Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ventricular tachycardia (VT) and ventricular fibrillation (VF) represent the most common rhythms documented in patients with sudden cardiac arrest. , Even in specific population subsets, such as patients with preexisting intraventricular conduction disease, ventricular tachyarrhythmias are the most common rhythm at the time of cardiac arrest. In patients with implantable cardioverter defibrillators (ICDs), sustained and nonsustained monomorphic VT episodes are substantially more common than VF and polymorphic VT events, which indicates that many out-of-hospital VF events may initially start as VT before becoming disorganized and converting to VF. Ventricular tachyarrhythmias usually occur in patients with underlying structural heart disease, with coronary artery disease (CAD) being the most common cause ; however, a high proportion of patients with lethal ventricular arrhythmias fail to show any conventional markers of high risk, which represents a significant challenge to efficiently preventing sudden cardiac death (SCD). The latter is consistently identified as one of the missing links in evidence-based management for risk stratification in a large number of people potentially at risk for ventricular arrhythmia. , These difficulties for risk stratification are related to the relatively low probability of a ventricular arrhythmic event compared with an immensely higher number of normal beats, even in the presence of an underlying cardiac disease. A common relevant initial event is the occurrence of a trigger, which results in ventricular arrhythmia after encountering a sensitive myocardial substrate that may be functional, structural, or both. These triggers are more likely to induce ventricular arrhythmias under high-risk conditions, such as acute ischemia or severe left ventricular systolic dysfunction. , Nevertheless, arrhythmia initiation and individual-specific risk windows are extremely difficult to predict in the vast majority of clinical scenarios, such as with dynamic functional abnormalities or cardiac conditions that are particularly sensitive to autonomic nervous system fluctuations, physical exertion, or stress.
Despite limitations to increasing specificity on predicting ventricular arrhythmias, during the last decades, a progressively growing body of scientific evidence has provided important insights into the mechanisms of complex arrhythmias like VT and VF. , Although reentry is the main mechanism of ventricular arrhythmias, especially in the setting of CAD and underlying ventricular scar, other mechanisms that include triggered activity become predominant in clinical scenarios, such as right ventricular outflow tract (RVOT) tachycardias in patients with structurally normal hearts. This chapter discusses the main mechanisms that trigger and maintain ventricular arrhythmias and the cardiac conditions that predispose patients to these potentially lethal ventricular events.
VT episodes in patients with infarct-related scarring constitute the clinical paradigm of reentry. Pathophysiologic changes in the infarct region include myocyte loss and replacement of the extracellular matrix with fibrosis, which establishes an intermingling of viable myocytes and collagen that settles a heterogeneous substrate with areas of potentially slow conduction, leading to reentrant ventricular arrhythmias. This process that leads to reentrant VT episodes starts early after ischemia and progressively evolves over months or years, , until reaching a substrate prone to VT initiation and maintenance. During the healing process after acute ischemia, necrotic regions are replaced with granulation tissue that matures into scar tissue. Myofibroblasts play a relevant role in this process by modulating collagen production and fibrosis in the healing myocardium. Interestingly, although myofibroblast activity progressively decreases after acute and subacute myocardial healing, myofibroblasts may still be documented in infarct-related human scars several years after the acute event. The latter suggests that scar remodeling is a long period that may last for years and progressively evolves toward structural and functional myocardial properties prone to sustain reentrant VT episodes. This is also supported by sequential cardiac magnetic resonance (CMR) imaging studies, which have revealed steady decreases in scar mass over the years after an acute coronary event. Importantly, scar heterogeneity also seems to increase over the years of scar remodeling, which also suggests a more sensitive substrate to sustain reentry upon an initial trigger. This is also consistent with a higher risk for VT episodes in patients with chronic ischemic cardiomyopathy after a period of more than 3 years after the myocardial infarction (MI).
