Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The mechanisms triggering brain damage after intracerebral hemorrhage (ICH) are pleiotropic and are in many respects distinct from those contributing to ischemic brain injury.
The toxicity of extravasated blood toward all structural components of the neurovascular unit represents a unique feature of ICH-mediated brain damage.
Inflammation and oxidative stress appear to play prominent roles in the pathobiology of ICH.
The secondary injury after ICH develops over days suggesting the presence of a considerably wide window for therapeutic intervention.
Approaches aimed at detoxification of blood-derived noxious components represent a promising target for the treatment of ICH.
Pre-clinical animal models provide useful guidance on the pathogenesis of ICH. However, better models to assess re-bleeding (hematoma enlargement) are urgently needed.
Intracerebral hemorrhage (ICH) is a devastating form of stroke with a high mortality and poor prognosis, for which no effective therapy is currently available. Rapid accumulation of blood within the brain parenchyma causes increased intracranial pressure and initial cell/tissue damage. Since only half of ICH-related deaths occur in the first 2 days after ICH, , the contributions of toxic hematoma-derived products (e.g., hemolysis products ), oxidative stress, and pro-inflammatory responses to secondary brain injury are clearly important. Indeed, as compared to ischemic stroke, ICH may have a wide therapeutic time window. This chapter will outline selected aspects of our current knowledge from experimental models of ICH, regarding cellular mechanisms of injury and experimental approaches to combat ICH-mediated brain injury ( Fig. 8.1 ).
Over the past two decades numerous animal models have been developed to study the pathobiology of ICH. However, most experimental studies of ICH have been conducted in two models, that is, autologous blood infusion or collagenase injection (reviewed in ). In the blood infusion model, arterial blood is directly infused into a specific brain structure, for example, basal ganglia in rodents or frontal white matter in pigs. In the collagenase model, the injected bacterial enzyme, collagenase, degrades the basal lamina that surrounds cerebral blood vessels causing them to rupture and bleed. An additional model in swine involves the creation of a cavity in white matter by balloon inflation followed by infusion of blood into the cavity.
All ICH models have their limitations. , Direct autologous blood infusion does not capture blood vessel rupture that is the basis for bleeding in human ICH. In the collagenase model, the bacterial enzyme induces considerable inflammation and has a significantly different time course, extent, and robustness of perihematomal blood-brain barrier (BBB) opening versus ICH in humans or in blood infusion models. Furthermore, collagenase also produces an injury volume that is considerably larger despite similar initial hematoma volumes. In the balloon inflation model, white matter tracts are “torn” and otherwise damaged in a manner different than that which occurs in blood infusion models and in human ICH. Lastly, unfortunately none of these models replicate the blood vessel pathologic changes that may be present with aging, for example, amyloidosis.
Despite their limitations, and as presented in this chapter, findings from these models (reviewed in , , , ) have provided significant new understanding of ICH pathophysiology and pathochemistry. In addition, these models support potential pharmacologic and surgical treatments, including the recent development of a minimally invasive surgical approach using magnetic resonance-guided focused ultrasound. It is noteworthy that findings directly from ICH animal models have provided the basis for several ongoing clinical therapy trials including iron chelation with deferoxamine (iDEF), , pioglitazone for hematoma resolution (SHRINC), and minimally invasive surgery plus thrombolysis for clot evacuation (MISTIE). ,
A considerable body of literature demonstrates the participation of inflammatory cells in the pathophysiologic processes following ICH, including blood-derived leukocytes, resident microglia, astrocytes, and mast cells. These cells can aggravate ICH-induced secondary brain injury by releasing a variety of toxic factors, including cytokines, chemokines, free radicals, and nitric oxide.
