Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Helicobacter pylori is a Gram-negative bacterial species that selectively colonizes gastric mucosa, and virtually all persons colonized by this organism develop coexisting gastritis. A signature feature of the inflammatory response to H. pylori is its capacity to persist for decades, which is in marked contrast to inflammatory reactions induced by other Gram-negative enteric pathogens, such as Salmonella , that either resolve within days or progress to eliminate the host. H. pylori has evolved numerous strategies to facilitate its persistence including limiting the bactericidal effects of pro-inflammatory molecules, varying the antigenic repertoire of surface-exposed proteins, and/or actively suppressing the host-adaptive immune response. However, microbial persistence implies linkage in which signals of the colonizing organism affect signals of the host and during its coevolution with humans, H. pylori has developed the ability to send and receive signals from gastric epithelium, allowing both host and bacteria to participate in a dynamic relationship.
There are biological costs incurred by the long-term relationship between H. pylori and humans in that chronic inflammation confers a significantly increased risk of serious disease, including peptic ulceration (gastric and/or duodenal), gastric adenocarcinoma, and non-Hodgkins lymphoma of the stomach. On the basis of epidemiological studies in humans and experimental studies in rodents, the World Health Organization has designated H. pylori a class I carcinogen for gastric cancer, and since virtually all infected persons have superficial gastritis, it is virtually certain that the organism plays a causative role early in this progression ( Fig. 63.1 ). Eradication of H. pylori significantly decreases the risk of developing gastric adenocarcinoma in colonized individuals, providing additional evidence that this organism influences early stages in gastric carcinogenesis. However, although persistent H. pylori infection is the strongest identified risk factor for peptic ulcer disease or malignancies that arise within the stomach, only a small percentage of colonized persons ever develop clinical sequelae, suggesting that disease risk involves specific and well-choreographed interactions between pathogen and host, which, in turn, are dependent upon strain-specific bacterial factors and/or inflammatory responses governed by host genetic diversity. Further, dietary factors such as high salt intake and low iron states can intensify H. pylori -induced injury. Recent evidence has also indicated that H. pylori -induced chronic gastritis, particularly when localized to the acid-secreting corpus, is associated with a reduced risk for developing gastroesophageal reflux disease, Barrett's esophagus, Barrett's-associated esophageal adenocarcinoma, and asthma and atopic diseases. Thus, a comprehensive understanding of how H. pylori initiates ulcer disease or gastric cancer necessitates understanding how H. pylori induces gastritis, and this chapter will focus on specific mechanisms through which H. pylori colonization leads to gastric inflammation and injury via manipulation of the host immune response.
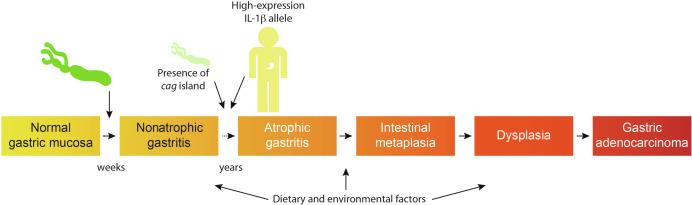
To successfully colonize the human stomach, H. pylori must initially overcome the barriers of gastric acidity and peristalsis. H. pylori possesses several mechanisms to elude these primary host defenses and establish persistent infection within the gastric niche ( Table 63.1 ). Acute ingestion of H. pylori by humans leads to transient hypochlorhydria, but when left untreated, microbial-dependent inhibition of acid secretion resolves within several months and intraluminal pH decreases to within the normal range. In some individuals, acid production continues to increase, which is likely to result from a compensatory rise in serum gastrin and fall in mucosal somatostatin levels induced by gastric inflammation.
