Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We honor the memory of Dr. Lionel H. Opie who contributed to previous versions of this chapter over the years.
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
The major function of cardiac muscle cells ( cardiomyocytes or myocytes ) is to execute cardiac excitation-contraction-relaxation that depends on the electrical calcium ion (Ca 2+ ) transport and contractile properties. , Cardiomyocytes constitute approximately 75% of total ventricular volume and weight, but only one third of the total number of cells there. Approximately half of each ventricular myocyte is occupied by myofibrils of the myofibers and 30% by mitochondria ( Fig. 46.1 and Table 46.1 ). A myofiber is a group of cardiomyocytes held together by surrounding collagen connective tissue, the latter being a major component of the extracellular matrix. Further strands of collagen connect myofibers to each other.
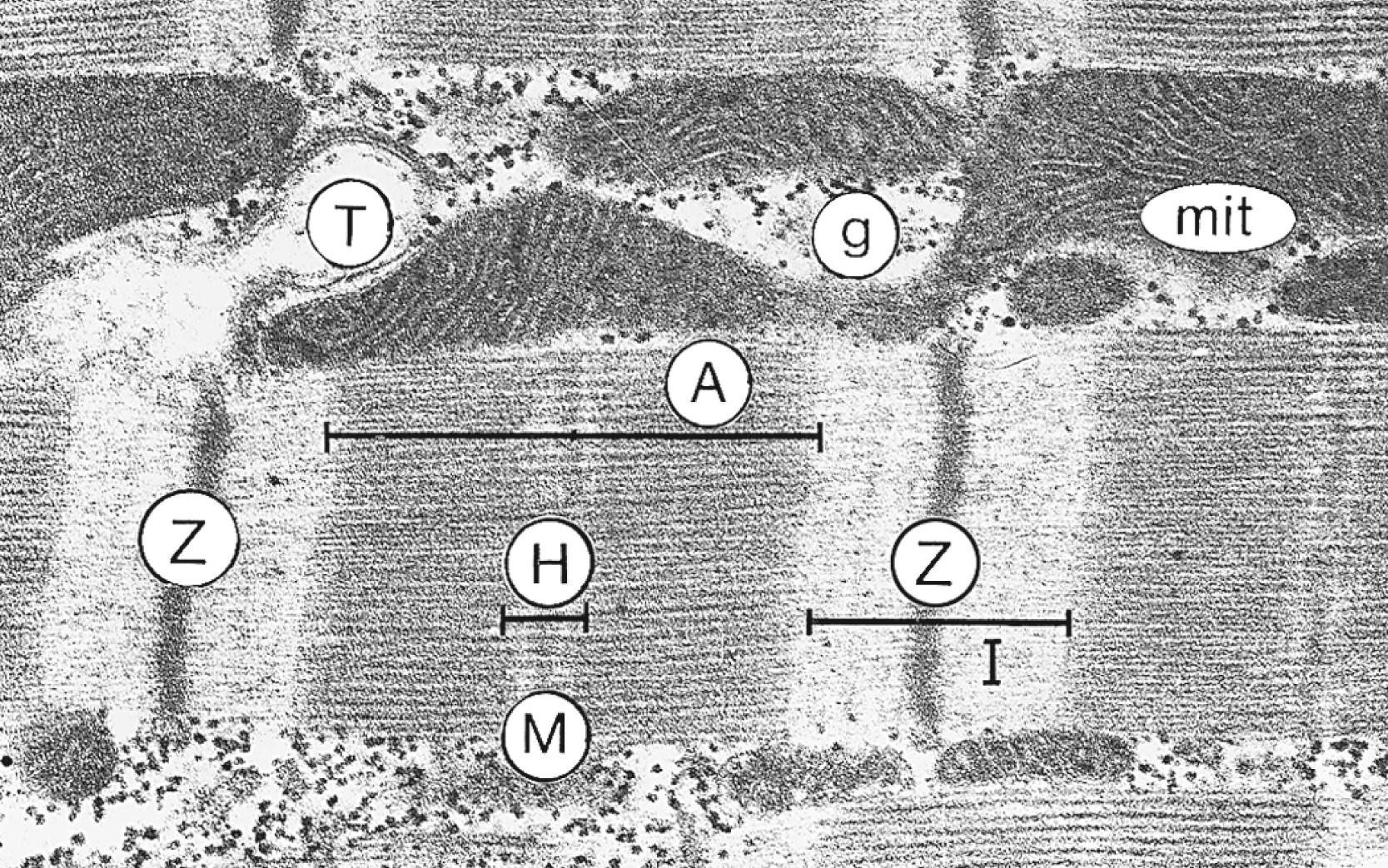
![FIGURE 46.1, Ultrastructural components of excitation-contraction coupling in ventricular myocytes, viewed anatomically ( A, with inset showing an end-on view of thick and thin filament organization) and schematically (B). The action potential is conducted along the surface sarcolemma and sarcolemma that extends into the T tubules. Ca 2+ current (I Ca ) at sites of junctional SR clefts trigger local Ca 2+ release, and the Ca 2+ diffuses throughout the cytosol to activate myofilament contraction. The [Ca 2+ ] i quickly declines at each beat because of Ca 2+ uptake via the SR Ca 2+ -ATPase (ATP/PLB), extrusion via sarcolemmal Na + /Ca 2+ exchange (NCX) and Ca 2+ -ATPase (and mitochondrial Ca 2+ uniport), allowing relaxation (diastole) to proceed. The myofibrils are bundles of contractile proteins that are organized into a regular sarcomeric array, bounded longitudinally by Z-lines that are immediately adjacent to T tubules that run in parallel. In diastole ( bottom ) the thin filaments (containing mainly actin) create a cage around the thick filaments (containing mainly myosin) that have cross-bridges (myosin heads) that extend toward the thin filament. Myosin molecule tails all face the center of the sarcomere, creating a zone around the M-line devoid of myosin heads. During systole, the myosin cross-bridges pull the thin filament “cage” toward the M-line, thus shortening the sarcomere length (additional details are in subsequent figures). ATP , Adenosine triphosphate; PLB , phospholamban; SR , sarcoplasmic reticulum; T tubules , transverse tubules. FIGURE 46.1, Ultrastructural components of excitation-contraction coupling in ventricular myocytes, viewed anatomically ( A, with inset showing an end-on view of thick and thin filament organization) and schematically (B). The action potential is conducted along the surface sarcolemma and sarcolemma that extends into the T tubules. Ca 2+ current (I Ca ) at sites of junctional SR clefts trigger local Ca 2+ release, and the Ca 2+ diffuses throughout the cytosol to activate myofilament contraction. The [Ca 2+ ] i quickly declines at each beat because of Ca 2+ uptake via the SR Ca 2+ -ATPase (ATP/PLB), extrusion via sarcolemmal Na + /Ca 2+ exchange (NCX) and Ca 2+ -ATPase (and mitochondrial Ca 2+ uniport), allowing relaxation (diastole) to proceed. The myofibrils are bundles of contractile proteins that are organized into a regular sarcomeric array, bounded longitudinally by Z-lines that are immediately adjacent to T tubules that run in parallel. In diastole ( bottom ) the thin filaments (containing mainly actin) create a cage around the thick filaments (containing mainly myosin) that have cross-bridges (myosin heads) that extend toward the thin filament. Myosin molecule tails all face the center of the sarcomere, creating a zone around the M-line devoid of myosin heads. During systole, the myosin cross-bridges pull the thin filament “cage” toward the M-line, thus shortening the sarcomere length (additional details are in subsequent figures). ATP , Adenosine triphosphate; PLB , phospholamban; SR , sarcoplasmic reticulum; T tubules , transverse tubules.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MechanismsofCardiacContractionandRelaxation/0_3s20B9780323722193000463.jpg)
| Microanatomy of Heart Cells | |||
|---|---|---|---|
| Ventricular Myocyte | Atrial Myocyte | Purkinje Cells | |
| Shape | Long and narrow | Elliptical | Long and broad |
| Length (μm) | 75–170 | 20–100 | 150–200 |
| Diameter (μm) | 15–30 | 5–6 | 35–40 |
| Volume (μm ) | 15,000–100,000 | 400–1500 | 135,000–250,000 |
| T tubules | Plentiful | Rare or none | Absent |
| Intercalated disc | Prominent end-to-end transmission | Side-to-side as well as end-to-end transmission | Very prominent abundant gap junctions Fast; end-to-end transmission |
| General appearance | Mitochondria and sarcomeres very abundant Rectangular branching bundles with little interstitial collagen |
Bundles of atrial tissue separated by wide areas of collagen | Fewer sarcomeres, paler |
| Composition and Function of Ventricular Cell | ||
|---|---|---|
| Organelle | Percentage of Cell Volume | Function |
| Myofibril | ≈50–60 | Interaction of thick and thin filaments during contraction cycle |
| Mitochondria | 16 in neonate 33 in adult rat 23 in adult man |
Provide ATP chiefly for contraction |
| T-system | ≈1 | Transmission of electrical signal from sarcolemma to cell interior |
| SR | 10 in neonate 2–3 in adult |
Takes up and releases Ca 2+ during contraction cycle |
| SR terminal cisternae | 0.33 in adult | Site of calcium storage and release |
| Rest of network of SR | Rest of volume | Site of calcium uptake en route to cisternae |
| Sarcolemma | Very low | Control of ionic gradients, channels for ions (action potential), maintenance of cell integrity, receptors for drugs and hormones |
| Nucleus | ≈3 | Transcription |
| Lysosomes | Very low | Intracellular digestion and proteolysis |
| Sarcoplasm (= cytoplasm) (includes myofibril but not mitochondria or SR) | ∼60 | Cytosolic volume within which [Ca 2+ ] i rises and falls |
Ventricular myocytes are roughly brick shaped, typically 150 × 20 × 12 μm (see Table 46.1 ), and are connected at the long ends by specialized junctions that mechanically and electrically couple the myocytes with each other. Atrial myocytes are smaller and more spindle shaped (<10 μm in diameter and <100 μm in length). When examined under a light microscope, atrial and ventricular myocytes have cross striations and are often branched. Each myocyte is bounded by a complex cell membrane, the sarcolemma (muscle plasma membrane), and is filled with rodlike bundles of myofibrils containing the contractile elements. The sarcolemma invaginates to form an extensive transverse tubular network ( transverse tubules [T tubules]) that extends the extracellular space into the interior of the cell (see Figs. 46.1 and 46.2 ). Ventricular myocytes are typically binucleate, and these nuclei contain most of the cell’s genetic information. Some smaller or more juvenile myocytes have one nucleus and some up to three to four nuclei. Rows of mitochondria are located between the myofibrils and also immediately beneath the sarcolemma. Mitochondria function mainly to generate the energy, in the form of adenosine triphosphate (ATP), that is needed to maintain cardiac contractile function and the associated ion gradients. The sarcoplasmic reticulum (SR) is a specialized form of endoplasmic reticulum that is critical for calcium (Ca 2+ ) cycling, which is the on-off switch for contraction. When the wave of electrical excitation reaches the T tubules, voltage-gated Ca 2+ channels open to provide relatively small entry of Ca 2+ , which triggers additional release of Ca 2+ from the SR via closely apposed Ca 2+ release channels. This is the Ca 2+ that initiates myocardial contraction. Ca 2+ sequestration by the SR and extrusion from the myocyte causes relaxation (diastole).