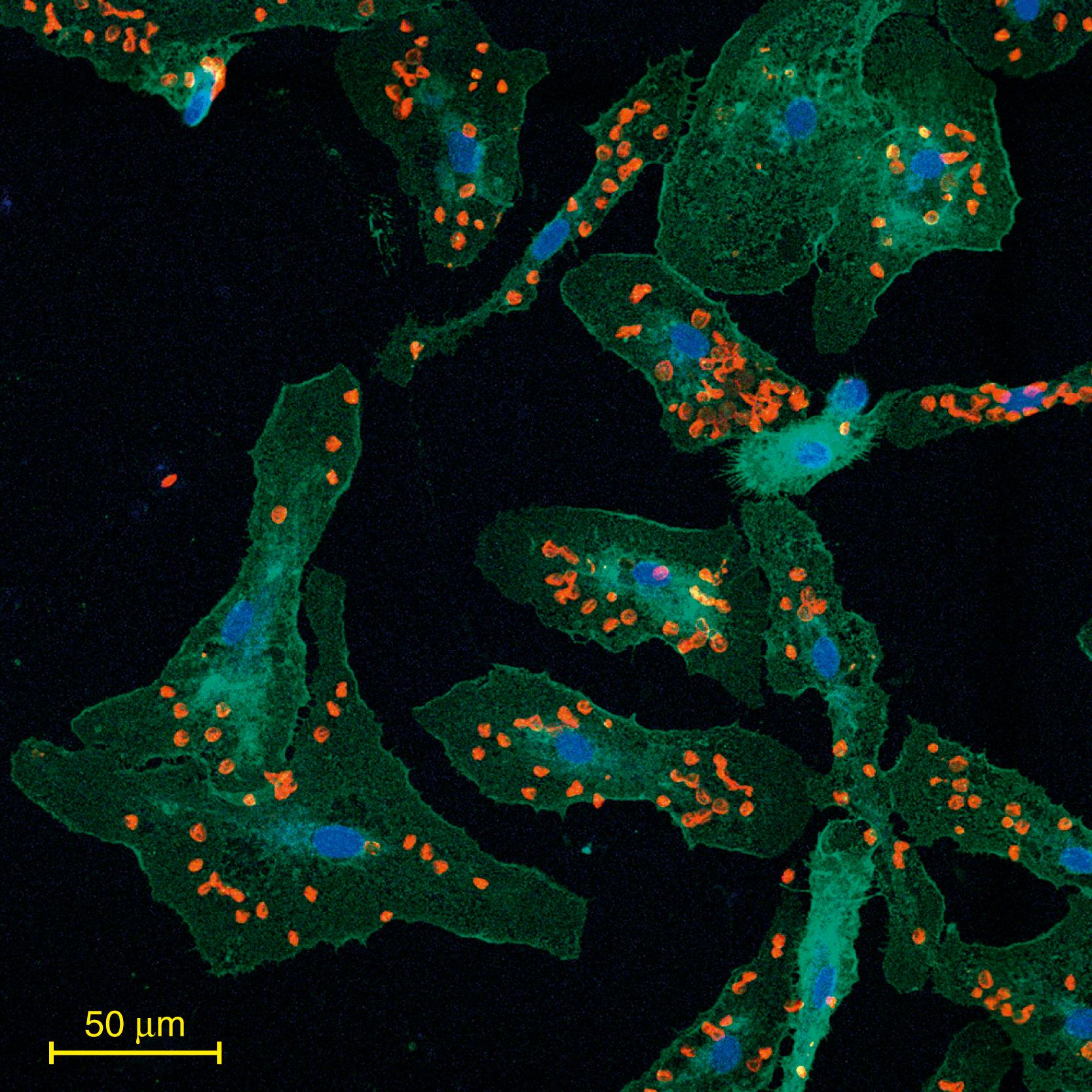
Activated microglia, which are likely the first non-neuronal cells to react to brain injury, undergo morphologic and functional changes. Microglial cells classified as the pro-inflammatory activated phenotype are involved in often deleterious processes following ICH, while the alternative “reparative” phenotype may contribute to “healing” processes. Microglial cells are activated within minutes after the onset of ICH. The initial activation of these cells results in the production of pro-inflammatory cytokines, including tumor necrosis factors (TNF)- α , interleukin (IL)-1 β , IL-6, , and various chemokines, proteases, and free radicals, which triggers neuroinflammation and leukocytes and other white blood cells’ brain infiltration. , , Several studies have shown that inhibition of microglia activation, for example by minocycline, tuftsin 1–3 fragment, or transforming growth factor-β, or microglial depletion reduces secondary brain injury and improves neurologic function in rodent models of ICH. , Timely clearance of the extravasated hematoma components and damaged tissue debris by activated microglia can reduce local damage from red blood cell (RBC) lysis, thereby favoring a nurturing environment and promoting tissue recovery. , , The phagocytic function of both microglia and blood monocyte-derived macrophages has been implicated in the process of clearance of the hematoma/blood components via cellular mechanisms involving scavenger receptors such as CD36 , or LRP1, , AXL/MERTK-assisted engulfment of eryptotic RBCs. The inhibition of CD47 that normally prevents phagocytosis-mediated engulfment may also play an important role. In vitro studies with microglia showed that a single phagocyte could engulf multiple RBC within a very short time ( Fig. 8.2 ). In both human and animal studies, multinucleated giant microglia/macrophages with strong phagocytosis capacity have been detected in the hematoma and perihematomal zone. Interestingly, it was shown that physical exercise after ICH contributes to hematoma clearance, as animals subjected to increased physical activities demonstrated faster hematoma resolution.