Reentry has been demonstrated in around 95% of VT episodes in patients with established MI. Classic reentry in infarct-related scar regions is a self-perpetuating mechanism by which a wavefront propagates repetitively throughout a closed rotational circuit long enough to allow the cardiac tissue to be excitable by the time the wavefront reaches it. This mechanism requires the presence of unidirectional conduction block, with successful conduction in only one direction, and a circuit cycle longer than any of the refractory periods throughout the circuit ( Fig. 46.1A ). Refractory periods and conduction velocity of the wavefront will determine the minimum length required for a reentrant circuit to be self-sustained. The unidirectional block can be anatomical, like fibrous tissue barriers and myocardial fiber disarrangement within the infarcted myocardium, but it can also be functional, as is the case with heterogeneous myocardial regions with dispersion of refractoriness because of acute ischemia. This reentry mechanism is commonly established within surviving channels of activation through scar tissue, which are defined as protected isthmuses (see Fig. 46.1B ). Conduction channels represent critical areas for maintaining VT episodes because slow conduction through specific regions of these pathways allows the wavefront to self-perpetuate the tachycardia.
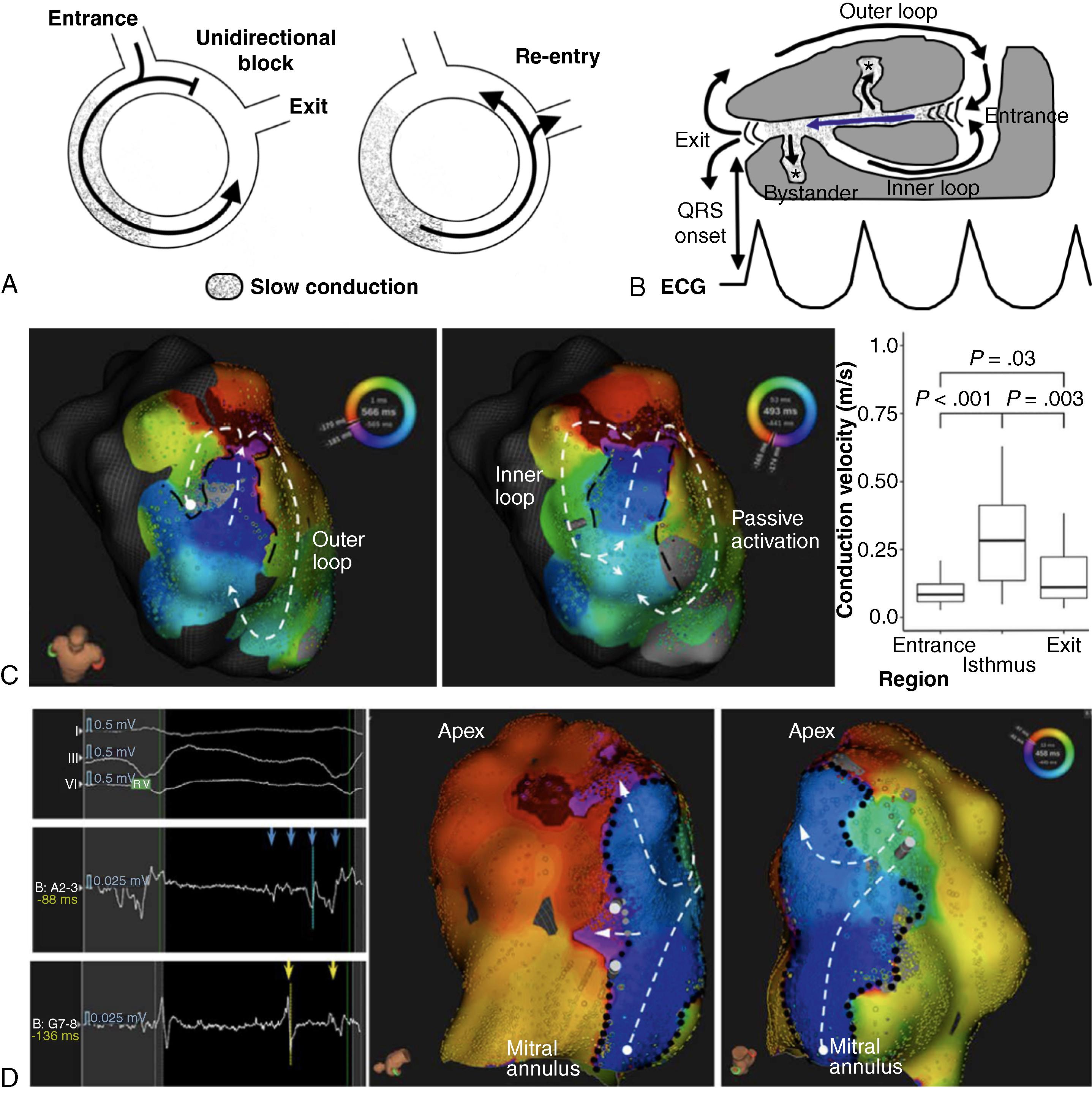
The myocardial substrate related to reentrant VTs in the setting of a prior MI has been predominantly located in surviving endocardial layers of the left ventricle. Nevertheless, reentry circuits may also be located deep into the myocardial wall and in the epicardium, which are more frequent in other myocardial substrates, including idiopathic dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy (ARVC), or in VTs associated with Chagas disease. Rarely, Purkinje fibers have also been identified as part of the reentry circuit in postinfarction VT. , Complete characterization of VT channels through scar tissue is difficult and often unmappable in clinical practice because of multiple inducible VT morphologies that interrupt the mapping process for one specific VT or because of hemodynamic instability during VT, which requires immediate cardioversion. Classic entrainment maneuvers, although limited in spatial resolution, have shown that VT circuits may be complex, with inner and outer loops, a common isthmus, and bystander loops or dead ends of activation (see Fig. 46.1B ).