Anatomically, the SR is a lipid membrane–bounded, fine interconnected network spreading throughout the myocytes. The Ca 2+ release channels (or ryanodine receptors [RyRs]) are concentrated at the part of the SR that is in very close apposition to the T tubular Ca 2+ channel. These are called terminal cisternae or the junctional sarcoplasmic reticulum (jSR). The second part of the SR, the longitudinal, free, or network SR, consists of ramifying tubules that surround the myofilaments (see Fig. 46.1 ) that take Ca 2+ back up into the SR and thus drive relaxation. Such Ca 2+ uptake is achieved by the ATP-consuming Ca 2+ pump known as SERCA (sarcoendoplasmic reticulum Ca 2+ –adenosine triphosphatase, or SR Ca-ATPase). The Ca 2+ taken up into the SR is then stored at high concentration, in part bound to Ca 2+ -buffering proteins, including calsequestrin, before being released again in response to the next wave of depolarization. Cytoplasm or sarcoplasm refers to the intracellular fluid and proteins therein, but excludes the contents of organelles such as the mitochondria, nucleus, and SR. The cytoplasm is crowded with myofilaments, but this is the fluid within which the concentration of Ca 2+ rises and falls to cause cardiac contraction and relaxation.
There are many microdomains and even nanometer-scale nanodomains involved in molecular signaling that convey messages within myocytes. These include the jSR-T-tubule junctions where T-tubular Ca 2+ channels are within 10 nm of a cluster of RyR channels in the jSR membrane to produce the synchronous Ca 2+ transients that control contraction. There are also sarcolemmal receptor complexes, such as beta-adrenergic receptors that have specific molecular partners (more below) that produce second messengers (cyclic nucleotides) that can diffuse to other functional targets in the myocyte. Caveolae (small, flask-shaped sarcolemmal invaginations) are also microdomains with key localized signaling cascades. Scaffolding proteins such as caveolin, A-kinase anchoring proteins (AKAPs), and the RyR itself bring interacting molecules closely together at these locations. These complexes can also release components that translocate and signal elsewhere in the cell, such as the nucleus, where they can signal for myocyte growth. Another type of subcellular shuttling is involved in transporting the ATP produced in mitochondria to sites where it is used (e.g., myofilaments), which is facilitated by the location of creatine kinase, an enzyme that converts creatine phosphate to ATP.
The typical ventricular myocyte has approximately 8000 mitochondria, each of which is ovate with a long axis measuring 1 to 2 μm and short axis of 300 to 500 nm. Mitochondria have two membranes: outer and inner mitochondrial membranes (OMM and IMM; Figs. 46.1 and 46.3 ). The IMM is “crumpled” into folds called cristae, which provide a large surface area within a small volume. The IMM also contains the cytochrome complexes that make up the respiratory chain, including F 0 -F 1 ATP synthase. The space within the IMM, the mitochondrial matrix, contains enzymes of the tricarboxylic acid (TCA) cycle and other key metabolic components. These components provide reducing equivalent protons that are pumped out of the matrix by the cytochromes, and it is this proton pumping that creates the very negative voltage with respect to cytosol (ψ m = −180 mV). The proton pumping out of the matrix also creates a trans-IMM [H + ] gradient, which together with the very negative ψ m creates a strong electrochemical gradient for protons to enter the matrix. The energy from this “downhill” proton flux is used by the F 0 F 1 ATP synthase to make ATP. However, in the absence of the normal proton and ψ m , this elegant F 0 -F 1 ATP synthase runs backward, consuming ATP. The ATP produced in the matrix is transported across the IMM by an adenine nucleotide transporter that exchanges mitochondrial ATP for cytosolic adenosine diphosphate (ADP). This system is exquisitely regulated to maintain cytosolic [ATP] and [ADP] constant during dramatic changes in cardiac workload. The multiple control mechanisms involved in this process are not fully understood, but one is relevant to excitation-contraction coupling. Increased cardiac work in a physiologic setting is usually driven by higher-amplitude and/or more frequent Ca 2+ transients. This elevation in average intracellular [Ca 2+ ] ([Ca 2+ ] i ) also increases mitochondrial matrix [Ca 2+ ] ([Ca 2+ ] m ), which activates key dehydrogenases in the TCA cycle and also pyruvate dehydrogenase to restore levels of reduced nicotinamide adenine dinucleotide (NADH), which drives cytochrome activity and helps restore (ATP) toward normal.
![FIGURE 46.3, Mitochondrial Ca 2+ regulation. The intramitochondrial matrix is very negative with respect to the cytosol (−180 mV). Ca 2+ enters mitochondria via the Ca 2+ uniporter in the inner mitochondrial membrane and is extruded by Na + /Ca 2+ exchange ( NCLX ). Na + is extruded via Na + /H + exchange (NHX). Protons (H + ) are pumped out of mitochondria by the cytochrome ( Cyto ) systems, thereby allowing H + to enter via F 0 F 1 ATP synthase ( ATP ). When mitochondrial [Ca] is increased, it activates mitochondrial dehydrogenases, which increase NADH levels and provide additional reducing equivalent protons to the electron transport chain. FIGURE 46.3, Mitochondrial Ca 2+ regulation. The intramitochondrial matrix is very negative with respect to the cytosol (−180 mV). Ca 2+ enters mitochondria via the Ca 2+ uniporter in the inner mitochondrial membrane and is extruded by Na + /Ca 2+ exchange ( NCLX ). Na + is extruded via Na + /H + exchange (NHX). Protons (H + ) are pumped out of mitochondria by the cytochrome ( Cyto ) systems, thereby allowing H + to enter via F 0 F 1 ATP synthase ( ATP ). When mitochondrial [Ca] is increased, it activates mitochondrial dehydrogenases, which increase NADH levels and provide additional reducing equivalent protons to the electron transport chain.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MechanismsofCardiacContractionandRelaxation/2_3s20B9780323722193000463.jpg)
This raises the issue of how mitochondria regulate [Ca 2+ ] m , because there is also a huge electrochemical gradient favoring entry of Ca 2+ into mitochondria. Indeed, [Ca 2+ ] m is typically similar to [Ca 2+ ] i and is kept at that level by a mitochondrial Na/Ca exchanger (NCLX), which uses the also steep Na + electrochemical gradient to pump Ca 2+ out of the mitochondria. However, this would load the mitochondria with Na + , so Na + must also be extruded from the mitochondria. This is accomplished by the mitochondrial Na/H exchanger in the IMM, but a consequence is that this influx of H + costs energy. That is, these protons could have entered the mitochondria via the F 0 -F 1 ATP synthase making ATP, but instead they were used to extrude Na + and Ca 2+ . Thus in a sense the mitochondrion can make ATP or extrude Ca 2+ . This becomes important when myocytes (or other cells) experience Ca 2+ overload. In the short term, mitochondria can take up large amounts of Ca 2+ to protect the cell from short-term Ca 2+ overload, but chronic high [Ca 2+ ] i has dire consequences. First, this Ca 2+ uptake can diminish ψ m and occurs at the expense of ATP production (as noted), thus hampering energetic recovery from such stress. Second, elevated [Ca 2+ ] i and [Ca 2+ ] m can facilitate opening of the mitochondrial permeability transition pore, which immediately dissipates ψ m , results in the F 0 F 1 ATP synthase consuming rather than making ATP, and allows the matrix contents to be released to the cytosol. This is usually the death knell for individual mitochondria, as well as the cells that rely on their robust function.