The roles of other blood-born inflammatory cells, such as leukocytes and monocyte-derived macrophages, have increasingly gained attention in ICH-induced inflammation. In preclinical animal models, neutrophils (polymorphonuclear leukocytes [PMNs]) are the earliest leukocyte subtype to infiltrate the hemorrhagic brain, occurring within 4–5 hours after the onset of ICH, and peaking at 3 days after hemorrhage induction. , PMNs can occlude capillaries, release various proteolytic enzymes, and generate NADPH-oxidase- and myeloperoxidase-dependent oxidative stress, which can damage local cells and compromise blood brain barrier. , The death of these infiltrating PMNs, without timely removal by phagocytes, may lead to secondary necrosis and further exacerbation of secondary brain injury by stimulating microglia/macrophages to release pro-inflammatory mediators. However, neutrophils at later stage after ICH could be beneficial, , including by releasing lactoferrin that can sequestrate iron and modulate microglia phenotype. , Recently, studies showed that fingolimod, an anti-inflammatory drug used as pharmacotherapy for multiple sclerosis, effectively reduced cerebral infiltration of T-lymphocytes, thereby inhibiting local inflammation and improving neurobehavioral and cognitive outcomes following experimental ICH and reduced perihematomal edema in ICH patients. Fingolimod and experimental T-lymphocyte deficiency protected BBB from damage in a mouse model of ICH. In contrast to most CD4 and CD8 T cells, regulatory T cells were demonstrated as important beneficial players in ICH pathogenesis, including through polarization of microglia/macrophages to a “healing” phenotype.
Activated astrocytes can secrete inflammatory mediators and increase production of glial fibrillary acidic protein (GFAP), causing so-called reactive gliosis, which can interfere with axonal regeneration. Astrocytes also express and release a variety of matrix metalloproteinases and chemokines that participate in brain inflammation. Thus, blocking microglia-astrocyte interactions might be a potentially effective strategy to minimize secondary brain damage following ICH. On the other hand, astrocytes may promote neuroprotection by modulating the production of microglial inflammatory mediators. , Additionally, the inhibition of mast cells has been reported to reduce brain edema and hematoma volume, which was associated with ameliorated neurologic deficits following experimental ICH. Hydrogen gas inhalation also diminished brain edema and enhanced blood–brain barrier preservation by reducing mast cell activation and degranulation in an ICH mouse model.
Accumulating evidence shows that cytokines exacerbate secondary injury after ICH. TNF-α and IL-1β are the two prominent mediators of pro-inflammatory responses in the progression of ICH-induced brain injury. , , , IL-10 and transforming growth factor-β are anti-inflammatory cytokines, which act to modulate and reduce inflammation. In patients, both have been associated with improved outcomes. , Following ICH, perihematomal levels of TNF-α are significantly increased, , , which contributes to brain edema and neurologic deficits. Clinical evidence is consistent with animal studies, supporting the theory that TNF-α can aggravate ICH-induced brain injury. Similarly, IL-1β has been found to be upregulated after ICH; and increased IL-β expression is associated with severe brain edema and blood–brain barrier disruption. Inhibition of IL-1β with the receptor antagonist, IL-1Rα, reduces ICH-mediated damage. In addition, IL-6 possesses both pro- and anti-inflammatory properties and may play a significant role in ICH pathophysiology.