| Bacterial Product | Functions | Targeted Host Defense |
|---|---|---|
| Urease | Neutralize acidity/regulate inflammation | Gastric acid |
| Flagellae | Motility/evade TLR5 | Gastric peristalsis/immune response |
| Adhesins | Adherence to gastric epithelium | Gastric peristalsis |
| Lewis antigens | Adherence to gastric epithelium/molecular mimicry | Host immune response |
| Arginase | Decrease nitric oxide | Inflammation |
| AhpC, NapA | Antioxidants | Oxidative stress |
| LPS | Evade TLR4 | Immune response |
| DNA | TLR9 signaling | Immune response |
| VacA | Suppresses T-cell activation | Immune response |
| CagY | Antigenic variation | Immune recognition |
One pH-altering mechanism within the H. pylori arsenal is urease, a nickel-containing hexadimer composed of two different subunits (60 and 27 kD) ( Table 63.1 ). Urease enzymatic activity, which converts urea to ammonia and carbon dioxide, is conserved among all known Helicobacter species and allows survival to pH as low as 1 in the presence of urea. Further, the primary structure of urease shows little divergence among H. pylori strains suggesting that urease is a necessary factor for the establishment of chronic infection. Indeed, several studies in mice have demonstrated that the activity of H. pylori urease is absolutely required for both successful initial colonization and maintenance of infection. In addition, infection of rhesus macaques with H. pylori producing low levels of urease activity results in expansion of a subpopulation expressing high levels of urease activity.
Seven genes comprise the H. pylori urease gene cluster: ureA and ureB encode the structural subunits of urease, while ureE , ureF , ureG , and ureH encode accessory proteins necessary for assembly and Ni 2 + insertion required to form active urease. Urease activity increases 10- to 20-fold as the pH falls from 6.0 to 5.0 and remains constant thereafter down to a pH of 2.5. Acid activation of cytoplasmic urease is mediated by expression of the third gene in the urease gene cluster, ureI , which encodes a H + -gated urea channel. UreI increases the permeability of the bacterial membrane to urea by at least 300-fold as the pH of the surrounding medium becomes acidic, and the presence of this acid-activated urea channel within the H. pylori inner membrane is necessary for efficient utilization of urea present in gastric juice. UreA/B forms a membrane complex with UreI, and assembly of the urease apoenzyme at the membrane surface facilitates access of urea to urease within the cytoplasm, which permits rapid neutralization of the periplasmic space. These data explain the requirement for both urease and UreI for survival of H. pylori at a medium pH of < 4.0 as well as for successful colonization of animal models. In addition to altering the local pH, urease has a role in the disruption of the tight junctions of gastric epithelial cells. A potential novel function for urease has been identified using H. pylori isogenic deletion mutants and recombinant proteins in H. pylori : macrophage coculture systems. These studies demonstrated that upregulation of inducible nitric oxide synthase and release of nitric oxide, a pro-inflammatory molecule, were dependent upon ureA expression, raising the hypothesis that urease may not only be required for ammonia production but may also regulate inflammation.
H. pylori can further alter the gastric pH by increasing host production of the pro-inflammatory cytokines. One such cytokine is interleukin-1β (IL-1β), which has significant acid-suppressive properties. Studies with H. pylori -infected Mongolian gerbils demonstrated that levels of gastric mucosal IL-1β increase after 6–12 weeks of infection. This increase corresponds to a reciprocal decrease in gastric acid output. Demonstration that administration of recombinant IL-1 receptor agonist normalizes acid output, implicates IL-1β as a key modulator of acid secretion. Host polymorphisms associated with increased expression of IL-1β have been linked with increased risk for atrophic gastritis and gastric adenocarcinoma. High-expression alleles of another pro-inflammatory cytokine with acid-suppressive properties, TNF-α, are also associated with an increased risk for gastric cancer.
To facilitate movement within gastric mucus and to counteract peristalsis, H. pylori possesses multiple unipolar flagella that are comprised of two major structural subunits: a 53-kD FlaA and a 54-kD FlaB. The genes encoding these two flagellins are located at distant sites on the H. pylori chromosome and are transcriptionally regulated by different promoters. Expression of flaB and a gene encoding a component of the flagellar basal body export apparatus ( flhA ) peaks early during bacterial growth phase and precedes expression of flaA. Flagellin-dependent motility is also regulated at a posttranscriptional level by an H. pylori bicistronic operon comprised of neuA , encoding a cytidyl monophosphate- N -acetylneuraminic acid synthetase, and flmD , which encodes a putative glycosyl transferase gene. Inactivation of flmD results in a motility-defective H. pylori mutant that is incapable of glycosylating FlaA.
