotic connective tissue, which creates a honeycomb-like fibrotic matrix composed of a network of collagen sheaths. , Fibrotic content within the SAN increases with aging and disease-induced remodeling. Traditionally, fibrotic content within the SAN could be quantified using standard histologic methods including Masson’s trichrome (see Fig. 28.1C–D ). Recently contrast-enhanced cardiovascular magnetic resonance (CE-CMR) has advanced the field by accurately identifying the human 3D SAN structure in vivo and ex vivo and quantifying fibrotic content (see Fig. 28.1D ). These sophisticated imaging techniques have opened new avenues to study the structure and contribution of the 3D collagenous matrix to the human SAN function, in normal and diseased hearts. The fibrotic scaffolding houses specialized SAN myocytes while providing mechanical protection to the SAN. Additionally, fibrosis, fatty tissue, and/or discontinuous myofibers create a structural border around the SAN, penetrated only by the SACPs. The structural border electrically insulates the SAN pacemaker cells from the hyperpolarizing effects of the surrounding myocardium, efficiently protecting normal sinus rhythm (SR). , , ,
A unique composition of ion channels and Ca 2+ -handling proteins maintains SAN pacemaker automaticity and conduction, distinguishing the SAN complex from the surrounding atria. Recent findings show that the expression and composition of ion channels and receptors varies between compartments. , This variation allows each compartment to respond to metabolic and pathologic stimuli, helping to preventing complete SAN arrest. Importantly, direct molecular 5,6 and ion current studies on the human SAN, rather than animal models, are limited. It is generally accepted that mammalian SAN pacemaker cells are depolarized (–60 mV) compared with the surrounding atrial myocardium (–85 mV) and lack a stable resting potential because of the absence of the background inward rectifier K + current (I K1 ). Instead, the SAN pacemaker cells exhibit slow diastolic depolarization caused by the synergistic membrane and Ca 2+ clock mechanisms. The membrane voltage clock is attributed to the hyperpolarization-activated funny current (I f ), whose molecular α-subunits are represented by hyperpolarization-activated cyclic nucleotide-gated channels (HCN1, HCN2, and HCN4). The Ca 2+ clock contributes to SAN diastolic depolarization through activation of the Na + /Ca 2+ exchanger current by spontaneous localized Ca 2+ release from the sarcoplasmic reticulum via type 2 ryanodine receptors. The slow diastolic depolarization leads to spontaneous generation of the SAN pacemaker action potential. Importantly, central SAN pacemaker cells lack the fast Na + current (I Na ) responsible for the fast depolarization and conduction in atrial and ventricular cells. Instead, SAN conduction is very slow, , , as action potential upstroke is generated by small, slow Ca 2+ current (I Ca,L ), which is modulated by sympathetic and parasympathetic stimulation.
The unique expression of gap junction proteins also contributes to slow conduction within the SAN complex. The SAN lacks the high conductance gap junction proteins connexins Cx43 and Cx40, which are the major gap junction proteins responsible for electrical coupling between cardiac myocytes in the working atrial myocardium ( Fig. 28.2 ). Instead, animal and human studies have shown the presence of Cx45, a low-conductance connexin that provides weak electrical coupling, in the SAN. , However, a weaker cell-to-cell electrical connection may increase the safety of conduction.