Thus, mitochondria can rapidly become agents of cell death as just described, as well as by producing excessive reactive oxygen species (ROS), which can promote necrotic cell death through the mitochondrial permeability transition pore and release of proapoptotic proteins (see Chapter 47 ). Mitochondria can also induce mitochondrial autophagy, or mitophagy , which selectively and adaptively clears damaged mitochondria. Increased oxidative stress and apoptotic proteases can inactivate mitophagy and thereby cause cell death. Mitochondria can also undergo fission, sometimes with one daughter mitochondrion being less healthy and targeted for mitophagy. They can also undergo fusion, to merge smaller ones into a larger mitochondrion. Fission, fusion and mitophagy are normal and healthy parts of mitochondrial life, and dysfunction of any of these can have pathologic consequences.
The two chief contractile proteins are the motor protein myosin on the thick filament and actin on the thin filament (see Figs. 46.1B and 46.2 ). Ca 2+ initiates the contraction cycle by binding to the thin filament regulatory protein troponin C to relieve the inhibition otherwise exerted by this troponin complex ( Fig. 46.4 ). The thin actin filaments are connected to the Z-lines at either end of the sarcomere , which is the functional contractile unit that is repeated through the filaments. The sarcomere is limited on either side by a Z-line, which with the thin filaments creates a “cage” around the thick myosin filament that extends from the center of the sarcomere outward toward the Z-line. During contraction, the myosin heads grab onto actin and pull the actin filaments toward the center of the sarcomere. The thin and thick filaments can thus slide over each other to shorten the sarcomere and cell length, without the individual actin or myosin molecules actually changing length (see Fig. 46.1B ). The interaction of the myosin heads with actin filaments that is switched on when Ca 2+ arrives is called cross-bridge cycling. As the actin filaments move inward toward the center of the sarcomere, they draw the Z-lines closer together so that the sarcomere length shortens. The energy for contraction is provided by breakdown of ATP (myosin is an ATPase).
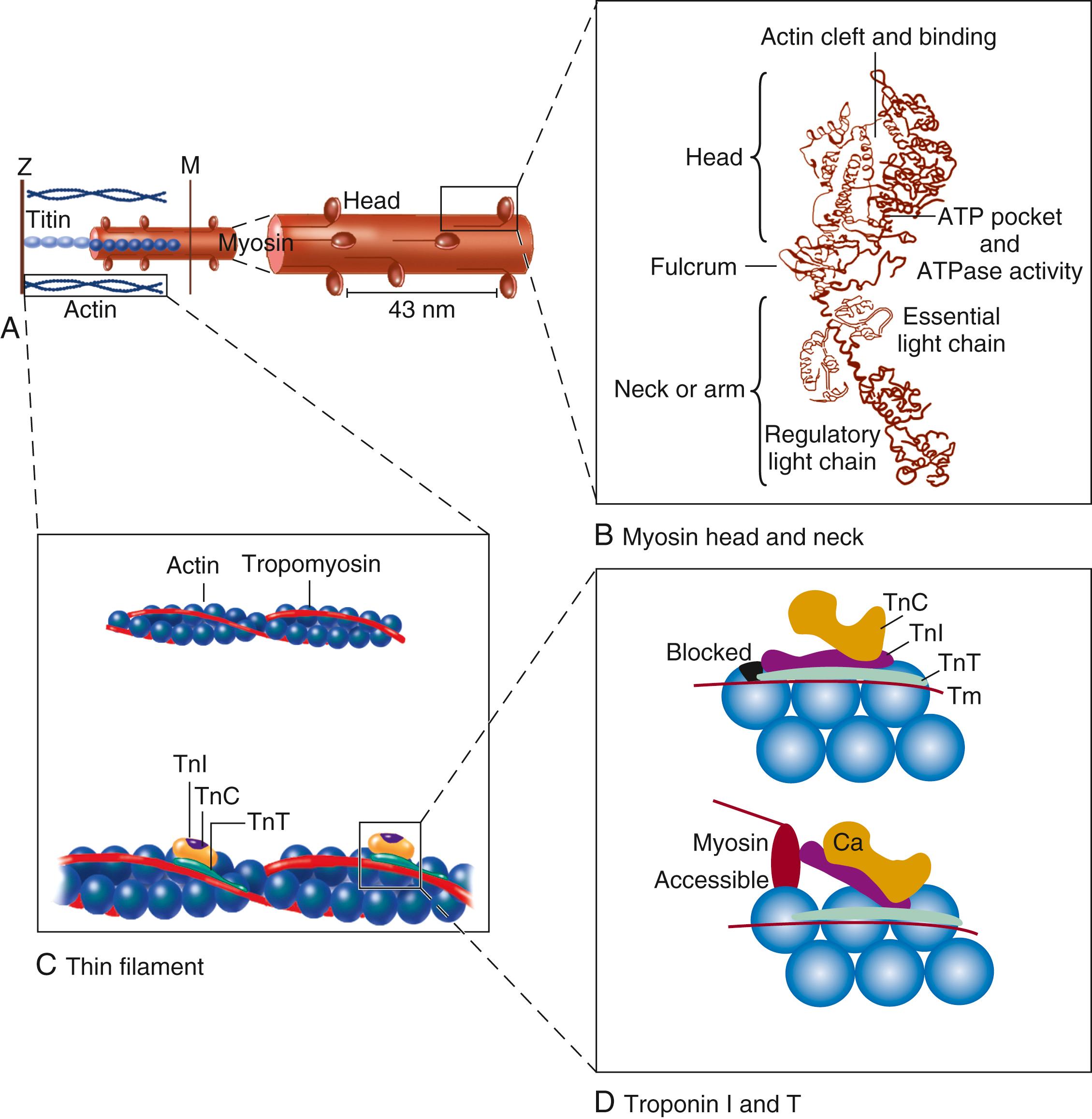
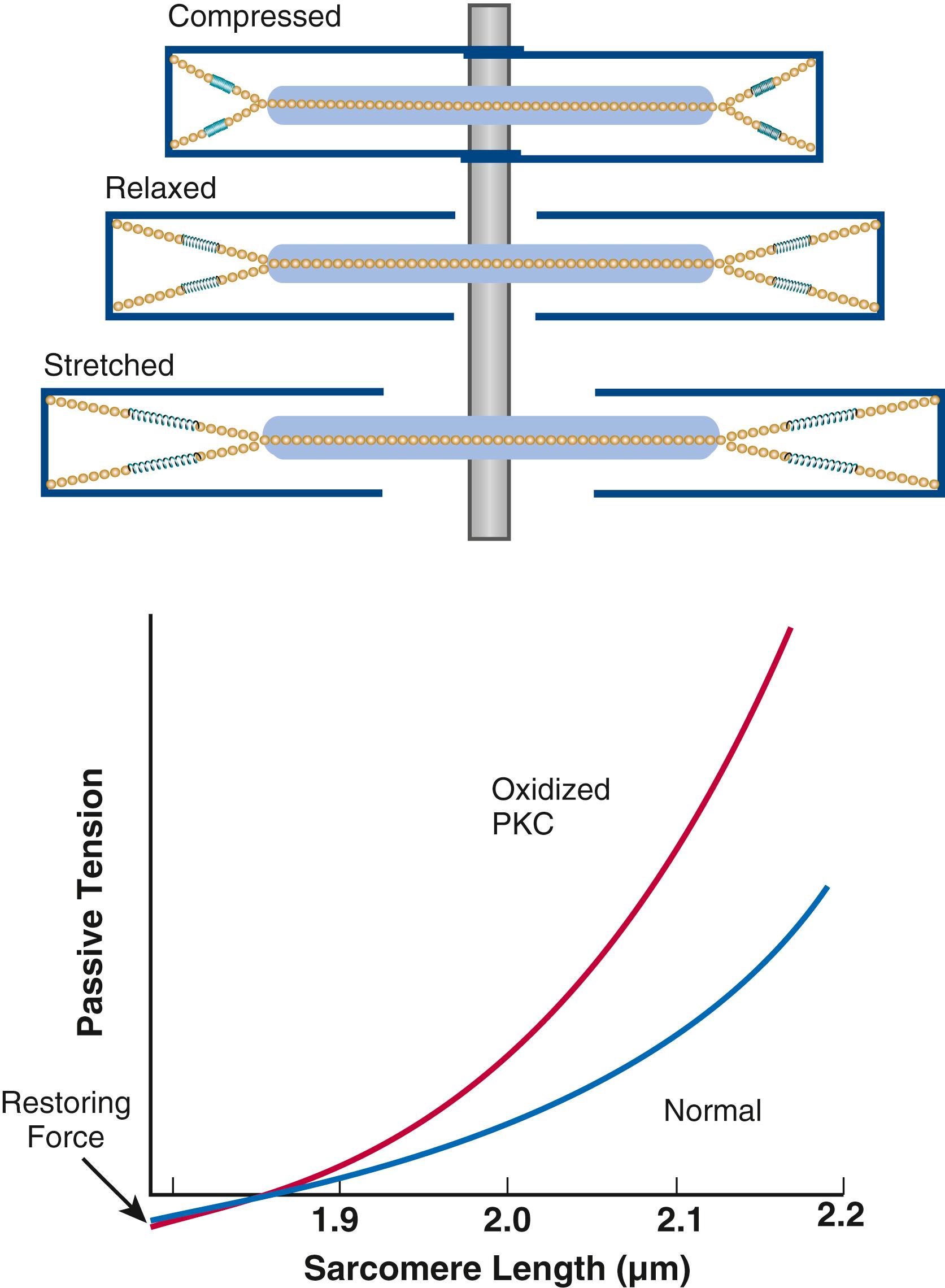
Titin is a giant molecule, the largest protein yet described. It is extraordinarily long, elastic, and slender ( Fig. 46.5 ). Titin extends from the Z-line into the thick filament, approaching the M-line, and connects the thick filament to the Z-line (see Fig. 46.1 ). Titin has two distinct segments: an inextensible anchoring segment and an extensible elastic segment that stretches as sarcomere length increases. Thus the titin molecule can stretch between 0.6 and 1.2 μm in length and has multiple functions. First, it tethers myosin and thick filaments to the Z-line, thereby stabilizing sarcomeric structure. Second, as it stretches and relaxes, its elasticity contributes to the stress-strain relationship of cardiac and skeletal muscle. At short sarcomere lengths, the elastic domain is coiled up on itself to generate restoring force (see Fig. 46.5 ), similar to a spring, helping to relengthen the sarcomere and aid early diastolic filling. These changes in titin help explain the series elastic element that was inferred from mechanics studies as elasticity in series with the myosin filaments. Third, the increased diastolic stretch of titin as the length of the sarcomere in cardiac muscle is increased causes the enfolded part of the titin molecule to straighten. This stretched molecular spring then limits overstretching of sarcomeres and end-diastolic volume (EDV) and returns some potential energy during systole as the sarcomeres shorten during cardiac ejection. Fourth, titin may transduce mechanical stretch into growth signals. Sustained diastolic stretch, as in volume overload, can cause titin-dependent signaling to muscle LIM protein (MLP) attached to the Z-line end of titin. MLP is proposed to be a stretch sensor that transmits the signals that result in the myocyte growth pattern characteristic of volume overload, and it may be defective in a subset of human dilated cardiomyopathy.