Toll-like receptor 4 (TLR4) recruits a specific set of adaptor molecules that interact with the TIR domain, such as MyD88 and TRIF, and subsequently activate a transcription factor, nuclear factor κB (NF-κB). The TLR4/NF-κB signaling pathway plays a major role in ICH-induced pathology. , Heme degradation products lead to production of TNF-α, IL-1 β , and IL-6 through activation of the TLR4 pathway. NF-κB is an important transcriptional regulator of pro-inflammatory cytokine production, including TNF-α and IL-1β. Activation of NF-κB occurs in the perihematoma within minutes, lasts for at least 1week after the onset of ICH, , and is positively associated with perilesional cell death after ICH in rats. TLR4/NF-κB inhibition, by significantly reducing the perihematomal inflammatory response and the infiltration of the peripheral inflammatory cells, is remarkably protective. In addition, treatment with peroxisome proliferator-activated receptor (PPAR)γ agonists, such as pioglitazone, rosiglitazone, or 15d-PGJ 2 , promoted phagocytosis of RBCs by microglia/phagocytes, accelerated hematoma resolution, and reduced neurologic deficits in both in vitro and in vivo ICH models. , A Phase 2 clinical study evaluating pioglitazone in ICH is currently being analyzed.
There are several shortcomings for studying ICH-induced inflammation in our current animal models. In both the collagenase and blood injection ICH models, inflammatory reactions are exacerbated by placing a needle into the animal’s brain. Moreover, collagenase itself may amplify inflammatory responses. Focused ultrasound or laser pulse-induced ICH via capillary rupture and endothelial damage might be necessary to improve the translatability of intracerebral injections of collagenase or blood products. Furthermore, establishing animal models of spontaneously occurring ICH, without the injection of foreign agents (such as collagenase), would be a substantial advancement in this field. Since preclinical ICH models are different from the human condition, human histopathologic studies or cellular analyses are required to confirm their validity. A better understanding of the inflammation signaling pathways underlying ICH, especially newly identified pathways or molecules should facilitate the identification of therapeutic targets for this malady.
Reactive oxygen species (ROS) levels dramatically increase following ICH. High levels of oxidative stress, as measured by protein carbonyl formation, have been found shortly after the intracerebral injection of autologous blood in pigs. , High levels of the ROS marker, ethidium or 4-hydroxynonenal, have been observed in the perihematomal brain region on day 1 and 3 after ICH in the mouse model. , ROS are produced as a natural byproduct of the oxygen metabolism. Iron and thrombin, released from the hematoma, can generate hydroxyl radicals. Recent study demonstrated that serum concentration of myeloperoxidase, a potent oxidizing enzyme, is associated with hematoma volume and National Institutes of Health stroke score, as well as the increased risk of unfavorable outcome at 6 months in ICH patients. One of the ROS sources after ICH is peripheral immune cells (e.g., neutrophils and monocytes), which start invading the brain shortly after the hemorrhage and participate in microglial activation. Subsequently, the activated microglia further enhance the generation of ROS.
Excess generation of ROS is lethal to cells. Hemoglobin degradation products can directly injure DNA by means of oxidative strand breaks. ROS also cause lipid peroxidation, protein oxidation, mitochondrial dysfunction, and altered signal transduction, eventually leading to cell death, including ferroptosis (see below). Beneficial effects of free radical scavengers in preclinical ICH models have been recently demonstrated, including α-phenyl-N-tert-butyl nitrone (PBN), NXY-059 (a derivative of PBN), and edaravone. , In addition, gp91phox KO mice with deleted NADPH oxidase, a key enzyme involved in ROS generation (and enzyme highly abundant in neutrophils), showed milder damage than wild-type mice in response to ICH.
Furthermore, given the potential sources of ROS production following ICH, other efforts targeting pro-oxidant heme or iron such as deferoxamine, porphyrin derivatives, or adaptaquin have gained increasing promise. , ,
In response to heme toxicity and the generation of free radicals, depletion/malfunction of scavenging antioxidant system may further enhance the oxidative injury of ICH. The pathway involving Kelch-like ECH-associated protein 1 (Keap1) and nuclear factor erythroid 2-related factor 2 (Nrf2) is currently recognized as the central endogenous antioxidant system. Nrf2 expression was significantly increased at 2 hours with a peak at 24 hours following intracerebral blood infusion, while Keap1 was decreased at 8 hours after ICH induction. The downstream antioxidative enzymes regulated by Nrf2, including haemeoxygenase-1 (HO-1), catalase, superoxide dismutase (SOD), glutathione (GSH), thioredoxin (TRX), and glutathione-S-transferase (GST-α1), increased to different degrees during the early stages of ICH. , , Nrf2 −/− mice exhibited more severe neurologic damage than did wild-type mice subjected to the whole-blood injection model of ICH. , , Conversely, Nrf2 inducer sulforaphane or dimethyl fumarate (DMF; medication use to treat multiple sclerosis) exerted to reduce oxidative damage, increased haptoglobin production (to improve hemoglobin elimination), reduced neutrophil amount, and improved behavioral deficits. , , , Oxidative stress contributes to grafted cell death after transplantation, in cell therapy for ICH. Genetic manipulation of grafted neural stem cells (NSCs) to overexpress copper/zinc-superoxide dismutase (SOD1), a specific antioxidant enzyme and normally gene target of Nrf2, could enhance survival of grafted NSCs and improve the outcome of ICH. Besides Nrf2, SOD1, SOD2, and catalase could also be upregulated as a result of PPARγ activation, including with TZD drugs such as pioglitazone, rosiglitazone, or exogenous activator 15-Deoxy-Delta(12,14)-prostaglandin J2. , , Recent study showed that MnSOD, a main intramitochondrial antioxidant enzyme, is upregulated in microglia upon transfer of astrocyte-derived mitochondria, or upon exposure to mitochondria-genome encoded small peptide humanin, and that humanin used as a therapeutic agent was effective in reducing neurologic damage in a mouse model of ICH.