The essential role for both flaA and flaB in the establishment of persistent colonization has been confirmed by demonstrating that aflagellate H. pylori strains only transiently colonized gnotobiotic piglets. In addition, studies in mice found that not only are the flagellar structures required for chronic colonization, but motility as well. The data further suggest that full motility requires urea such that the pH is increased thereby decreasing the mucin viscoscity. Ultrastructural studies have demonstrated that the H. pylori flagellum is cloaked by a sheath that is an extension of the bacterial outer membrane, which likely protects the acid-labile flagellar structure from gastric contents. Similar to urease production, motility is required for persistent infection, and FlbA, a component of the flagellar secretion apparatus that regulates flagellar biosynthesis, also mediates urease activity ; thus, FlbA may couple urease production and motility, bacterial phenotypes that are necessary for establishment and maintenance of gastric colonization.
In addition to motility, chemotaxis plays an important role in the ability of H. pylori to establish residence within the stomach. H. pylori expresses four chemotaxis receptors to sense external stimuli: TlpA, TlpB, TlpC, and TlpD. TlpA is a receptor for arginine, sodium bicarbonate, and senses acid gradients, TlpB may be involved in pH taxis, TlpC is responsive to media with nickel or zinc, and TlpD senses both acidic as well as basic pH. Unlike FlaA and FlaB, loss of individual Tlp proteins does not prevent colonization in wild-type-infected animals although levels of colonization may be lower. In addition, mutations in tlpB result in significantly less inflammation in infected C57BL/6 mice and gerbils. However, H. pylori that lack expression of TlpA and TlpD are unable to sense acid, resulting in a 100-fold decrease in colonization. Interestingly, when acid production in the stomach is blocked with omeprazole, the ability of tlpAD mutants to colonize is restored. In addition, the coupling proteins CheV1 and CheW, kinase CheY, and response-regulator Y are also critical for chemotaxis.
Though the vast majority of H. pylori in colonized hosts are free-living within the gastric mucous layer, ~ 20% bind to gastric epithelial cells. Adherence is important for colonization likely due to the need for acquisition of nutrients from the host and for resistance to shedding of the mucus gel layer. Adhesion by H. pylori to gastric epithelium is highly specific in vivo and when H. pylori is found in the duodenum, it only overlays islands of gastric metaplasia and not intestinal-type cells. H. pylori expresses greater than 30 outer membrane proteins (OMPs), including the Hop family of proteins, that includes several known and predicted adhesins to facilitate epithelial cell binding. One of these proteins is BabA that binds the host histo-blood group carbohydrate Lewis b and is associated with increased production of IL-8. Bugaytsova et al. have demonstrated that BabA binding is pH sensitive and has a role in H. pylori adaptation to changes in the gastric environment during disease progression and/or decreased adherence to cells shed into the gastric lumen. BabA adaptation to pH occurs via mutation and recombination events with related genes in such a way that changes in adherence are fully reversible.
The host receptor sialyl-LewisX is induced and bound by the H. pylori OMP SabA. The gene-encoding SabA can be in an “on” or “off” configuration, which is regulated by slipped strand mispairing within a group of CT dinucleotide repeats in coding region and a homopolymeric T tract in the promoter region of the gene and transcription of sabA is part of an acid responsive regulon. Recently, the adhesin HopQ has been shown to bind host CEACAM molecules, glycoproteins that regulate numerous cell functions, including adhesion. Additional OMPs with adhesin properties present in most, if not all, H. pylori strains include HopZ, AlpA, and AlpB. All of these H. pylori adhesins have roles in colonization and adherence to the gastric epithelium.
The O-antigen of H. pylori lipopolysaccharide (LPS) contains human Lewis antigens, including Lewis x , Lewis y , Lewis a , and Lewis b . Inactivation of the bacterial genes encoding Lewis x and Lewis y results in an inability of H. pylori to colonize mice, suggesting that H. pylori Lewis antigens may also mediate adhesion ( Table 63.1 ). In vitro, preincubation of H. pylori with anti-Lewis x monoclonal antibodies inhibits bacterial binding to gastric epithelial cells. H. pylori strains that strongly express Lewis x/y are associated with enhanced neutrophilic infiltration and higher colonization densities than strains that only weakly express Lewis x/y Since H. pylori Lewis antigens undergo phase variation (i.e., random and reversible high-frequency alteration of phenotype), a functional role for such changes in Lewis phenotype may be detachment of H. pylori from host epithelial cells that would subsequently facilitate transfer to a new host.