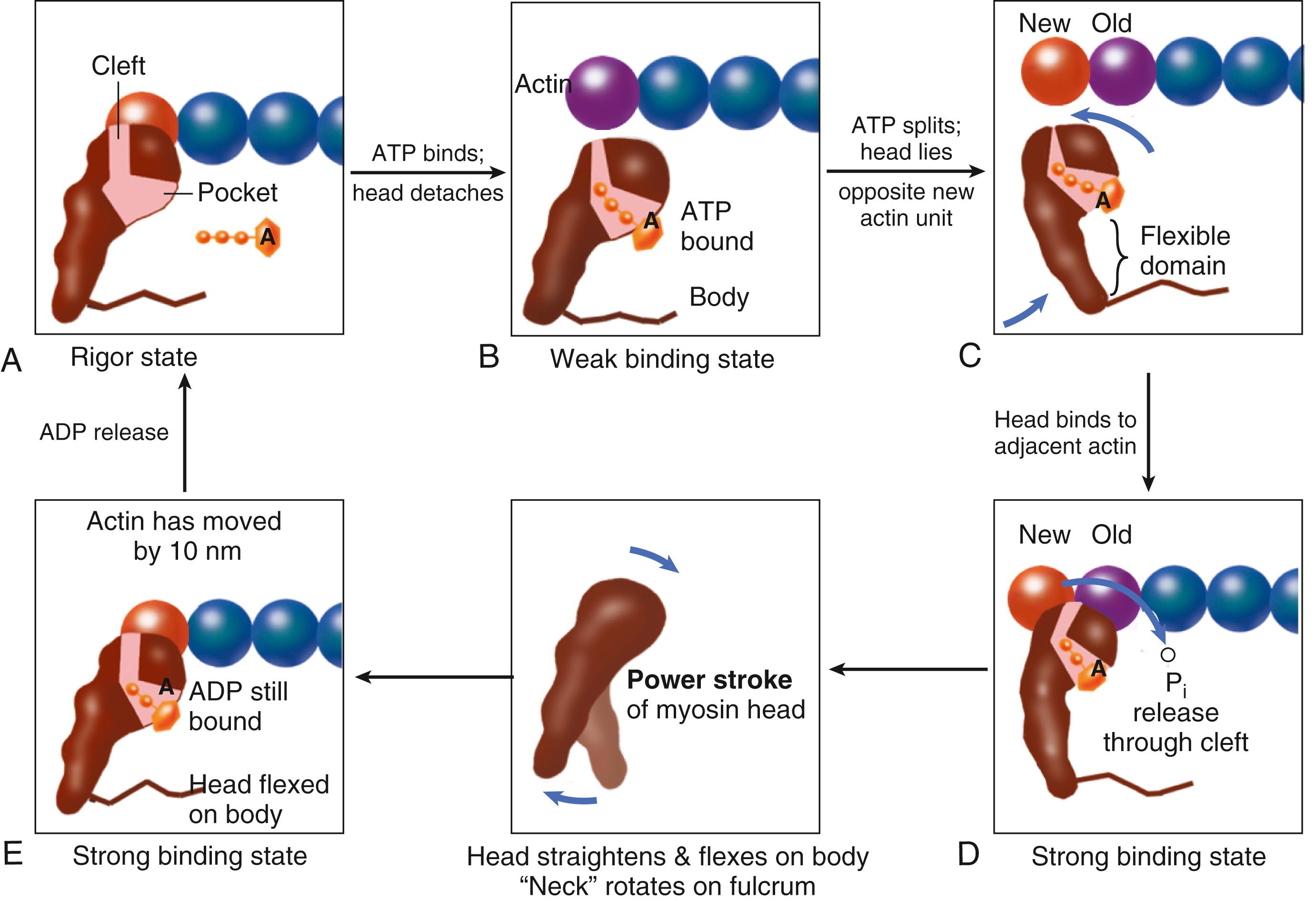
Although the molecular level details underlying the cross-bridge cycle are complex, cross bridges appear to exist in either a strong or a weak binding state (but a super-relaxed state also exists). During diastole, myosin heads normally have ATP bound ( Fig. 46.6B ) and hydrolyzed to ADP plus inorganic phosphate (Pi), although ADP-Pi is not yet released and the energy of ATP is not yet fully consumed ( Fig. 46.6C ). Thus the cross bridges are poised and ready to bind to actin. This interaction is permitted when Ca 2+ arrives and binds to troponin C, shifting the position of the troponin-tropomyosin complex on the actin filament (see Fig. 46.4C, D ). This enables the poised myosin heads to form strong binding cross bridges with actin molecules ( Fig. 46.6D ) and use the energy stored in myosin-ADP-P i to rotate the myosin head while bound to actin in the power stroke (and release P i ) while still in the strong binding state ( Fig. 46.6D and E ). Once a particular cross bridge proceeds through the power stroke (using the energy previously stored in the ATP molecule), it will remain in the strong binding or rigor state ( Fig. 46.6A ) until ATP binds again to myosin, causing a shift back to the weak binding state and allowing cross-bridge detachment and ATP hydrolysis ( Fig. 46.6C ). As long as [Ca 2+ ] i and [ATP] remain high, the cycle can continue with myosin-ADP-Pi binding to a new actin molecule. The weak binding state predominates when [Ca 2+ ] i falls and Ca 2+ dissociates from troponin C, allowing relaxation during diastole. If intracellular (ATP) declines too far (e.g., during ischemia), ATP cannot bind and disrupt the rigor linkage, leaving cross bridges locked in the strong binding state (as in rigor mortis).
The Ca 2+ on-switch of cross-bridge cycling is mediated by a series of interactions within the troponin, tropomyosin, and actin complex (see Fig. 46.4C, D ). Thin filaments are composed of two helical intertwining actin filaments, with a long tropomyosin molecule that spans seven actin monomers located in the groove between the two actin filaments. Also, at every seventh actin molecule (38.5 nm along this structure) there is a three-protein regulatory troponin complex: troponin C (Ca 2+ binding), I (inhibitory), and T (tropomyosin binding).
When [Ca 2+ ] i is low, the position of tropomyosin blocks the myosin heads from interacting effectively with actin. As a result, most cross bridges are in the “blocked position,” with a few visiting the weak binding state. Ca 2+ binding with troponin C causes troponin C to bind more tightly to troponin I (see Fig. 46.4D ), which allows tropomyosin to roll deeper into the thin filament groove, thereby opening access to allow myosin binding to actin. This allows the cross-bridge cycle to proceed (see Fig. 46.6 ). As they form, strong cross bridges can nudge tropomyosin deeper into the actin groove, allowing cross-bridge attachment at one site to enhance actin-myosin at its “nearest-neighbor” sites. This cooperatively spreads activation farther along the myofilaments. ,
Each myosin head is the terminal part of the myosin heavy chain molecule. The other ends of two myosin molecules (tails) intertwine as a coil that forms the bulk of the thick filament. Also, a short “neck” leads to the myosin head that protrudes out from the filament (see Fig. 46.4 ). According to the Rayment model, the base of the head and/or neck region changes configuration during the power stroke previously described. Each head has an ATP-binding pocket and a narrow cleft that extends from the base of this pocket to the actin-binding face (see Fig. 46.6 ). During the power stroke when there is no mechanical load on the muscle, the myosin head flexes and can move the actin filament by approximately 10 nm. When the pocket releases ADP and binds ATP, the cross bridge releases back to an orientation more perpendicular to the direction of the thin and thick filaments. During isometric (or isovolumic) contraction, the cross bridges rotate but cannot fully move the actin filament, and the stretched strong binding cross bridges bear force. During shortening (ejection), the actin filament moves during the power stroke, accompanied by decreases in sarcomere length and ventricular volume.
Note that myosin heads stick out from the thick filament in six directions in an organized array to allow interactions with each of six actin filaments that surround each thick filament (see Fig. 46.1A ). The myosin molecules are also oriented in reversed longitudinal directions on either side of the M-line (which itself contains only myosin tails), such that each side is trying to pull the Z-lines toward the center. That is, when cross bridges are in the strong binding or rigor linkages, they form “chevrons” (or arrows ) pointing toward the Z-line on that side of the M-line.