More complex details of VT circuits have been shown using current technology and high-resolution mapping. Electroanatomic mapping systems and multipolar mapping with small and closely spaced electrodes have demonstrated that slow conduction is mainly localized at VT entrance and exit zones but is relatively preserved in the mid common isthmus (see Fig. 46.1C ). This deceleration of activation wavefronts seems related to the wavefront curvature around the lateral borders of the isthmus, which are often perpendicular to fiber orientation. The abrupt change in activation vector, along with a high degree of fiber disorganization, an increased axial resistivity, and a potentially myocardial thickness gradient are likely to contribute to wavefront propagation slowing. A greater slowing of activation in entrance zones has also been documented, probably related to collision of opposing wavefronts in double-loop circuits. Further characterization of VT isthmuses using high-density mapping in humans has shown a median isthmus length of 36.8 mm and a median minimum width of 9.5 mm, similar to what had been reported in animal models. Interestingly, many VT circuits are also bordered, at least in part, by regions of very slow activation, which are sufficiently slow to protect the central isthmus (see Fig. 46.1D ). The increasing evidence for the role of functional block in VT circuits provides an explanation for the observation of more than one VT breaking out from the same region of scar. Thus, a region of slow conduction, which acts as a lateral border for one VT, may act as the entrance or exit zone for another. ,
Abnormal automaticity and triggered activity have been described in VT episodes of patients with established MI. These focal VTs have been predominantly identified at the basal or mid cavity of the left ventricle, although focal tachycardias can also originate from Purkinje fibers. During the electrophysiologic study, an automatic mechanism can be suggested by failure of induction with programmed stimulation, transient suppression during rapid pacing, and the presence of warm-up and cool-down phenomena. Triggered activity because of delayed afterdepolarizations is more difficult to demonstrate, although micro-reentry will be unlikely if the tachycardia cannot be entrained after induction with burst pacing and programmed stimulation. Using enough mapping resolution, the lack of low-voltage areas or areas of slow conduction surrounding the focal sites further makes reentry unlikely. More robust characterization of the underlying VT mechanism in the clinic can be obtained to attempt resetting during stable tachycardia and to assess the response of the return cycle (defined as the interval from the extrastimulus to the onset of the next beat) at different coupling intervals of extrastimuli during programmed stimulation.