Studies that have focused on adherence of H. pylori to gastric epithelium have also provided mechanistic insights through which this organism colonizes different parts of the stomach. For example, Syder et al. studied bacterial tropism in transgenic mice deficient in acid-secreting parietal cells. Mice were colonized for 8 weeks with an H. pylori strain that expresses adhesins recognized by epithelial NeuAcα2,3Galβ1,4 glycan receptors. In control mice, H. pylori exhibited tropism for gastric mucosa that did not contain parietal cells (e.g., the boundary between the squamous epithelium and the proximal glandular epithelium), and lymphocytic infiltration was found preferentially in this area. In mice having a genetic ablation of parietal cells, epithelial progenitor cells synthesized NeuAcα2,3Galβ1,4 glycans, which was accompanied by an expansion of bacterial colonization and lymphoid aggregates within the glandular epithelium. Histopathologic studies in infected human subjects initially noted that H. pylori binds to particular areas within the gastric epithelium, and that bacterial density is greatest in the upper portion of the gastric glands. This pattern of colonization mirrors the distribution of trefoil factor 1 (TFF1), and Clyne et al. demonstrated that H. pylori binds avidly to TFF1 dimers, suggesting that TFF1 may act as a receptor for this organism in vivo. In contrast, a specific component of gastric mucin (α1,4-linked N -acetylglucosamine O -glycan) that is confined to the deeper glandular regions has been shown to exert antimicrobial effects against H. pylori in vitro by inhibiting the biosynthesis of cholesteryl- d -glucopyranoside, a major H. pylori cell wall component. However, recent studies using 3D reconstructions of confocal in vivo microscopy images have identified microcolonies of replicating H. pylori within the progenitor and stem cell compartments of both human and mouse stomachs. These findings indicate that the host has evolved mechanisms to restrict H. pylori to certain regions of the gastric mucosa.
If a bacterial species is to persistently colonize a mammalian host, its most formidable challenge is evasion of immune clearance. One mechanism through which H. pylori may persist is by limiting the bactericidal effects of pro-inflammatory molecules, such as nitric oxide (NO). Inducible NO synthase (iNOS, NOS2) has been shown to be upregulated by H. pylori in macrophages in vitro as well as in vivo . Further, macrophages can kill H. pylori in coculture in an NO-dependent manner even when there is no contact between the macrophages and bacteria. However, H. pylori expresses a functional arginase, RocF , which effectively siphons l -arginine away from the competing host enzyme, iNOS. This, in turn, limits the production of NO by limiting the availability of the l -arginine substrate. H. pylori further limits NO production by inducing expression of the host enzyme ARG2, which also uses l -Arg as its substrate.
H. pylori may also encounter reactive oxygen or nitrogen species generated by activated neutrophils that can induce oxidative DNA damage, which can not only harm the bacteria but also injure host tissue. H. pylori encodes multiple proteins to overcome this environmental stress, including AhpC, a protein that catalyzes the reduction of organic peroxides to alcohols; NapA, an iron-binding neutrophil-activating protein; and SodB, a superoxide dismutase. Bacterioferritin comigratory protein, encoded by bcp , is a thiol peroxidase that utilizes linoleic acid as its substrate. Inactivation of each of these genes resulted in enhanced sensitivity to either paraquat and cumene hydroperoxide ( ahpC − and bcp ) or oxygen ( napA − ), respectively, while an ahpC − / napA − double mutant exhibited the highest sensitivity to growth inhibition by oxygen and organic peroxides. Interestingly, a novel H. pylori protein (HP0630) was upregulated in the ahpC − / napA − mutant, suggesting that it may function as a compensatory oxidative stress-resistance constituent. This group also investigated whether ahpC, bcp, or another gene involved in antioxidant defense, tpx (encoding thiolperoxidase), were required for in vivo colonization. Inactivation of any of these genes led to a severe impairment in the ability of H. pylori to infect mice, suggesting that oxidative resistance is a critical factor for successful colonization. Interestingly, NapA is not required for colonization of mice, although napA − mutants do not survive well in competition experiments with wild-type H. pylori.