Each cycle of the cross bridge consumes one molecule of ATP, and this myosin ATPase activity is the major site of ATP consumption in the beating heart. Thus, when the heart is more strongly activated, the level of ATP consumption is similarly increased. The two myosin heads that stick out from an intertwined pair of myosin molecules seem to work through a hand-over-hand action such that the myosin dimer never fully releases the thin filament during the activation period. There are also two main myosin isoforms in cardiac myocytes, alpha and beta, which have similar molecular weight but exhibit substantially different cross-bridge cycle and ATPase rates. The beta-myosin heavy chain (β-MHC) isoform exhibits a slower ATPase rate and is the predominant form in adult humans. In small mammals (rats and mice), the faster α-MHC form normally predominates but shifts to the β-MHC pattern during chronic stress and heart failure. β-MHC has been targeted therapeutically using both gain and loss of function approaches. Mavacamten is a novel therapeutic myosin inhibitor that targets the excessive contractility and impaired relaxation, myocardial energetics and compliance in patients with obstructive hypertrophic cardiomyopathy (oHCM). In the PIONEER clinical trial (NCT03470545), mavacamten improved exercise capacity, left ventricular (LV) outflow tract obstruction, New York Heart Association (NYHA) functional class, and health status in patients with oHCM (see also Chapter 54 ). Omecamtiv mecarbil is a novel therapeutic that activates myosin ATPase and enhances myosin cross-bridge formation and duration, thereby prolonging myocardial contraction. The GALACTIC-HF trial demonstrated that treatment with the selective cardiac myosin activator omecamtiv mecarbil reduced the incidence of a composite of a heart-failure event or death from cardiovascular causes in patients with heart failure and reduced EF (see also Chapter 49 ).
Each myosin molecule neck also has two light chains (see Fig. 46.4A ). The essential myosin light chain (MLC-1) is more proximal to the myosin head and may limit the contractile process by interaction with actin. The regulatory myosin light chain (MLC-2) is a potential site for phosphorylation (e.g., in response to beta-adrenergic stimulation) and may promote cross-bridge cycling. In vascular smooth muscle, which lacks the troponin-tropomyosin complex, contraction is activated by the Ca 2+ -dependent myosin light chain kinase (MLCK) rather than by Ca 2+ binding to troponin C (as in striated muscle). Myosin-binding protein C appears to traverse the myosin molecules in the A-band, thereby potentially tethering the myosin molecules and stabilizing the myosin head with respect to the thick and thin filaments. Defects in myosin, myosin-binding protein C, and several other myofilament proteins are genetically linked to familial hypertrophic cardiomyopathy.
The myofilaments are activated in a graded rather than all-or-none manner as a function of [Ca 2+ ] i ( Fig. 46.7 ), such that as [Ca 2+ ] i rises force of contraction increases going up the curve. Then as [Ca 2+ ] i declines relaxation proceeds (back to the diastolic point). The dynamics and regulation of Ca 2+ transients in cardiac myocytes are discussed in the following section, but a major physiologic mechanism for regulating cardiac contractility (e.g., during sympathetic activity) is to increase peak [Ca 2+ ] i and more fully activate the myofilaments. The higher the [Ca 2+ ] i , the more fully saturated are the Ca 2+ binding sites on troponin C, and consequently, more sites are available for cross bridges to form. When more cross bridges are working in parallel, the myocyte (and heart) can develop greater force (or ventricular pressure). There is high cooperativity in this process, in large part because of the “nearest-neighbor” effect mentioned earlier. That is, Ca 2+ bound to a single troponin C molecule encourages local cross-bridge formation, and both Ca 2+ binding and cross-bridge formation directly enhance the likelihood of cross-bridge formation in the seven actin molecules controlled by one tropomyosin molecule. Furthermore, the openness of that domain directly enhances that of the neighboring domain with respect to both Ca 2+ binding and cross-bridge formation. This cooperativity means that a small change in [Ca 2+ ] i can have a great effect on the strength of contraction.
![FIGURE 46.7, Myofilament Ca 2+ sensitivity. Active force development in cardiac muscle depends on the cytosolic free [Ca] i . As [Ca] i rises during systole, force develops as dictated by the sigmoidal myofilament Ca 2+ sensitivity curve ( solid curve ; Force = 100/(1+[600 nm]/[Ca] i ) 4 ). As [Ca] i declines relaxation ensues and force declines. If peak [Ca] i increases (as in inotropy) the peak force can reach a higher value. At shorter sarcomere length (SL), acidosis, and troponin I (TnI) phosphorylation, the myofilament Ca 2+ sensitivity is reduced, and the former two also decrease maximal force ( dashed curve ). FIGURE 46.7, Myofilament Ca 2+ sensitivity. Active force development in cardiac muscle depends on the cytosolic free [Ca] i . As [Ca] i rises during systole, force develops as dictated by the sigmoidal myofilament Ca 2+ sensitivity curve ( solid curve ; Force = 100/(1+[600 nm]/[Ca] i ) 4 ). As [Ca] i declines relaxation ensues and force declines. If peak [Ca] i increases (as in inotropy) the peak force can reach a higher value. At shorter sarcomere length (SL), acidosis, and troponin I (TnI) phosphorylation, the myofilament Ca 2+ sensitivity is reduced, and the former two also decrease maximal force ( dashed curve ).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MechanismsofCardiacContractionandRelaxation/6_3s20B9780323722193000463.jpg)
Besides [Ca 2+ ] i , the other major factor influencing the strength of contraction is sarcomere length at the end of diastole (preload), just before the onset of systole. Both Otto Frank and Ernest Starling observed that the more the diastolic filling of the heart, the greater the strength of the heartbeat. The increased heart volume translates into increased sarcomere length, which acts by a length-sensing mechanism. A part of this Frank-Starling effect has historically been ascribed to increasingly optimal overlap between the actin and myosin filaments. Clearly, however, there is also a substantial increase in myofilament Ca 2+ sensitivity with an increase in sarcomere length (see Fig. 46.7 ). A plausible mechanism for this regulatory change may reside in the decreasing interfilament spacing as heart muscle is stretched. That is, the myocyte is at constant volume (over the cardiac cycle), so as the cell shortens, it must thicken, and conversely, when it is stretched, the cell becomes thinner and filament spacing becomes narrower. This attractive lattice-dependent explanation for the Frank-Starling relationship has been challenged by careful x-ray diffraction studies, which found that reducing sarcomere lattice spacing by osmotic compression failed to influence myofilament Ca 2+ sensitivity. Although several mechanisms could contribute to myofilament Ca 2+ sensitization at longer sarcomere length, the issue is unresolved.
When changes in diastolic length (or preload) are the cause of altered contractile strength, it is said to be a Frank-Starling (or Starling) effect. Conditions in which contraction is strengthened independent of sarcomere length (e.g., typically by increased Ca 2+ transient amplitude) are referred to as positive inotropic states or enhanced contractility . The distinction between these heterometric (Starling) and homeometric (inotropic) mechanisms of altered cardiac strength is functionally and therapeutically important.
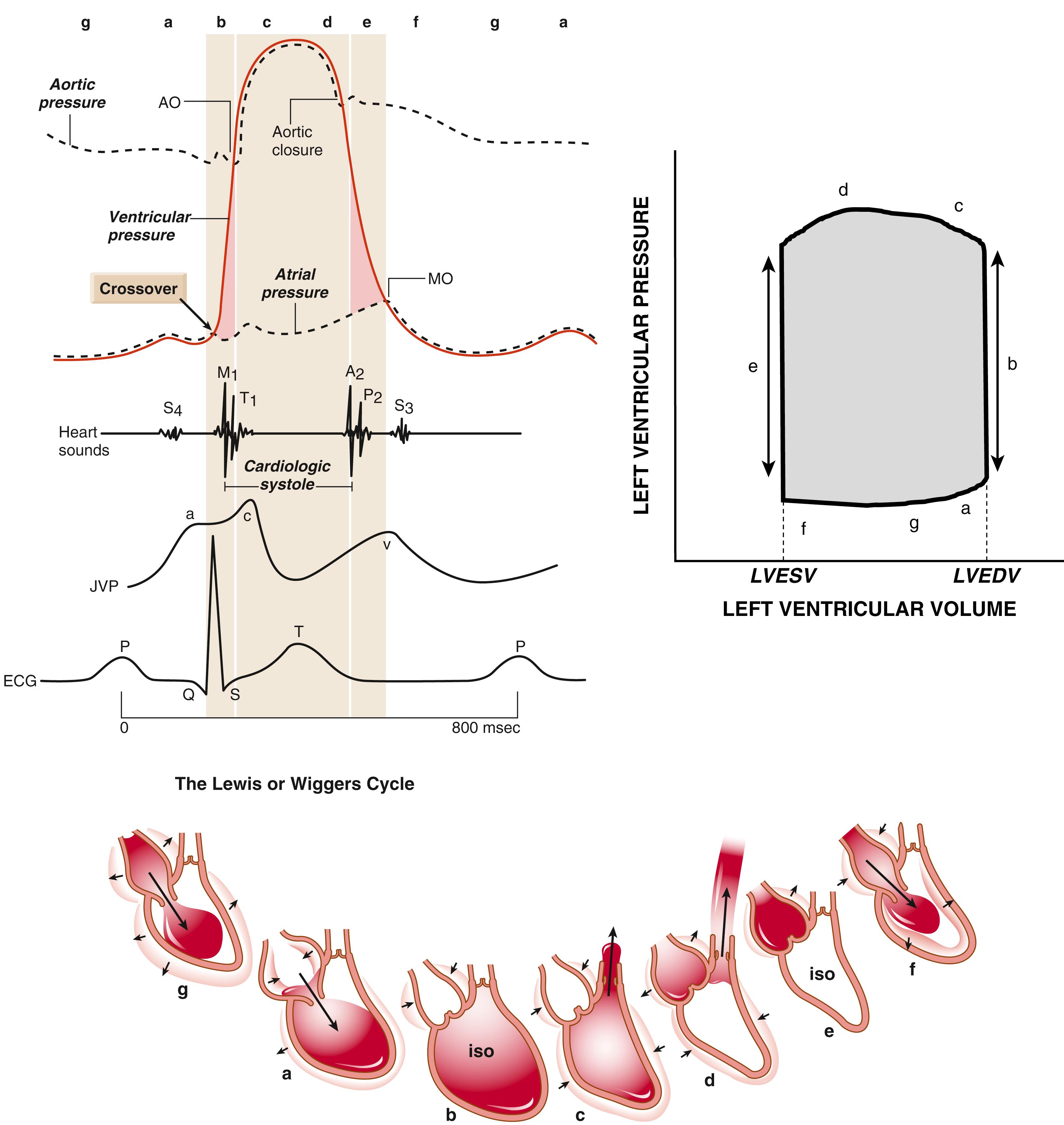
The cardiac cycle of Wiggers (see Fig. 46.16 ) must be distinguished from the cross-bridge cycle. The cardiac cycle reflects the overall changes in pressure in the left ventricle, whereas the cross-bridge cycle is the repetitive interaction between myosin heads and actin. During isovolumic contraction (before aortic valve opening), the sarcomeres do not shorten appreciably, but cross bridges are developing force, although not all simultaneously. That is, at any given moment, some myosin heads will be flexing or flexed (resulting in force generation), some will be extending or extended, and some will be attached weakly to actin and some detached from actin. Numerous such cross-bridge cycles, each lasting microseconds, are integrated to produce the resulting force (and pressure). When ventricular pressure (sum of cross-bridge forces) reaches aortic pressure (afterload), ejection begins and is associated with the cross bridges actively moving the thin actin filaments toward the center of the sarcomere (M-line), thereby shortening the sarcomere. Note that as ejection proceeds (and sarcomeres shorten), myofilament Ca 2+ sensitivity declines (see Fig. 46.7 ). Thus, both [Ca 2+ ] i decline and shortening cause a progressive decline in the contractile state as systole gives way to diastole. Both the Ca 2+ transient properties and the myofilament Ca 2+ sensitivity and cross-bridge cycling rate are altered under physiologic conditions, such as sympathetic stimulation and local acidosis or ischemia, as discussed later.