Nonischemic cardiomyopathies include a large number of functional and morphologic phenotypes, including dilated cardiomyopathy, hypertrophic cardiomyopathy, and left ventricular noncompaction. In the clinic, VTs are commonly described in cardiomyopathies with a dilated phenotype and predominant left ventricular involvement with systolic dysfunction. In this context, nonischemic cardiomyopathies show less scar confluence, a basal predilection in the left ventricle, and less predominant endocardial involvement compared with infarct-related scarring. , Compact fibrosis without transmural extension has been described in a very low percentage (around 3%) of myocardial samples from patients with nonischemic cardiomyopathy. Conversely, histopathology analyses have shown that patchy and interstitial fibrosis are the most common structural patterns, with areas of fiber disorganization that may facilitate reentry ( Fig. 46.2A ), similar to what has been described in ischemic-related substrates. In fact, reentry represents the main underlying mechanism in around 80% of patients with nonischemic cardiomyopathy and mappable VT episodes. Occasionally, triggered activity and VTs originating from the His-Purkinje system can be the predominant mechanism in specific cases. It is worth mentioning that the relative contribution of bundle branch reentry to inducible monomorphic sustained VT is higher in nonischemic cardiomyopathies (from 16.7%–41%) than in ischemic etiologies (from 4.5%–6%).
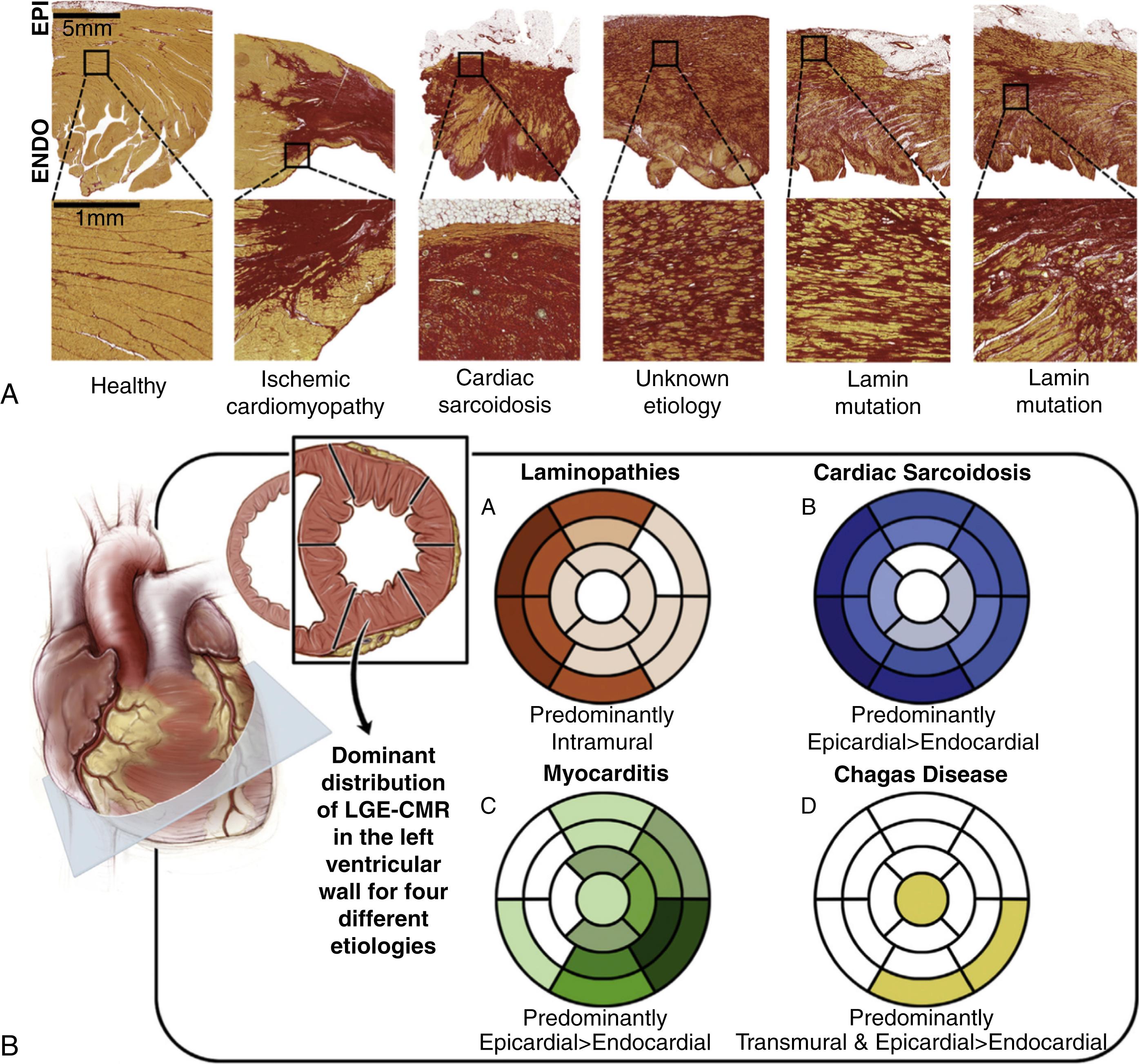
The group of nonischemic dilated cardiomyopathies commonly shows a steady progression of fibrotic remodeling, which is intrinsic to the disease process. Marchlinski et al. have described these progressive changes in the myocardial substrate using unipolar and bipolar endocardial mapping in patients with nonischemic cardiomyopathy undergoing multiple catheter ablations of ventricular arrhythmias. The authors documented a predominant increase in abnormal unipolar voltage values in around 50% of patients after a mean time interval between procedures of 32 months. The latter suggests that the scarring process involves the midmyocardium and the epicardium to