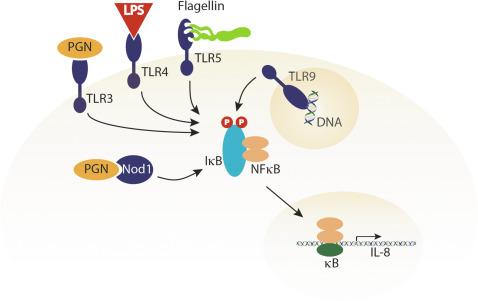
Another level of host defense that must be circumvented by H. pylori is innate immunity. Toll-like receptors (TLRs) are an evolutionarily conserved family of eukaryotic receptors that function in innate immunity via recognition of invariant regions in bacterial molecules termed pathogen- or microbe-associated molecular patterns ( Fig. 63.2 ). Thirteen different TLRs have been identified in mammals and although the bacterial ligands for TLRs are distinct, the intracellular signaling pathways activated by these receptors all appear to eventuate in NF-κB activation and pro-inflammatory gene expression ( Fig. 63.2 ). It is becoming increasingly clear; however, that H. pylori has evolved strategies to avoid global activation of this system. For example, TLR4 generally recognizes bacterial LPS, yet H. pylori LPS is relatively anergic compared with that of other enteric bacteria (e.g., Escherichia coli ). This is primarily due to lipid A core modifications as H. pylori LPS contains only four fatty acids compared with the more typical six-component fatty acid structure present in other bioactive LPS molecules. Further, H. pylori can upregulate TLR4 and utilize it as a receptor to adhere to epithelial cells. Bacterial flagellin is typically recognized by TLR5, which is present on the gastric epithelium. However, in contrast to flagellins expressed and secreted by Gram-negative mucosal pathogens such as Salmonella or E. coli , which robustly activate TLR5-mediated pro-inflammatory responses, H. pylori flagellin is not secreted and is noninflammatory. Finally, recent evidence has demonstrated that H. pylori can activate TLR9 via DNA translocation resulting in an antiinflammatory response.
Chronic gastric inflammation induced by H. pylori is considered a T-helper lymphocyte 1 (Th1) response leading to increased production of interferon gamma (IFN-γ). H. pylori has been reported to interfere with IL-4-dependent Stat6 signaling and blunt IFNγ-mediated activation of gastric epithelial cells in vitro , and H. pylori infection is associated with increased mucosal levels of IL-10, an antiinflammatory peptide that inhibits secretion of pro-inflammatory chemokines from macrophages and neutrophils.
Experimental infection of IL-10 − / − mice with either H. pylori or a related Helicobacter species, Helicobacter felis , leads to a significant decrease in colonization in conjunction with a more severe inflammatory response compared to infected IL-10 + / − heterozygotes or wild-type controls, suggesting that microbially induced IL-10 may attenuate an appropriately targeted gastric inflammatory response that has the capacity to eradicate H. pylori from the stomach. Further, ex vivo experiments using H. pylori -infected human biopsies demonstrated that binding of IL-12 to its receptor leads to STAT4 phosphorylation and increased levels of IFN-γ, indicating its role in the H. pylori - associated Th1 phenotype. In addition to production of IL-12 by macrophages and dendritic cells (DCs), studies have indicated that the p40 heavy chain subunit is also expressed in gastric epithelial cells and T cells.
IL-17 levels are also higher in human and mouse gastric tissue compared with uninfected samples. Mice treated with an anti-IL-17 antibody exhibited lower levels of H. pylori colonization as well as inflammation. In H. pylori -infected mice lacking an IL-17 receptor subunit, IL-17RA, transcript levels of IL-17 were higher as was H. pylori colonization density and inflammation. Together, these data suggest that IL-17 induction may be important in both H. pylori colonization and pathologic responses.