Volume and pressure overload may have different effects on myocardial growth because of different patterns of force transmission. Whereas increased diastolic force is transmitted longitudinally by titin to reach MLP, the postulated sensor (see earlier), increased systolic force may be transmitted laterally (i.e., at right angles) by the Z-disc and cytoplasmic actin to reach the cytoskeletal proteins and cell-to-matrix junctions, such as the focal adhesion complex. This mechanical force is translated into signals by the dystrophin and integrin protein complexes that mediate force transmission between the intracellular cytoskeleton, the extracellular matrix, and neighboring cells. These can activate intrinsic short-term adaptive such as the Anrep effect, as well as signaling to the nucleus to activate the growth pathways via altered gene regulation, as addressed in other chapters.
Genetic-based hypertrophic and dilated cardiomyopathies not only produce hearts that look and behave very differently but also have diverse molecular causes. These cardiomyopathies in general are linked to mutant genes that cause abnormalities in the force-generating system, such as β-MHC, MLCs, myosin-binding protein C, troponin subunits, and tropomyosin (see Chapter 52 ). One hypothesis is that mutations that increase myofilament calcium sensitivity, contractility, and energy demand result in concentric hypertrophy, whereas mutations that reduce myofilament calcium sensitivity or force generation or that result in non–force-generating cytoskeletal proteins (e.g., dystrophin, nuclear lamin, cytoplasmic actin, titin) lead to a dilated cardiomyopathy. Although useful, such broad distinction between the two types of cardiomyopathy is oversimplified, with several examples of overlapping mechanisms.
Ca 2+ is central to cardiac contraction and relaxation, and the associated Ca 2+ fluxes that link contraction to the wave of excitation (excitation-contraction coupling) are now well understood and accepted. , Each QRS complex in the electrocardiogram (ECG) represents the synchronization of ventricular myocyte action potentials (APs) that trigger Ca 2+ transients and consequent contraction-relaxation in each myocyte ( Fig. 46.8A ). Relatively small amounts of Ca 2+ (trigger Ca 2+ ) enter and leave the cardiomyocyte during each cardiac cycle, with larger amounts being released and taken back up by the SR (see Fig. 46.8B ). Each AP depolarization opens voltage-gated L-type Ca 2+ channels in the T tubules that are physically near the junctional SR, and that local Ca 2+ influx activates SR Ca 2+ release channels (RyRs) to release additional Ca 2+ which can diffuse to cause a whole-cell Ca 2+ transient that activates contraction. In this Ca 2+ -induced Ca 2+ release mechanism, a smaller amount of Ca 2+ entering via the calcium current (I Ca ) triggers the release of a larger amount of Ca 2+ into the cytosol. , In the human ventricle and large mammals, SR Ca 2+ release is three to four times larger than Ca 2+ influx by I Ca . In rat and mouse myocytes, however, SR Ca 2+ cycling is more than 10 times greater than sarcolemmal Ca 2+ flux. The combined Ca 2+ release and influx elevates [Ca 2+ ] i and promotes binding of Ca 2+ to troponin C and thus contractile activation. Contraction is terminated mainly by Ca 2+ reuptake into the SR by SERCA and extrusion from the myocyte by Na + /Ca 2+ exchange (NCX) which return [Ca 2+ ] i to the diastolic level.
![FIGURE 46.8, Myocyte Ca 2+ fluxes during excitation-contraction (E–C) coupling. Rapid depolarization during the action potential (AP) triggers the Ca 2+ transient that activates contraction (A). B, Crucial features are (1) Ca 2+ entry via the voltage-activated L-type Ca 2+ channels, which triggers release of more Ca 2+ from the SR; (2) a tiny amount of Ca 2+ may enter via Na + /Ca 2+ exchange early in the action potential; and (3) removal of Ca 2+ ions from the cytosol is mainly via the SR Ca-ATPase (SERCA; 75%) and Na + /Ca 2+ exchange (24%), with tiny amounts transported by mitochondrial Ca 2+ uniport and the sarcolemmal Ca-ATPase (1%). The sodium pump (Na + /K + -ATPase) extrudes the Na + ions that entered during Na + current and Na + /Ca 2+ exchange action. Note that extracellular and intra-SR [Ca 2+ ] (1 to 2 mm) is much higher than diastolic [Ca 2+ ] i (0.10 μm). Mitochondria can act as a buffer against excessive changes in cytosolic Ca 2+ . FIGURE 46.8, Myocyte Ca 2+ fluxes during excitation-contraction (E–C) coupling. Rapid depolarization during the action potential (AP) triggers the Ca 2+ transient that activates contraction (A). B, Crucial features are (1) Ca 2+ entry via the voltage-activated L-type Ca 2+ channels, which triggers release of more Ca 2+ from the SR; (2) a tiny amount of Ca 2+ may enter via Na + /Ca 2+ exchange early in the action potential; and (3) removal of Ca 2+ ions from the cytosol is mainly via the SR Ca-ATPase (SERCA; 75%) and Na + /Ca 2+ exchange (24%), with tiny amounts transported by mitochondrial Ca 2+ uniport and the sarcolemmal Ca-ATPase (1%). The sodium pump (Na + /K + -ATPase) extrudes the Na + ions that entered during Na + current and Na + /Ca 2+ exchange action. Note that extracellular and intra-SR [Ca 2+ ] (1 to 2 mm) is much higher than diastolic [Ca 2+ ] i (0.10 μm). Mitochondria can act as a buffer against excessive changes in cytosolic Ca 2+ .](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MechanismsofCardiacContractionandRelaxation/8_3s20B9780323722193000463.jpg)
Electron and fluorescence microscopy studies show that the SR is a continuous network surrounding the myofilaments with connections across Z-lines and transversely between myofibrils. Moreover, the lumens of the entire SR network and nuclear envelope are connected in adult cardiac myocytes. This allows relatively rapid diffusion of Ca 2+ within the SR to balance free [Ca 2+ ] within the SR ([Ca 2+ ] SR ). , The total SR Ca 2+ content is the sum of [Ca 2+ ] SR plus Ca 2+ bound to intra-SR Ca 2+ buffers (especially calsequestrin). SR Ca 2+ content is critical to normal cardiac function and electrophysiology, and its abnormalities contribute to systolic and diastolic dysfunction and arrhythmias. [Ca 2+ ] SR dictates the SR Ca 2+ content and driving force for Ca 2+ release and also regulates RyR release channel gating.