Drugs with antioxidant properties are promising candidates for ICH therapy. However, conventional antioxidant cannot neutralize ROS formed intracellularly, because the native enzymes cannot come across the membrane of neurons and astrocytes. Thus, different alternatives of enzyme delivery have been designed to improve fusion of enzymes, such as nanoparticles, PEGylation, and lecithinization. More recently, mitochondrial ROS amplified the inflammatory response by triggering NLRP3 inflammasome activation after ICH. Thus, the inhibition of the NLRP3 inflammasome may effectively block the interactions between oxidative stress and inflammation following ICH. The novel free radical neutralizers with clear pharmacokinetics still need to be explored. Another study with transgenic mice observed that E3 ubiquitin ligase ring finger protein 34 (RNF34) overexpression exacerbated brain injury after ICH by facilitating mitochondrial dysfunction-mediated oxidative stress. Dexmedetomidine, which is an inhibitor of mitochondrial dysfunction-derived oxidative stress, alleviated neurologic dysfunction after ICH in mouse.
As described below, studies have shown that RBC lysis and coagulation cascade activation are major factors leading to brain edema, BBB disruption, neuronal death, and neurologic deficits following ICH.
RBCs within a clot preserve their normal biconcave configuration for a few days after ICH. Thereafter, they lose their normal shape and start to lyse. RBC lysis appears to begin very early in the brain after hemorrhage. In rodent models of brain hemorrhage, for example, RBCs start to lyse within 24 hours, which can be detected by MRI. In ICH patients, hemoglobin levels in the cerebrospinal fluid (CSF) increase during the first few days after ictus. However, lysis of RBCs occurs mostly several days after ICH, , , which may result from either depletion of intracellular energy reserves or formation of membrane attack complex after activation of the complement system, or both. RBC lysis causes edema formation, oxidative stress, and neuronal death following ICH. , A clinical study of edema and ICH indicates that delayed brain edema is related to significant midline shift after ICH in humans. This delayed brain edema (in the second or third weeks after the onset in humans) is probably due to hemoglobin and its degradation products. A recent study showed that haptoglobin, an acute response protein and a key hemoglobin neutralizing component, is neuroprotective against ICH-induced brain injury.
Hemoglobin-induced brain injury may be direct or the result of heme degradation products. , Heme is degraded by heme oxygenases in the brain into iron, carbon monoxide, and biliverdin. An intracerebral injection of hemoglobin or its degradation products caused brain damage. Studies have shown that heme oxygenase-1 protein levels are increased after brain hemorrhage , and heme oxygenase inhibitors, tin-mesoporphyrin, and zinc protoporphyrin reduce perihematomal edema, neuronal loss, and neurologic deficits in ICH animal models.
In addition, other RBC components also can cause brain injury. For example, carbonic anhydrase-1, one of fourteen carbonic anhydrase isozymes, is present at high concentrations in RBCs. Extracellular carbonic anhydrase-1 also contributes to BBB disruption and ICH-induced brain injury. , Peroxiredoxin-2 is the 3rd most abundant protein in RBC, and brain peroxiredoxin-2 levels are increased in a model of ICH. Intracerebral injection of peroxiredoxin-2 causes BBB disruption, inflammation, and neuronal death, suggesting that extracellular peroxiredoxin-2 released from the hematoma can result in brain damage.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here