H. pylori can also facilitate its own persistence by varying the antigenic repertoire of surface-exposed proteins and by actively suppressing the host-adaptive immune response. Since Lewis antigens are expressed on gastric epithelial cell surfaces, a potential biological role for such antigens is molecular mimicry ( Table 63.1 ). This may allow H. pylori to escape host immunological detection by preventing the formation of antibodies directed against shared bacterial and host epitopes, thus facilitating persistent infection. Initially, human and animal studies supported this paradigm by demonstrating a significant concordance between the Lewis phenotype of the host and the infecting Helicobacter strain. However, two studies challenged these observations by failing to show a significant relationship between the human host Lewis phenotype and that of the corresponding colonizing H. pylori isolates. Moreover, strains that express both Lewis x and Lewis y can be isolated from a single human host. An in vivo experiment with Le b -expressing transgenic mice challenged with Le x and Le y positive strains led to the recovery of H. pylori that expressed only Le b and this change was associated with a putative galactosyltransferase. These data suggest that Lewis antigens may facilitate molecular mimicry and permit H. pylori to evade recognition by host immune defenses.
Finally, H. pylori can survive and replicate within epithelial cells and macrophages, thereby evading host clearance. The ability of H. pylori to invade epithelial cells has been investigated using differential interference contrast video and immunofluorescence microscopy. Using these techniques, H. pylori was found to reside within large cytoplasmic vacuoles, and following vacuole evolution, the reappearance of H. pylori in the extracellular environment was observed in parallel with the disappearance of intravacuolar bacteria, suggesting release from intravacuolar sites. At the ultrastructural level, the entry process was reported to occur via a zipper-like mechanism, as internalized bacteria were bound within phagolysosomes in close association with condensed filamentous acti. Zheng and Jones reported that virulent H. pylori strains can inhibit phagosome development following bacterial ingestion by macrophages, which is associated with retention of coronin 1. However, despite these multiple strategies for evading host clearance, substantial immune activation still occurs as manifested by epithelial pro-inflammatory cytokine release, infiltration of the gastric mucosa by inflammatory cells, and cellular and humoral recognition of H. pylori antigens.
The gastric inflammatory response induced by H. pylori consists of neutrophils, lymphocytes (T and B cells), plasma cells, and macrophages, along with varying degrees of epithelial cell degeneration and injury. One potential mechanism for induction of inflammation is that H. pylori secrete substances that stimulate mucosal inflammation from afar. For example, urease has been detected within the gastric lamina propria, and the urease complex of H. pylori stimulates chemotaxis by both monocytes and neutrophils and activates mononuclear cells as well. Similarly, H. pylori porins and low-molecular-weight molecules, such as Paf-acether, possess chemotactic properties. Finally, H. pylori water extracts promote neutrophil-endothelial cell interactions in vitro and increase leukocyte adherance via CD11a/CD18 and CD11b/CD18 interactions with intercellular adhesion molecule type-1 (ICAM-1).
The presence of acute inflammatory components within H. pylori -infected mucosa suggests that soluble mediators that are capable of attracting polymorphonuclear cells (PMNs) are key regulators in the development of gastritis. Therefore, another mechanism through which H. pylori may induce inflammation is via direct contact with gastric epithelial cells and stimulation of cytokine release. Gastric epithelium from infected persons contains increased levels of IL-1β, IL-2, IL-6, IL-8, and TNF-α compared with gastric mucosa from uninfected persons, and within this group of pro-inflammatory mediators, IL-8 has been studied most intensively as a mediator of H. pylori pathogenesis.
IL-8 is a potent neutrophil-activating chemotactic cytokine (chemokine) that is secreted by gastrointestinal (GI) epithelial cells in response to infection with pathogenic bacteria. IL-8 produced by activated enterocytes binds to the extracellular matrix and establishes a haptotactic gradient that directs inflammatory cell migration toward the epithelial cell surface. Consistent with observations for infected intestinal epithelial cells, expression of IL-8 within H. pylori -colonized gastric mucosa localizes to gastric epithelial cells, and levels of IL-8 are directly related to the severity of gastritis. In vitro, H. pylori stimulates IL-8 expression and release from gastric epithelial cells, and these events are dependent upon an active interplay between viable bacteria and epithelial cells. Thus, a paradigm for the acute component of H. pylori -induced gastric inflammation is that contact between bacteria and epithelial cells stimulates IL-8 secretion, which then regulates neutrophil infiltration into the gastric mucosa.