The RyR channels that mediate SR Ca 2+ release are mainly located in the jSR membrane at the junctions with the T tubule. Each junction has 50 to 250 RyR channels on the jSR that are directly under and nearly touching a cluster of 20 to 40 sarcolemmal L-type Ca 2+ channels across a 15-nm junctional gap (that is crowded with protein). RyR2 (the cardiac isoform) functions both as a Ca 2+ channel and as a scaffolding protein that localizes numerous key regulatory proteins to the jSR. , On the large cytosolic side, these include proteins that can stabilize RyR gating (e.g., calmodulin [CaM], FK-506 binding protein [FKBP-12.6]); kinases that can regulate RyR gating by phosphorylation (e.g., protein kinase A [PKA], Ca 2+ /CaM-dependent protein kinase II [CaMKII]); and the protein phosphatases PP1 and PP2A, which dephosphorylate the RyR. Inside the SR, the RyR also couples to several proteins (e.g., junctin, triadin, and via these, calsequestrin) that similarly regulate RyR gating and, in the case of calsequestrin, provides a local reservoir of buffered Ca 2+ close to the release channel. The actual RyR channel is made up of a symmetric tetramer of RyR molecules, each of which may have the aforementioned regulatory proteins associated with it. Thus the RyR receptor complex is very large (>7000 kDa; Fig. 46.8 ). When the T tubule is depolarized, one or more L-type Ca 2+ channels open, and local cleft [Ca 2+ ] increases sufficiently to activate at least one local jSR RyR (multiple channels here ensure high-fidelity signaling). The Ca 2+ released from these first openings recruit additional RyRs in the junction through Ca 2+ -induced Ca 2+ release to amplify release of Ca 2+ into the junctional space. The Ca 2+ diffuses out of this space throughout the sarcomere to activate contraction. Each of the approximately 20,000 jSR regions in the typical ventricular myocyte seems to function independently in response to local activation by I Ca . Thus the global Ca 2+ transient in the myocyte at each beat is the spatiotemporal summation of SR Ca 2+ release events from thousands of jSR regions, synchronized by the upstroke of the AP and activation of I Ca at each junction.
Ca 2+ -induced Ca 2+ release is a positive feedback process, but it is now known that SR Ca 2+ release turns off when [Ca] SR drops by approximately 50% (i.e., from a diastolic value of 1 mM to a nadir of 400 μM). Elegant studies have documented how I Ca is inactivated by high local [Ca 2+ ], and this robust calcium-dependent inactivation is mediated by binding of Ca 2+ to the CaM that is already associated with that channel. When Ca 2+ binds to CaM, it alters channel conformation such that I Ca inactivation is favored. I Ca is also subject to voltage-dependent inactivation during the AP plateau, and thus inactivation limits further entry of Ca 2+ into the cell.
As for Ca 2+ -dependent RyR activation, several mechanisms may contribute to breaking its inherent positive feedback. Although not necessarily most compelling, one mechanism is analogous to Ca 2+ /CaM-dependent inactivation of I Ca . That is, binding of Ca 2+ to CaM that is prebound to RyR2 favors closure of RyR channels and inhibits reopening ( Fig. 46.9 ). A second mechanism, undoubtedly important, is that RyR2 gating is also sensitive to luminal [Ca 2+ ] SR such that high [Ca 2+ ] SR favors opening and low [Ca 2+ ] SR favors closure. Indeed, release of Ca 2+ from the SR during normal Ca 2+ transients is robustly turned off when [Ca 2+ ] SR falls to approximately half its normal value (400 μM, which is still 500 times higher than bulk [Ca 2+ ] i ), almost regardless of the rate of SR Ca 2+ release. , A third and related mechanism is that as Ca 2+ release proceeds and [Ca 2+ ] SR declines, Ca 2+ flux through the RyR falls and junctional [Ca 2+ ] also falls, all of which tend to disrupt the positive feedback. That is, the RyR is less sensitive to activating Ca 2+ (because [Ca 2+ ] SR is low) and lower [Ca 2+ ] on the cytosolic side also activates more weakly.
CaM has four Ca 2+ -binding sites, resembles troponin C, and participates in many different cellular pathways, from ion channels to transcriptional regulation. In many cases (e.g., L-type Ca 2+ , Na + , and some K + channels; RyR and inositol 1,4,5-triphosphate receptors), CaM is already prebound or “dedicated” such that elevation of local [Ca 2+ ] i can rapidly induce Ca 2+ -CaM effects on their gating (see Fig. 46.9 ). , Indeed, more than 90% of the CaM in myocytes is already bound to cellular targets before Ca 2+ binds to and activates it. Nevertheless, many myocyte CaM targets (e.g., CaMKII, calcineurin, nitric oxide synthase [NOS]) compete for this limited pool of “promiscuous” CaM. Thus, CaM signaling in myocytes is complex and is further complicated by the effects of CaMKII, which influences some of the same targets and processes as CaM itself does. ,
In addition to SR Ca 2+ release triggered by I Ca during normal excitation-contraction coupling, there is a finite probability that a given RyR will open stochastically. Because of local Ca 2+ -induced Ca 2+ release in the junctional cleft, this can lead to spontaneous local SR Ca 2+ release events known as Ca 2+ sparks. , Under normal resting conditions, these Ca 2+ sparks have a low probability (approximately 10 -4 ), which means that at any moment there might be one or two Ca 2+ sparks per myocyte. Because local [Ca 2+ ] i declines rapidly as Ca 2+ diffuses away from the initiating cleft, the resulting local [Ca 2+ ] i at the next cleft (1 to 2 μm away) is normally too low to trigger that neighboring site. Thus, Ca 2+ sparks are very local events (within 2 μm in the cell). However, the probability of Ca 2+ sparks is greatly enhanced when [Ca 2+ ] i or [Ca 2+ ] SR is elevated or under conditions in which the RyR is otherwise sensitized (e.g., by oxidation or CaMKII). These conditions can greatly enhance the likelihood that SR Ca 2+ release from one junction will be sufficient to trigger neighboring junctions 1 to 2 μm away and result in propagating Ca 2+ waves throughout the whole myocyte. These Ca 2+ waves can be arrhythmogenic. The Ca 2+ wave can activate substantial inward current through NCX (see later), which can depolarize the membrane potential and contribute to both early and delayed afterdepolarizations (EADs and DADs) during the AP plateau or during diastole, respectively. EADs result in prolongation of the AP duration, and DADs can initiate premature ventricular complexes (PVCs).
![FIGURE 46.9, Role of CaM and CaMKII in regulating intracellular [Ca 2+ ]. The rising cytosolic Ca 2+ concentration in systole activates the Ca 2+ regulatory system whereby Ca 2+ -CaM causes inactivation of L-type Ca 2+ current and RyR release. This negative feedback system limits cellular Ca 2+ gain. The effects of CaMKII can also modulate these systems. 22 For example, (1) CaMKII limits the extent of Ca 2+ -dependent inactivation and enhances Ca 2+ current amplitude, (2) it increases the fraction of SR Ca 2+ released from the RyR in response to the Ca 2+ current trigger (which can be arrhythmogenic), (3) it phosphorylates PLB to enhance SR Ca 2+ uptake by SERCA, and (4) it can modulate Na + and K + channel gating in ways that are also proarrhythmic. 22 , 23 FIGURE 46.9, Role of CaM and CaMKII in regulating intracellular [Ca 2+ ]. The rising cytosolic Ca 2+ concentration in systole activates the Ca 2+ regulatory system whereby Ca 2+ -CaM causes inactivation of L-type Ca 2+ current and RyR release. This negative feedback system limits cellular Ca 2+ gain. The effects of CaMKII can also modulate these systems. 22 For example, (1) CaMKII limits the extent of Ca 2+ -dependent inactivation and enhances Ca 2+ current amplitude, (2) it increases the fraction of SR Ca 2+ released from the RyR in response to the Ca 2+ current trigger (which can be arrhythmogenic), (3) it phosphorylates PLB to enhance SR Ca 2+ uptake by SERCA, and (4) it can modulate Na + and K + channel gating in ways that are also proarrhythmic. 22 , 23](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MechanismsofCardiacContractionandRelaxation/9_3s20B9780323722193000463.jpg)
Ca 2+ is transported into the SR by SERCA, which constitutes nearly 90% of the SR protein. Its molecular weight is 115 kDa, with 10 transmembrane domains and large cytosolic and small SR-luminal domains. Three isoforms exist, but in cardiac myocytes the dominant form is SERCA2a. For each molecule of ATP hydrolyzed by this enzyme, two calcium ions are taken up into the SR ( Fig. 46.10 ; see also Fig. 46.9 ). SR Ca 2+ uptake is the primary driver of cardiac myocyte relaxation, and reuptake starts as soon as [Ca 2+ ] i begins to rise. Because Ca 2+ removal is slower than Ca 2+ influx and release, a characteristic rise and fall in [Ca 2+ ] i called the Ca 2+ transient takes place. As [Ca 2+ ] i falls, Ca 2+ dissociates from troponin C, which progressively switches off the myofilaments. A reduction in SERCA expression or function (as seen in heart failure or energetic limitations) can thus directly result in slower rates of cardiac relaxation. In addition, the strength of SR Ca 2+ uptake directly influences the diastolic SR Ca 2+ content and [Ca 2+ ] SR , which dictates both the sensitivity of the RyR and the flux rate of SR Ca 2+ release. Thus, SR Ca 2+ uptake and release are an integrated system.