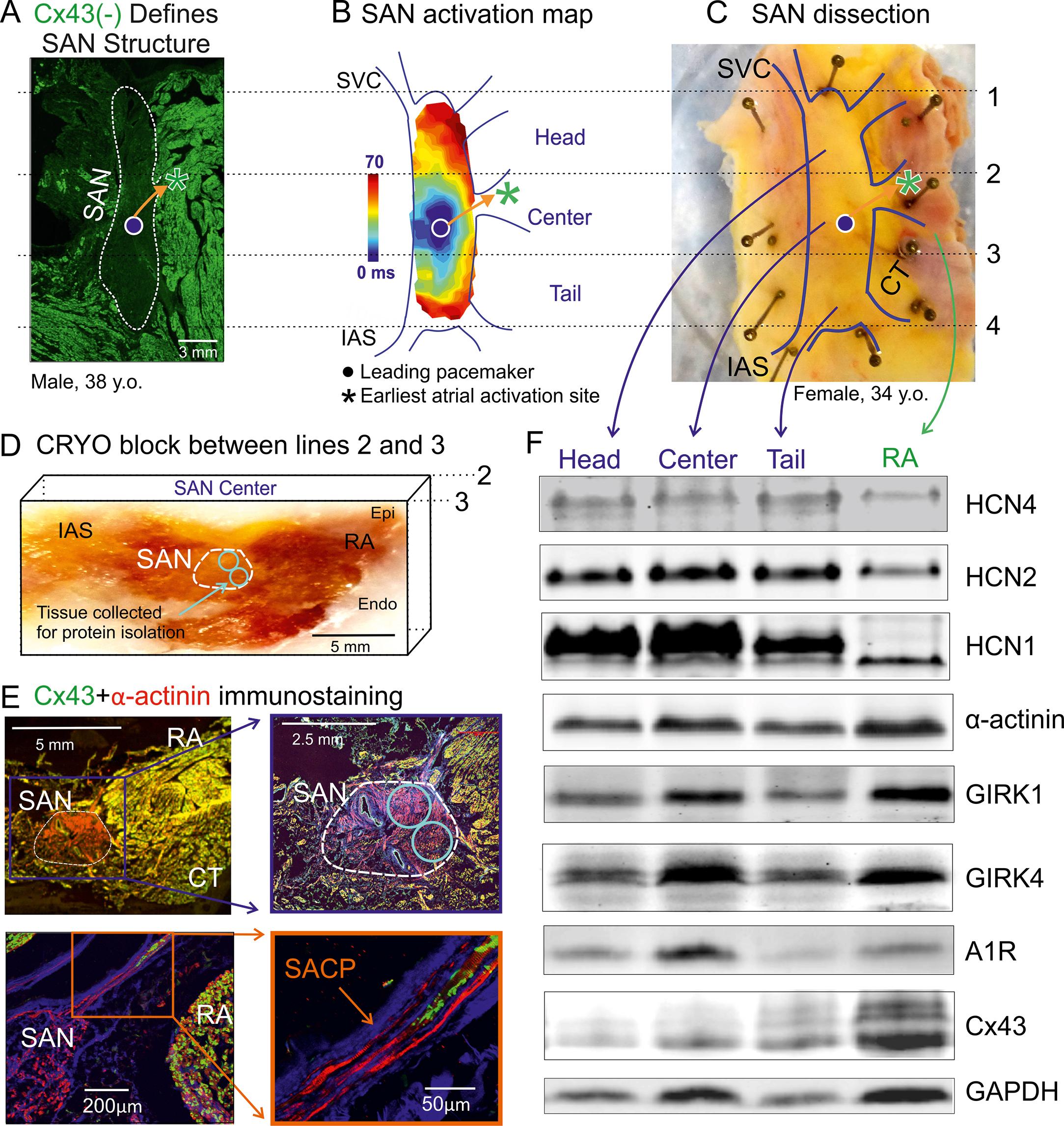
The specialized SAN structure and connexin distribution, along with the combination of ion channels responsible for SAN automaticity, present multiple molecular markers to distinguish the SAN from surrounding atrial tissue. , The high fibrosis level in the SAN (40%–50% tissue composition) compared with the atria (5%–20%) allows Masson’s trichrome or immunostaining of fibroblasts to identify the SAN (see Fig. 28.1C–D ). The absence of Cx43 and potentially Cx40 can also be used as molecular markers of human SAN tissue (see Fig. 28.2A ). A recent molecular mapping study of HCN isoform distribution in the human heart revealed that HCN1 may be exclusively expressed in the SAN pacemaker compartment, emphasizing its functional importance and potential utility as a new specific molecular marker of the human SAN (see Fig. 28.2E ).