a greater extent than the endocardium, as demonstrated in human histopathology studies. Moreover, these progressive changes in the myocardial substrate were also associated with a progressive decrease in left ventricular systolic function. Altogether, these data explain that VT recurrences may be common in these patients, , despite a successful ablation procedure, because new reentry circuits may develop over time.
Predominant intramyocardial and subepicardial fibrotic patterns in nonischemic dilated cardiomyopathies (see Fig. 46.2A ) may explain complex three-dimensional (3D) VT circuits with frequent location of critical VT isthmuses within the myocardial wall. This reentry complexity has been documented using entrainment mapping to localize critical circuit components (entrance, isthmus, or exit sites) in patients with ischemic and nonischemic cardiomyopathies and hemodynamically tolerated VT episodes. Entrainment maneuvers can identify critical components of the VT circuits in the majority of patients with ischemic cardiomyopathy, which are mainly located in the endocardium of the left ventricle. Conversely, mapping the endocardium of the left ventricle helps identify critical circuit components in less than half of mappable VTs in patients with nonischemic cardiomyopathy. Moreover, although in patients with ischemic cardiomyopathy the critical VT isthmus is mainly located in the endocardium of the left ventricle, this protected isthmus is difficult to identify in patients with nonischemic cardiomyopathy (<30% of mappable VTs), even after performing epicardial mapping. The data support that critical VT isthmuses are frequently intramyocardial in tolerated VT episodes in the setting of nonischemic cardiomyopathy. Moreover, intramyocardial VT isthmuses seem predominantly frequent in anteroseptal scar patterns compared with inferobasal scarring. This highlights current technical challenges to identifying and successfully targeting complex VT circuits within the basal and midseptal region of the ventricular septum, which is also reflected in lower success rates and higher VT recurrences after radiofrequency delivery compared with inferobasal ablation targets.