![FIGURE 46.10, Ca 2+ uptake into the SR by SERCA2a. An increased rate of uptake of Ca 2+ into the SR enhances the rate of relaxation ( lusitropic effect ). PLB, when phosphorylated ( P ), removes the inhibition exerted on the Ca 2+ pump by its dephosphorylated form. Thereby, Ca 2+ uptake is increased either in response to enhanced cytosolic [Ca 2+ ] or in response to beta-adrenergic agonists or CaMKII activation (which can be secondary to the beta-adrenergic system). 1 , 23 , 32 FIGURE 46.10, Ca 2+ uptake into the SR by SERCA2a. An increased rate of uptake of Ca 2+ into the SR enhances the rate of relaxation ( lusitropic effect ). PLB, when phosphorylated ( P ), removes the inhibition exerted on the Ca 2+ pump by its dephosphorylated form. Thereby, Ca 2+ uptake is increased either in response to enhanced cytosolic [Ca 2+ ] or in response to beta-adrenergic agonists or CaMKII activation (which can be secondary to the beta-adrenergic system). 1 , 23 , 32](https://storage.googleapis.com/dl.dentistrykey.com/clinical/MechanismsofCardiacContractionandRelaxation/10_3s20B9780323722193000463.jpg)
Phospholamban (PLB) was so named by its discoverers Tada and Katz to mean “phosphate receiver.” PLB is a single-transmembrane pass protein that binds directly to SERCA2a. Under basal conditions, this reduces the affinity of SERCA for cytosolic Ca 2+ , which results in slower SR Ca 2+ uptake at any given [Ca 2+ ] i . However, when PLB is phosphorylated by either PKA or CaMKII (at Ser16 or Thr17, respectively), the inhibitory effect is relieved, thereby resulting in increased rates of SR Ca 2+ uptake, cardiac relaxation (lusitropic effect), and increased SR Ca 2+ content, which drives stronger contraction (inotropic effect; see Fig. 46.10 ).
The Ca 2+ taken up into the SR is stored within the SR before the next release. Calsequestrin is a highly charged, low-affinity Ca 2+ buffer (K d = 600 μM) found primarily inside the jSR, where it enhances the local availability of Ca 2+ for release through the nearby RyR. Calreticulin is another Ca 2+ -storing protein that is similar to calsequestrin in structure and function. There is also evidence that calsequestrin and two other proteins located in the SR membrane (junctin and triadin) may regulate the properties of the RyR and be part of the mechanism by which higher [Ca] SR enhances RyR opening. Reuptake by SERCA occurs everywhere in the SR membrane, including the network SR that surrounds the myofilaments. Diffusion of Ca 2+ within the SR is relatively fast, which allows restoration of [Ca 2+ ] SR at the jSR to occur quickly after Ca 2+ is taken back up everywhere. Indeed, during normal Ca 2+ release, intra-SR Ca 2+ diffusion is rapid enough to limit Ca 2+ gradients between SR release sites in the jSR and the Ca 2+ uptake sites. This diffusion also ensures that [Ca 2+ ] SR is relatively uniform throughout the myocyte, which facilitates the uniformity of SR Ca 2+ release and myofilament activation throughout the cell.
Excitation-contraction coupling is initiated by voltage-induced opening of the sarcolemmal L-type Ca 2+ channels. The channels are pore-forming macromolecular proteins that span the sarcolemmal lipid bilayer to allow a highly selective pathway for transfer of ions into the heart cell when the channel changes from a closed to an open state. Ion channels have two major properties: gating and permeation. Ca 2+ and Na + channels have two functional “gates,” activation and inactivation. At the normal resting membrane potential, the activation gates are closed and the inactivation gate is open, so the channels are available to open on depolarization in their characteristic voltage-gated manner. On activation, the inactivation gate starts to close, and the kinetics of inactivation depends on voltage, time, and local [Ca 2+ ] i . Recovery from inactivation (which makes the channels available for activation again) is also time, voltage, and Ca 2+ dependent. Thus, after the AP ends, time is required for the Ca 2+ and Na + channels to recover from inactivation.
Permeation (or conductance) refers to the actual flow of ions or current through the open channel. Ca 2+ and Na + channels are highly selective for Ca 2+ and Na + , respectively, relative to other physiologic ions. However, nonphysiologic ions can also permeate; barium (Ba 2+ ) and strontium (Sr 2+ ) readily permeate Ca 2+ channels, and lithium (Li + ) permeates Na + channels, and these ions are sometimes used experimentally to study I Ca and I Na . The concentration of the permeant ion influences the conductance, and in simple Ohm’s law terms (I Ca = g Ca [E m − E Ca ]), current is the product of conductance (g Ca ; which depends on gating and permeation) times the electrochemical driving force (E m − E Ca ), which is the difference between the membrane potential (E m ) and the potential that exactly counterbalances the transmembrane [Ca 2+ ] gradient (E Ca , typically +120 mV but changes as [Ca] i changes). Thus, depolarization activates both Ca 2+ and Na + channels but also decreases the driving force for the currents.
Both Ca 2+ and Na + channels contain a major alpha subunit with four transmembrane domains (I to IV), each of which has six transmembrane helices (S1 to S6) and a pore loop between S5 and S6. Each channel also has associated auxiliary subunits (α2δ, β, and γ for Ca 2+ channels) that may influence trafficking and gating. Activation is now understood in molecular terms as outward movement of the charged S4 transmembrane segment (called the voltage sensor ) in each of the four domains of Na + and Ca 2+ channels. This S4 voltage dependence differs among channels, and Na + channels are activated at more negative E m than are Ca 2+ channels. Inactivation is more complex and involves multiple channel domains, and channels accumulate in this state during prolonged depolarization. The open state is typically the last of a sequence of multiple molecular closed conformations. However, there is typically a binary switch between closed and open such that the single-channel conductance is either near zero or at a constant open conductance. This stochastic nature means that it is often better to speak of the probability of channel opening for a single channel, while the whole-cell current integrates flux through all the stochastic channels.
The cardiovascular system has two major types of sarcolemmal Ca 2+ channels, T-type and L-type channels. T (transient)–type channels open at a more negative voltage, have short bursts of opening, and do not interact with conventional Ca 2+ antagonist drugs. In adult ventricular myocytes, there is normally little T-type I Ca (except under pathophysiologic conditions). Even when expressed in ventricular myocytes, T-type channels do not seem to target the regions where RyRs are, and consequently do not participate in excitation-contraction coupling per se. However, measurable T-type I Ca is present in neonatal ventricular myocytes, Purkinje fibers, and some atrial cells (especially pacemaker cells). In these locations the negative activation voltages may allow T-type I Ca to contribute to pacemaker function. Thus, in ventricular myocytes, L-type currents predominate.
L (long-lasting)–type Ca 2+ channels are concentrated in the T tubules at jSR sites, where they are positioned for Ca 2+ -induced Ca 2+ release from the RyR. A fraction of L-type Ca 2+ channels are also localized in caveolae, where they may participate in local Ca 2+ signaling, which is distinct from triggering of SR Ca 2+ release. L-type Ca 2+ channels are inhibited by Ca 2+ channel blockers such as verapamil, diltiazem, and the dihydropyridines. I Ca is rapidly activated during the rising phase of the AP, but the combination of Ca 2+ influx via I Ca itself and local SR Ca 2+ release causes rapid Ca 2+ -dependent inactivation of I Ca . Voltage-dependent inactivation also contributes to I Ca decline during the AP, but I Ca continues at low levels throughout the AP. Inward I Ca is an important contributor to the plateau phase of the cardiac AP, and excess I Ca or failure of inactivation can prolong the duration of the AP and participate in EADs.
During beta-adrenergic stimulation, cyclic adenosine monophosphate (cAMP) and PKA activity increases and results in phosphorylation of the Ca 2+ channel and alteration of its gating properties. Notably, most of the molecular components of this beta-adrenergic receptor–cAMP-PKA and phosphatase pathway are localized directly at the L-type Ca 2+ channel, which facilitates rapid sympathetic activation of I Ca . PKA-dependent phosphorylation of the channel shifts activation (and inactivation) to more negative voltages and increases the open time of the channel. This combination can greatly increase I Ca , which increases both the fraction of SR Ca 2+ release and the Ca 2+ load of the cell and SR (to enhance further the Ca 2+ transient amplitude and inotropic state).
Voltage-gated cardiac Na + current is carried mainly by the Nav1.5 cardiac isoform, but a minor component is attributed to several other, neuronal isoforms. The Nav1.5 channels are especially concentrated at the ends of the myocyte near intercalated discs, but the overall density of I Na is relatively uniform between the T tubule and surface membrane. Depolarization activates I Na , and peak I Na is very large and drives the upstroke of the cardiac AP. Voltage-dependent inactivation of I Na is very rapid, and under normal conditions, Na + channels inactivate within 4 milliseconds of depolarization. However, a tiny fraction of Na + channels remain open (or reopen), thereby creating a small but persistent influx of Na + throughout the plateau of the AP. This so-called late sodium current (I NaL ) is characterized by ultraslow, voltage-independent inactivation and reactivation. Although the amplitude of I NaL is small (<1% of peak I Na ), because peak I Na is so large, this I NaL still constitutes a significant inward current during the plateau phase of the AP. Under pathophysiologic conditions, the amount of I NaL can increase significantly, which can result in acquired long-QT (LQT) syndrome and also cause Na + and Ca 2+ loading of myocytes, which carries additional arrhythmogenic potential. Thus, I NaL has emerged as a potentially important therapeutic target. ,
CaMKII is known to be upregulated and chronically activated in numerous pathophysiologic conditions (e.g., ischemia-reperfusion, heart failure, ROS). Also, CaMKII-dependent Na + channel phosphorylation causes increased I NaL , which may produce an acquired form of LQT3 syndrome in patients with genetically normal Na + channels (see Fig. 46.9 ). , At the same time, CaMKII also shifts Na + channel availability to more negative voltages, enhances intermediate inactivation, and slows recovery from inactivation, all loss-of-function effects that could cause an acquired Brugada syndrome–like condition. Indeed, this can foster both phenotypes, depending on the heart rate (HR): LQT syndrome at a lower HR and Brugada syndrome at a higher HR. CaMKII also modulates Ca 2+ and potassium (K + ) channel currents, which can further promote arrhythmogenesis through EADs and enhanced transmural dispersion of repolarization.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here