Studies also show that protein expression may vary significantly between different compartments of the SAN (head vs. center vs. tail), and that their unique compartment-specific ratios can predetermine the leading pacemaker locations. , , One such signaling pathway that can affect human SAN function via its heterogeneous expression pattern is the adenosine receptor (A1R)-mediated pathway along with its downstream components, the G protein–coupled inward rectifying potassium channel (IK.Ado/ACh) subunits, GIRK1 and GIRK4. , Adenosine is an endogenous metabolite of the heart, which contributes to the daily regulation of SR and is abundantly produced during ischemia and heart failure. Adenosine has also been shown to directly affect the SAN through negative chronotropic depression, especially in patients with SND; excessive bradycardic response and atrial pauses (2–23 s) have been reported in response to adenosine bolus. , Alternatively, adenosine receptor blockers, such as theophylline , , and the IK.Ado/ACh blocker tertiapin, can also be used to treat SND, confirming the role of adenosine receptors in the human SAN. Recently near-infrared optical mapping (NIOM) studies also revealed heterogenous adenosine-induced inhibition of pacemaking and conduction across the human SAN complex. Specifically, the central intranodal pacemaker compartment consistently demonstrates a high sensitivity to adenosine and a relatively profound bradycardic response compared with the other intranodal pacemakers. Correlating with these functional findings, the expression of A1R and GIRK4 has been shown to be higher in the central compartment (see Fig. 28.2 ) than the head and tail regions. The differential expression of A1R and GIRK4 subunits confers varying sensitivities to adenosine, ensuring robust protection of SR. SAN regions with lower A1R expression function as backup pacemakers even when the highly sensitive leading pacemaker regions such as the central compartments are depressed. Furthermore, variations in the ratio and expression patterns of A1R and GIRK4 protein across the different regions of the SAN pacemaker complex could direct the heart-specific intranodal leading pacemaker shifts. Additionally ex vivo human heart studies also show that the difference in A1R and GIRK4 protein expression between the SAN and atria is greater in human than in canine hearts. This relatively high gradient may underlie the incidence of adenosine-induced SAN arrest in humans, which was not observed in canine hearts.
Although the roles of several ion channels in SAN functions have been well documented, the contributions of voltage-gated sodium channels (Na V ), which are major contributors to cardiac and neuronal excitability, have remained controversial in human SAN pacemaking and conduction. Animal models have shown that the expression of cardiac isoform (cNa V ) Na V 1.5 is significantly lower in the SAN versus the atria. , However, some symptomatic SND patients have been found to harbor loss-of-function mutations in the SCN5A gene, which encodes for the cNa V 1.5 α-subunit, providing indirect evidence that the voltage gated I Na maybe required for human SAN function as well. One recent study of ex vivo human hearts