Some nonischemic cardiomyopathy etiologies show characteristic patterns of scar distribution that also affect the complexity of reentry circuits and ablation outcomes (see Fig. 46.2A–B ). Cardiac sarcoidosis, lamin-A (LMNA), and sarcomeric mutations are good examples of intramural complex substrates (interstitial, patchy, and compact) that affect the basal ventricular septum and are capable of sustaining multiple reentry circuits. Fibrous tissue distribution in cardiac sarcoidosis is also highly dependent on the anatomic location of inflammation, which also affects the right ventricle with confluent endocardial and epicardial scar (see Fig. 46.2A ). Reentry and sustained monomorphic VT are common findings in healed viral myocarditis and in Chagas cardiomyopathy. , Scar distribution predominantly affects the subepicardial lateral wall and the inferolateral basal segments (mainly transmural or subepicardial scar) of the left ventricle in myocarditis- and Chagas-related substrates, respectively (see Fig. 46.2B ). Although less common, the left ventricular apex can also show fibrous tissue in Chagas disease. In both, Chagas and myocarditis-related myocardial substrates, abnormal electrograms, and critical VT circuit components are frequently identified and targeted from the epicardium. The latter increases the success rates on ablation procedures and decreases VT recurrences during the follow-up compared with other substrates with more intramural and complex VT circuits.
ARVC is another well-characterized morphologic phenotype of nonischemic cardiomyopathies. The disease shows a predominant right ventricular involvement with classic fibrous or fibro-fatty replacement of the myocardium and VT episodes with typical left bundle branch morphology because of right ventricular origin. Most patients have one or more pathogenic variants in genes encoding desmosomal proteins, although the intercalated disc as a whole is affected. Genetically abnormal desmosomes may lead to disruption of intercellular junctions, with myocyte detachment and cell death. Mechanical uncoupling of myocytes can be aggravated by physical exercise, which increases wall stress to a greater extent in the thinner wall of the right ventricle. A growing body of evidence also supports the hypothesis that exercise plays a major role in the pathogenesis and progression of the disease, especially in individuals with very high genetic risk as carriers of multiple mutations. Despite a predominant right ventricular involvement, most patients with ARVC also develop pathologic features in the left ventricle, which can often be detected using CMR imaging. , In fact, a left ventricular ejection fraction (LVEF) of 49% or less represents a major risk factor for developing potentially lethal ventricular arrhythmias. Moreover, specific pathogenic variants in genes encoding desmosomal proteins have been associated with a higher probability of left ventricular dysfunction, as described for desmoplakin mutation carriers.
The fibrofatty tissue that replaces myocardium contributes to the development of ventricular arrhythmias by slowing intraventricular conduction and generating a substrate for scar-related macro-reentry, , similar to that observed after MI. Conduction slowing has been reported at elevated heart rates during endocardial ventricular pacing at intervals close to the ventricular refractory period. More advanced approaches combining body surface mapping (i.e., electrocardiographic imaging [ECGI]) and CMR imaging data have also reported a heterogeneous prolongation of repolarization by means of prolongation of epicardial activation recovery intervals. Moreover, premature ventricular complexes (PVCs), potentially triggering VT initiation, occur more frequently as the heart rate increases ( Fig. 46.3A ). The earliest epicardial activation of these premature beats has been predominantly identified in right ventricular scar areas with prolonged repolarization (see Fig. 46.3B ).