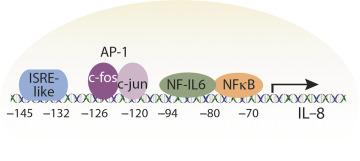
Pro-inflammatory cytokine expression is often regulated at the mRNA level by soluble transcription factors and the human IL-8 gene contains several motifs within its promoter region including binding sites for NF-κB, NF-IL-6, AP-1 (which is comprised of the binding elements c- fos and c- jun ), as well as an element that is homologous to an interferon-stimulated responsive element (ISRE; Fig. 63.3 ). NF-κB constitutes a family of transcription factors sequestered in the cytoplasm, whose activation is tightly controlled by a class of inhibitory proteins termed IκB's. Multiple signals, including microbial contact, stimulate phosphorylation of IκB by IκB kinase β (IKKβ), which leads to proteasome-mediated degradation of phospho-IκB, thereby liberating NF-κB to enter the nucleus where it regulates transcription of a variety of genes, including immune response genes ( Fig. 63.3 ). Stimulation and activation of NF-κB does not require protein synthesis, thereby allowing efficient activation of target genes, such as IL-8. This system is particularly utilized in immune and inflammatory responses where rapid activation of defense genes following exposure to pathogens such as bacteria is critical for survival of an organism. Several studies have demonstrated that contact between H. pylori and gastric epithelial cells results in brisk activation of NF-κB, which is followed by increased IL-8 expression ( Fig. 63.3 ). Smith et al. delineated additional upstream mediators of H. pylori -induced NF-κB activation by transfecting HEK293 cells with TLR2 as well as an NF-κB reporter gene. Cotransfection of TLR2 conferred the ability to activate NF-κB and induce IL-8 production in response to different H. pylori strains. The ability of H. pylori to activate NF-κB in vitro has been corroborated in vivo as activated NF-κB is present within gastric epithelial cells of infected, but not uninfected patients, which mirrors the distribution pattern of increased IL-8 protein within colonized mucosa.
Mitogen-activated protein kinases (MAPK) also mediate H. pylori -dependent IL-8 expression. MAPK are signal transduction networks that target transcription factors such as AP-1 and participate in a diverse array of cellular functions, including cytokine expression. MAPK cascades are organized in three-kinase tiers consisting of a MAPK, a MAPK kinase (MKK), and a MKK kinase (MKKK), and transmission of signals occurs by sequential phosphorylation and activation of components specific to a respective cascade. In mammalian systems, at least five MAPK modules have been identified and characterized to date; these include extracellular signal-regulated kinases 1 and 2 (ERK 1/2), p38, and c-Jun N-terminal kinase (JNK). H. pylori activates p38, ERK 1/2, and JNK in gastric epithelial cells in vitro, and activation of ERK1/2 is dependent upon transactivation of the EGF receptor, a receptor tyrosine kinase.
Cell culture studies have revealed that H. pylori -induced IL-8 expression is dependent upon both NF-κB and MAPK activation of AP-1, and cross-talk between NF-κB and MAPK pathways has been demonstrated previously. For example, MEKK1, a hierarchical MAPK kinase, can directly activate the IκB kinase signalsome. However, inhibition of ERK 1/2 attenuates H. pylori -induced IL-8 secretion without affecting NF-κB, raising the possibility that synergistic interactions between AP-1 and NF-κB occur within the IL-8 promoter. Consistent with these data, H. pylori activation of ERK 1/2 results in enhanced c- fos transcription, indicating that ERK 1/2 may exert regulatory effects on IL-8 production that are primarily dependent upon AP-1 ( Fig. 63.3 ). Another layer of complexity exists in that maximal H. pylori -induced IL-8 gene transcription requires activation of NF-κB, AP-1, and ISREs within the IL-8 promoter ( Fig. 63.3 ). Finally, recent exciting data have shown that H. pylori can activate another pathway that mediates IL-8 production, which involves the adaptor protein TIFA. Activation of TIFA is a strain-specific effect that is dependent upon the cag pathogenicity island and translocation of a bacterial metabolite termed heptose-1,7-bisphosphate (HBP), an intermediate of heptose biosynthesis. Mutant H. pylori strains unable to synthesize HBP retain the ability to translocate CagA, but IL-8 induction is reduced by 95%. Collectively, these data indicate that the mucosal inflammatory reaction that develops in response to H. pylori involves multiple different pathways converging on the IL-8 promoter and that H. pylori -mediated host signaling is of central importance for understanding the inflammatory response to this pathogen, which if left untreated over decades, may progress to peptic ulceration or gastric cancer.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here