found that multiple Na V transcripts, of both cNa V and neuronal isoforms (nNa V ), are heterogeneously expressed within the human SAN and, more importantly, that they perform isoform-specific distinct functions. The study showed that although cNa V 1.5 is highly expressed in the atria relative to the SAN, it is important for both SAN pacemaking and conduction, especially during conditions of metabolic or pacing-induced stress. In contrast, several nNa V are highly expressed in the SAN relative to the atria. Thus nNa V , especially Na V 1.6, can be recruited mainly to preserve intranodal conduction and prevent SAN exit block rather than pacemaking or atrial conduction. Interestingly, nNa V expression in human SAN could be vulnerable to diseases including heart failure, hypertrophy, and lifestyle factors including alcohol consumption, which could lead to SAN arrhythmias.
SAN pacemaker clusters require anatomical and functional barriers for protection from the hyperpolarizing influence of the surrounding atria, yet a direct myofiber connection between the two structures must still exist for SAN cells to excite the right atrium. The idea of a discrete connection between the SAN and the right atria has been proposed by multiple structural and functional studies, , , , , but a lack of resolution of both functional and structural mapping techniques has led to debates regarding the connection between the SAN and the atria. One hypothesis is that a structural border of fibrosis, fat layers, and myocyte discontinuity surround and electrically insulate the SAN, and that functional and structural connection between the SAN and atria is limited to discrete SACPs. , , , , An alternative hypothesis is that SAN and atrial cells are extensively connected by diffuse interdigitations of the SAN border with the atrial myocardium, and that no discrete pathways exist. ,
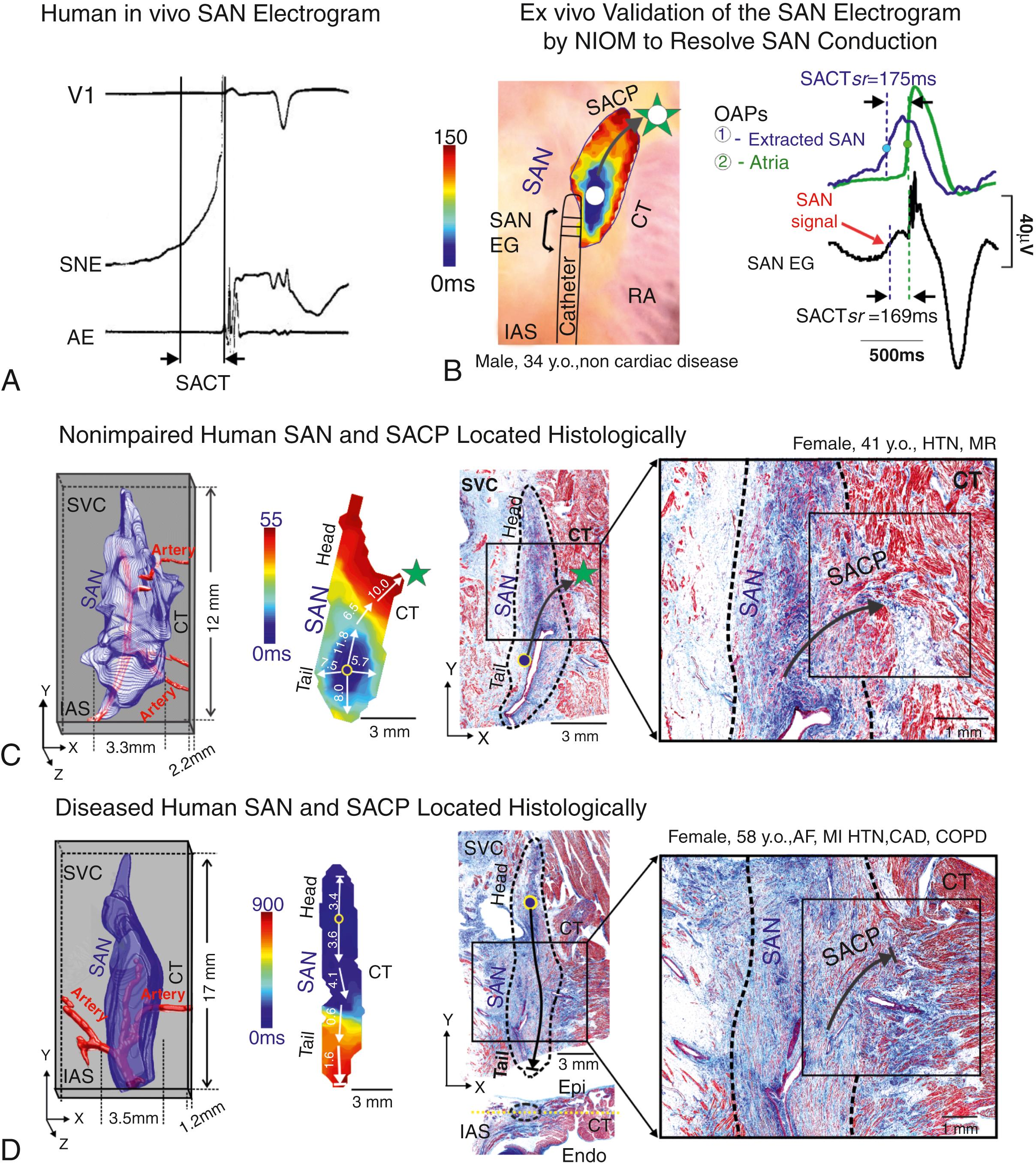
One study used serial high-resolution histology sections to reconstruct optically mapped SANs, allowing identification and definition of the structural features of the functionally identified SACPs. In the nondiseased hearts studied, several discrete branching myofiber tracts formed the SACP structure, which made continuous, uninterrupted physical connections between the SAN and atria. These myofiber tracts were composed of cells with transitional morphology (including shape and cell diameter) between SAN and atrial cells. Furthermore, Cx43 immunolabeling showed a progressive transition from Cx43 negative SAN pacemaker cells to intermediate expression in the SACP region and distinct Cx43 gap junction expression in the atria (see Fig. 28.2F ).
The 3D histologic computational analyses show that the superior SACP composed of functional myocardium intermingled with fibrotic strands that facilitated SAN conduction into the atria ( Fig. 28.3C–D ). However, cardiac disease and drug-induced structural and molecular remodeling can significantly alter pacemaker cell excitability and intracellular coupling in the SAN, which may lead to variations from the classic hierarchy of head-tail leading pacemakers , and the emergence of latent atrial pacemakers. , ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here