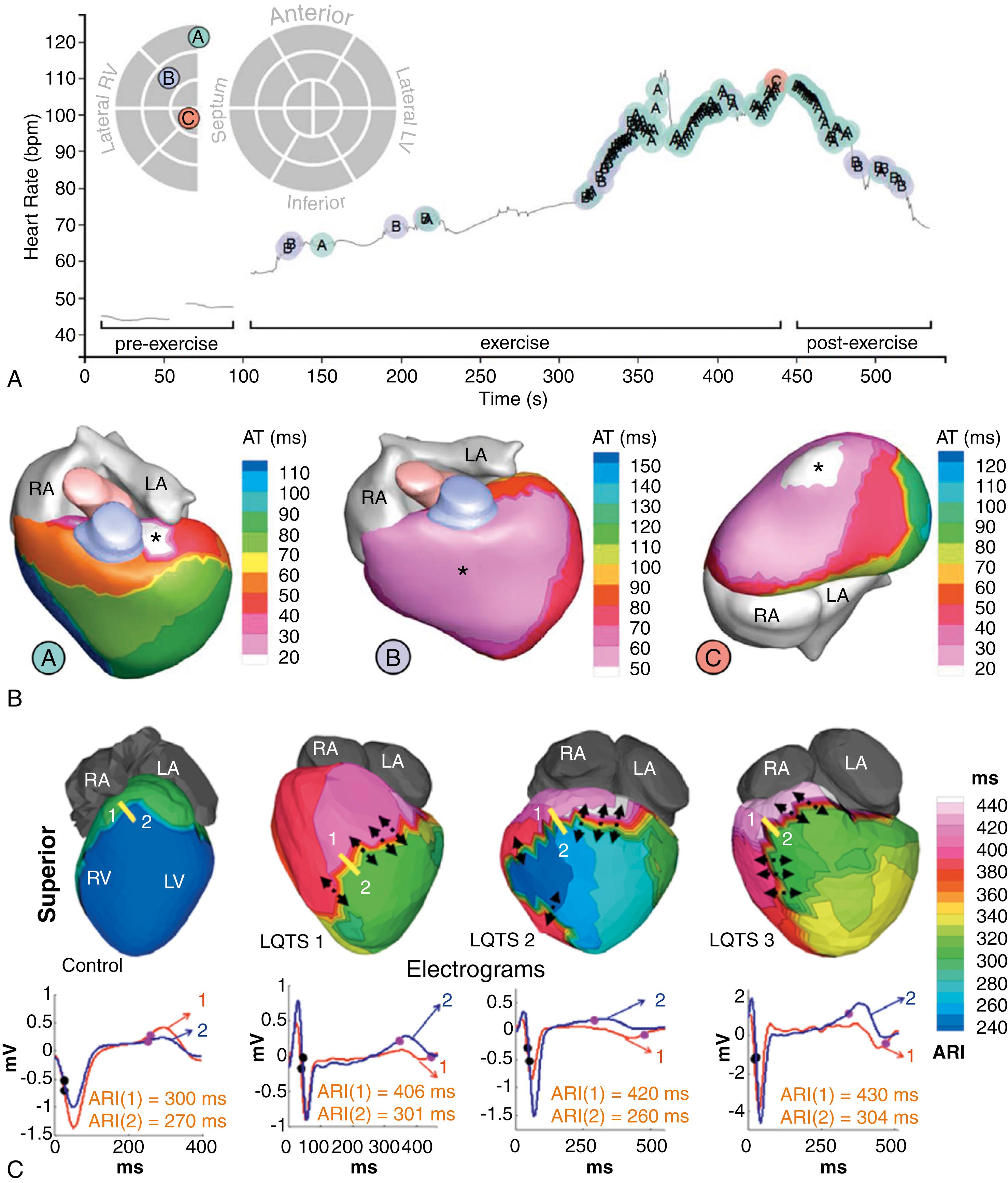
Altogether, electrophysiologic alterations in ARVC support reentry as the main mechanism of sustained VT episodes. These alterations also explain the clinical data that indicate that physical exercise plays a critical role in triggering malignant ventricular arrhythmias, which are frequently reported as exercise-related palpitations and/or syncope or sudden cardiac arrest during exercise. Data by the Marchlinski group have shown that in most patients with ARVC and VT episodes requiring repeat ablation, endocardial unipolar and bipolar voltage-derived scar regions remained stable (≤10% increase) after a median follow-up of around 4 years. Nevertheless, patients with larger voltage-derived scar areas at the baseline electrophysiologic study showed a significant correlation with progressive right ventricular dilation. Interestingly, the ventricular regions targeted to ablate the recurrent VT were confined to the defined area of the voltage-derived scar in the baseline study. This relative scar and voltage-derived stability of the arrhythmogenic substrate is consistent with long-term control of VT recurrences after endocardial and adjuvant epicardial substrate ablation in the right ventricle.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here